Facile synthesis ofporous Pd nano flowers with excellentcatalytic activity towards CO oxidation☆
Tareque Odoom-Wubah ,Mingming Du ,Williams Brown Osei,Daohua Sun ,Jiale Huang ,*,Qingbiao Li,2,3,*
1 Department of Chemical and Biochemical Engineering,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361005,China
2 Environmental Science Research Center,College of the Environment&Ecology,Xiamen University,Xiamen 361005,China
3 College of Chemistry&Life Science,Quanzhou Normal University,Quanzhou 362000,China
Keywords:Pd nano flowers Microorganism CO oxidation CTAC
ABSTRACT Microorganism-mediated,hexadecyltrimethylammonium chloride(CTAC)-directed(MCD)method was employed in this work to synthesize Pd nano flowers(PdNFs).Proper Pichia pastoris cells(PPCs)dosage,ascorbic acid(AA),Pd(NO3)2 and CTAC concentrations were essential for the growth of the PdNFs.The size of the assynthesized PdNFs could be tuned by adjusting the amount of Pd(NO3)2 solution and dosage of PPCs used.Characterization techniques such as X-ray diffraction,transmission electron microscopy,and X-ray photoelectron spectroscopy were used to verify the nature of the PdNFs.Finally the PdNF/PPC nanocomposites were immobilized onto TiO2 supports to obtain bio-PdNF/TiO2 catalysts which showed excellent catalytic activity for CO oxidation,obtaining 100%conversion at 100°C and remaining stable over a period of 52 h of reaction time.
1.Introduction
The specific characteristics exhibited by metal nanostructures are attributed to their size,shape,composition,crystallinity,and structure[1-4].Their unique catalytic,optical,thermal,electrical and photonic properties at the nanoscale,make them preferable to their bulk counterparts[5-7].Metal nanostructures are mainly fabricated by a variety of chemical and physical methods[8-12].However,with increased impetus for environmentally benign processes,biogenic production of nanoparticles,as a novel method,has gained much attention due to its‘eco-friendly’nature,inexpensiveness and easy availability of materials.As a result,designing,synthesis,and characterization of stable metal nanostructures using biological means are of significant interest.In contrast to synthetic templates,using microbial cells,the cell surface layers have the advantages of nanoscale dimensions,versatility,and specificity[13-18].However,the shape of metal nanostructures could not be effectively controlled using only microbial cells.Therefore,in our previous studies,microorganism-mediated,surfactant-directed(MSD)approach was used to tune the morphology ofmetalnanostructures.For example,it was possible to synthesize well-defined Au nanowires[19,20]and Au nanohorns[21,22]using the MSD method.
There has been extensive research on various shapes of Pd nanoparticles ranging from nanothorns[23],nanoboxes,nanocages[24], nanorods,nanowires[25],and branched nanocrystals etc.[26].In addition,there have been some reports on flower-like three dimensional metal,metaloxides,and metalsul fide nanomaterials,which possess the advantages of larger surface area and more active centers,making them preferable to spherical and normal particles[27,28].More recently Yin et al.demonstrated the rapid assembly of porous Pd nanoflowers with average size of 50 nm with enhanced methanol activity employing 1,2-hexadecanediol as the reduction agent at 180°C[29].Wang et al.also produced porous Pd nanoflowers using N-2-hydroxyethylpiperazine-N9-2-ethanesulfonic acid.The as-produced Pd nano flowers showed high electrocatalytic activity towards methanoloxidation[30].However,despite these works,it still remains a challenge to generate anisotropic Pd nanoparticles.
Hence in this study,the microorganism-mediated,surfactant directed approach was extended to synthesis of Pd nanostructures. Specifically,Pd nano flowers(PdNFs)with tunable diameters were fabricated by the microorganism-mediated,CTAC-directed approach.The as-produced Pd nano flowers were characterized using transmission electron microscopy(TEM),X-ray diffraction(XRD),and X-ray photoelectron spectroscopy(XPS)analysis.
Supported noble metal catalysts have been extensively investigated because they are efficient and durable for CO oxidation.Supported Pd catalyst is one of the extensively used catalysts for CO oxidation[31-34]as such we immobilized the as-produced bio-PdNFs onto TiO2support to form supported bio-PdNF/TiO2catalyst,through the sol-immobilization method.The results from the test showed that,assynthesized bio-PdNF/TiO2catalyst had excellent catalytic performance for CO oxidation outperforming two other supported catalysts.The first catalyst is prepared as in our previous report[35]and is denoted as(PdNS/TiO2-IM),and the second supported Pd hexagonal catalyst(PdHS/TiO2)as in Fig.3(a).
2.Experimental Section
2.1.Materials and chemicals
Palladium nitrate(Pd(NO3)2)was purchased from Sinopharm Chemical Reagent Co.Ltd,China,Hexadecyltrimethylammonium chloride(CTAC,99.9%)and ascorbic acid(AA,99%)were purchased from Tianjin Guangfu Fine Chemical Research Institute and Sangon Biotech(Shanghai)Co.,Ltd,respectively.All chemicals were used as received,without further purification.
2.2.Cultivation of PPCs and preparation of their powder
The medium used for growing the PPCs contained yeast extract(10 g·L-1),peptone(20 g·L-1),and glucose(20 g·L-1)in deionized water.After the cells were incubated for 48 h at30°C and 150 r·min-1,they were harvested through centrifugation,and then dried in an oven(60°C)for 24 h.The dried cells were then crushed into fine powders,which were screened through a 100-mesh sieve.The screened cell powder was stored in a desiccator.
2.3.Synthesis of the PdNFs
First different amounts of dried PPCs(0.005-0.01 g)was added to aqueous CTAC(5.0 mmol·L-1)in a flask,followed by the addition of 50-200 μl aqueous(Pd(NO3)2)(0.07425 mol· L-1)and then 50 μl of AA(0.2 mol·L-1)to form the initial reaction solutions.The contents of the flasks were then shaken on a shaker(at 30 °C,150 r·min-1)for 1-24 h.The color of the solutions turned from yellow to dark brown 15 min after the addition of the AA.The resulting solutions were sampled and centrifuged at 2000 r·min-1for 10 min.The supernatants were decanted and the precipitates dispersed in 200 μl deionized water for further characterization.
2.4.Catalyst synthesis
The as-produced bio-PdNFs were immobilized onto TiO2supports by sol-immobilization method to form supported bio-PdNF/TiO2catalyst.After the preparation of the bio-PdNFs,weighed amounts of TiO2were added to the solution and replaced in the shaker to allow immobilization for 10 h.The suspension after the immobilization process was filtered,washed thoroughly with DI water and then placed in an oven at 70°C for 6 h.Thereafter the catalysts were calcinated at different temperatures(300 °C,375 °C,450 °C,550 °C,and 650 °C)for 6 h prior to testing.All other catalyst were prepared in this manner except for the one labeled bio-PdNFs untreated,which was not calcinated to ascertain the effect of calcination on the performance of the catalyst.For comparison a catalyst was prepared as described in our previous report[35](PdNS-IM,Fig.S4).Supporting information(SI)and Pd hexagonal shaped nanostructures as in Fig.3(a)(PdHNs)were supported on TiO2and dried in the oven at 70 °C and later calcinated at 375 °C.
2.5.Characterization of the PdNFs
Transmission electron microscopy(TEM)examinations were conducted on an electron microscope(Tecnai F30,FEI;Netherlands)with an accelerating voltage of 300 kV.Size distribution measurements of the bio-PdNFs were done by calibrating their TEM images scale bar on the SigmaScan Pro software(SPSS Inc.,Version 4.01.003)as in our previous reports.Then their diameters were estimated using the software.XRD investigation of the atomic structure of the crushed samples was done on an X-ray crystallography(Phillips;Netherlands)provided with Cu Kαradiation(40 kV,30 mA).X-ray photoelectron spectroscopy(XPS)study to determine the electronic state of the samples was carried out on an American Perkin-Elmer PHI-1600 with a monochromatised microfocused Al X-ray source.
2.6.Catalytic test
The experimental process is same as in our previous reports[36,37].The catalytic tests were carried out in a half-inch-diameter vertical fixed-bed stainless-steel reactor using a feed volume concentration of 1%/1%/98%of CO(99.999%),O2(99.999%)and N2(99.999%)and 0.2 g of catalyst at a space velocity of 18,000 ml·h-1·(g·cat)-1.The temperature was measured by using a glass tube covered Cr-Al thermocouple located in the center of the catalyst bed.Analysis of the gas leaving the reactor was done online,via a chromatograph.Having a thermal conductivity detector(TCD),using a MS 5A packed column(2 mm×3 m)with the ability to detect temperature programmed CO+O2curves.The catalyst was heated in the reaction mixture from 30 to 350 °C at a heating rate of about 10 °C min-1.The CO conversion was then determined using the equation below.
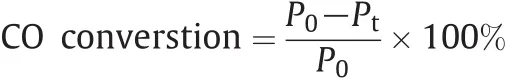
where Pois the partial pressure of CO in the inlet and Ptis the partial pressure in the outlet recorded at a certain temperature.
3.Results and Discussions
3.1.Synthesis of the PdNFs
Uniform and well-defined Pd nano flowers were synthesized by reducing different amounts of aqueous Pd(NO3)2solution of concentration(0.74 mmol·L-1),with AA(1.0 mmol·L-1)in the presence of dried PPC(0.005 g)and CTAC(5.0 mmol·L-1).The as-produced PdNFs were formed from the interconnection of primary building Pd nanoparticles of size 4.0 nm as seen in Fig.S3(b),SI.
Corresponding TEM images of the as-produced bio-PdNFs from different amounts of Pd(NO3)2precursor solutions(50 μl to 150 μl)are shown in(Fig.1(a)-(c)insert particle diameters).It can be seen from the images that,uniform bio-PdNFs could be produced from the reduction of aqueous Pd(NO3)2(0.74 mmol·L-1)with AA(1.0 mmol·L-1)in the presence of dried PPC(0.005 g)and CTAC(5.0 mmol·L-1).Their estimated statistic diameters were 35.6±4.6,43.7±6,51.0±10 nm,corresponding to 50μl,100μl,and 150μlofaqueous Pd(NO3)2solutions respectively.The results from the XRD analysis in(Fig.2(a))show clear diffraction peaks,which corresponds to 40.3°,46.7°,68.0°,82.2°and 86.4°.These peaks can respectively be assigned to{111},{200},{220},{311},and{222}crystal facets for face centered cubic(fcc)of the crystal structure of Pd(JCPDS standard 05-0681).The XPS spectrum(Fig.2(b))of the as-produced bio-PdNFs shows Pd 3d region(Pd 3d5/2and Pd 3d3/2)energy values.The binding energy values are 335.5 eV for Pd 3d5/2and 340.8 eV for Pd 3d3/2respectively.
Anisotropy,one of the primary characteristics of crystals has been reported to highly depend on experimental factors[38,39].As such,further inquiry experiments were conducted to verify the effects of(PPCs,Pd(NO3)2,AA and CTAC)on the morphology of the resulting Pd nanostructures.
3.2.Effect of PPCs
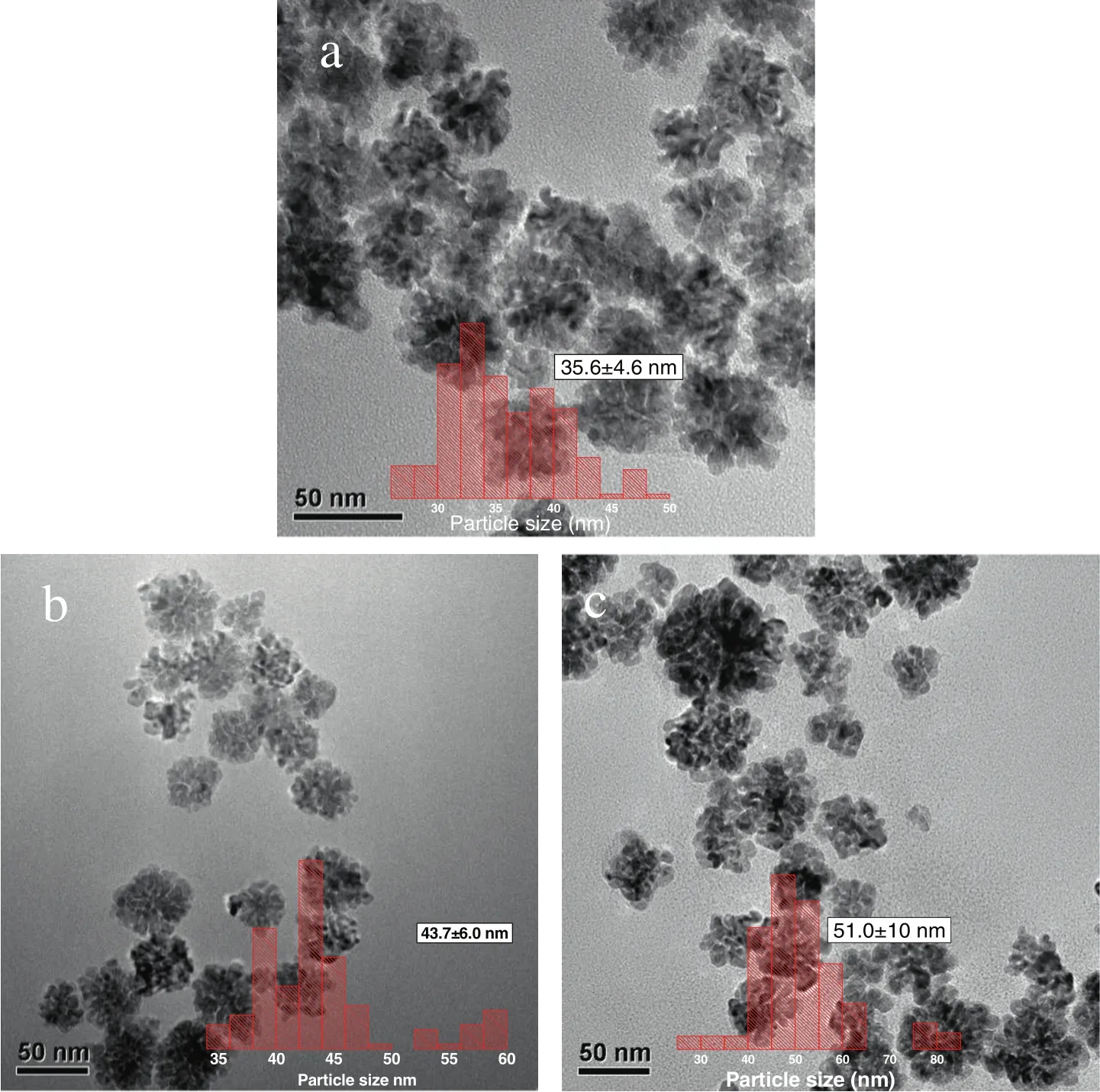
Fig.1.TEMimage(a-c)of the as-produced PdNFs synthesized through the reduction of aqueous 50μl,100μl and 150μlPd(NO3)2(0.74 mmol·L-1)respectively with AA(1.0 mmol·L-1)in the presence of dried PPC(0.005 g)and CTAC(5.0 mmol·L-1).
The function of the microorganism(PPC)in the formation of the bio-PdNFs was closely related to biosorption of Pd2+ions as in previous reports[19].It acted as a preferential nucleation site for Pd clusters.The assertion was further proven using FTIR studies which showed that,oxygenous-and nitrogenous-active groups of carboxylate anion of amino-acid residues(side-chains of polypeptide backbone)and peptide bond(amides I and II)were responsible for the biosorption process.The effect of the PPCs on the morphology of the as-produced PdNFs was verified by altering their usage(amount used)in the fabrication process while keeping the other parameters constant.From Fig.3(a),it is observed that,reducing Pd(NO3)2,in the presence of CTAC but without PPCs resulted in the formation of hexagonal Pd structures with diameters 20.1±2.4 nm and thus the production of the bio-PdNFs was impossible without the PPCs.This phenomenon was also observed in our previous reports utilizing the MSD strategy to synthesize AuNWs[19,20].However,as seen in Fig.3(b)-(d),uniformly formed bio-PdNFs could be fabricated using different amounts of the PPC biomass in the synthesis process.In addition,it is seen that,the sizes of the as-formed PdNFs increased with increasing the amount of PPC used in the synthesizing process.Thus their diameters could also be tuned by adjusting the amount of microbial biomass used,an occurrence consistent with our previous reports.The sizes of the as-produced bio-PdNFs were 24.4±6.6,35.1±4.4 and 54.2±7.6 nm for 0.025,0.005 and 0.01 g of PPCs respectively.
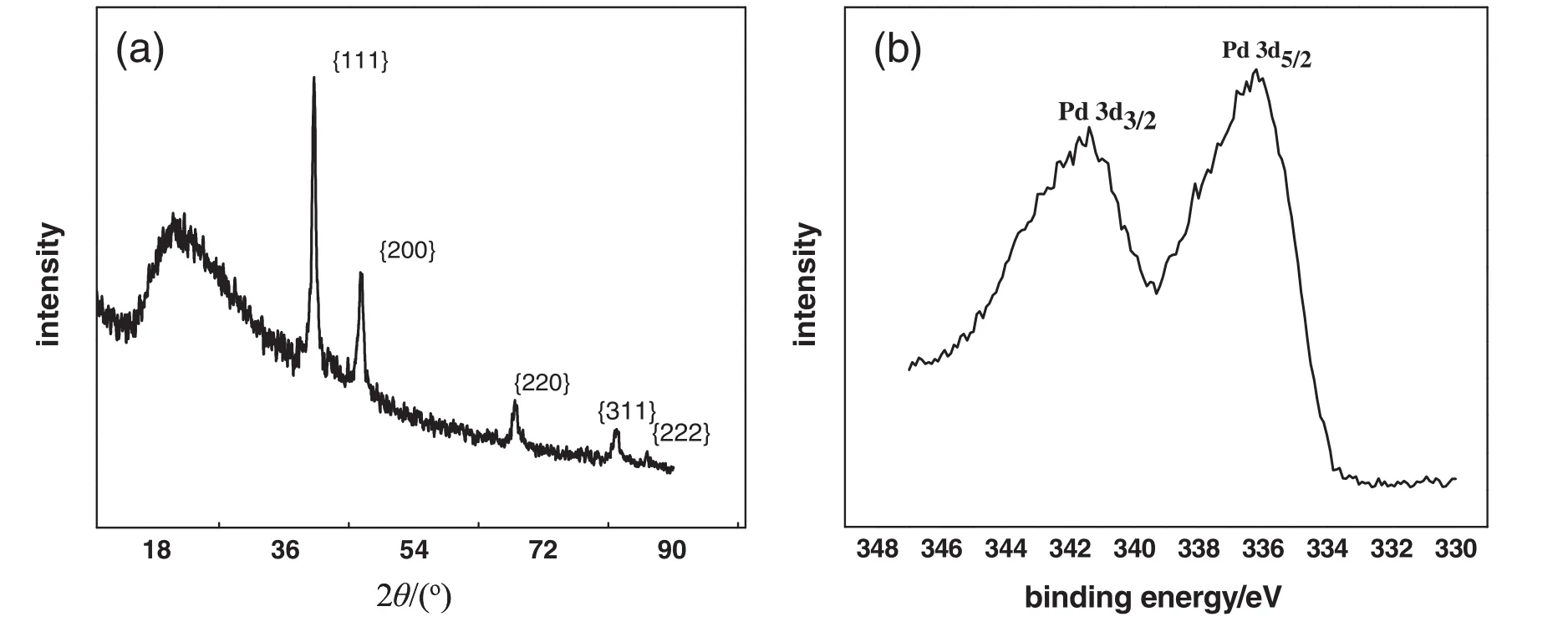
Fig.2.(a)XRD and(b)XPS pattern of the as-produced PdNFs,synthesized through the reduction of aqueous Pd(NO3)2(0.74 mmol·L-1)with AA(1.0 mmol·L-1)in the presence of the dried PPCs(0.005 g)and CTAC concentration of 5.0 mmol·L-1.
3.3.Effect of CTAC
Previous accounts have shown that wetting or stabilizing agents are essential to the evolution and stability of different morphologies of metal nanostructure,[40,41]since they lower the surface tension of water.Keeping all other parameters constant but leaving out CTAC resulted in the formation of many tiny bits of Pd nanoparticles as seen in Fig.S2(a),SI.In contrast adding a proper concentration of 5.0 mmol·L-1CTAC resulted in the formation of the well-formed PdNFs in Fig.1(a)-(c).Lowering the concentration to 2.5 mmol·L-1resulted in the formation of broken bio-PdNFs coupled with otherplate-like Pd nanostructures Fig.4(a).On the other hand increasing the CTAC concentration to 10.0 mmol·L-1resulted in the formation of several Pd nanostructures consisting of cube,spherical and plate-like as shown in Fig.4(b).The role of CTAC was elucidated in our previous studies in the production of AuHNs.CTAC was shown to produce CTA+and Cl-in aqueous solution with the charged CTA+head groups bound to the metal surface and the alkyl tails pointed towards water.Since Cl-hardly ionizes,the shaping of the flower-like structure is ascribed to CTA+which can easily attach itself to the Pd crystalline metal surface reducing the growth rate in favor of the anisotropic growth.Therefore in the case of lower CTAC concentration(2.5 mmol·L-1),the production of the broken Pd nano flowers was probably caused by insufficient CTA+to direct the shape evolution process.In the case of higher concentration of CTAC,the abundance arrested the effectiveness of the secondary nucleation process thereby limiting the formation of the bio-PdNFs.
3.4.Effect of the precursor Pd(NO3)2 concentration
The amount of Pd(NO3)2precursor solutions used was crucial in determining the final morphology of the Pd nanostructures.Well defined bio-PdNFs as in Fig.1(a)could be produced using 50 μl of precursor solution with an average diameter of 35.6±4.6 nm.Halving the amount(25 μl)resulted in the production of numerous particles instead of the bio-PdNFs(Fig.S1(a)).This occurrence can be attributed to insufficient Pd nanoparticlesto be consumed in the Ostwald ripening process,causing excess CTAC as compared to Pd(NO3)2.The excess CTAC thus suppressed the number of Pd nanoparticles that could have been obtained from secondary nucleation for the further development of the bio-PdNFs.However,doubling(100 μl)and tripling(150 μl)the amount could also produce similar bio-PdNFs as seen in Fig.1(b)and(c)with average diameters of 43.7±6 and 51.0±10 nm.Thus the morphology of the as-produced PdNFs could be tuned by adjusting the amount of Pd(NO3)2precursor solutions used in the experiment.Tang et al.reported a similar occurrence where increasing the precursor(K2PdCl4)concentration between 1.25 and 12.5 mmol·L-1increased the average diameter of as-formed spherical porous palladium nanostructures from 40 to 100 nm[42].Quadrupling(200 μl)the amount of precursor solution used resulted in the production of similar PdNFs as can been seen in Fig.1(a)-(c).Nevertheless,the bio-PdNFs formed employing 200 μl of the precursor solution severely agglomerated(Fig.S1(b)).This could be ascribed to the excess availability of Pd nanoparticles in solution compared to CTAC.At 200 μl the protection of CTAC was insufficient to provide shield against agglomeration as such the observation in(Fig.S1(b)).
3.5.Effect of AA
The reaction system can be said to be kinetically controlled as compared to thermodynamic since the reaction was carried out under a relatively low temperature of30°C.As such the morphology of the PdNFs was kinetically influenced by the reducing agent ascorbic acid(AA).As shown in Fig.5(a),compared to the well-defined bio-PdNFs prepared at 1.0 mmol·L-1AA in Fig.1(a)-(c),without AA only particles reduced on and around the biomass of the microorganism were observed(Fig.5(a))and thus the reductant was indispensible in the fabrication of the PdNFs.In addition halving the AA concentration to 0.5 mmol·L-1(Fig.5(b))resulted in the formation of numerous Pd spherical nanostructures.Moreover,if the AA concentration was doubled(2.0 mmol·L-1),uniform PdNFs were also obtained.However,they tended to agglomerate probably caused by the accumulation of excess Pd nanoparticles concentrated in a particular place within the reaction solution.Therefore the ideal concentration of the reductant(AA)was 1.0 mmol·L-1;lowering or increasing it was detrimental to the formation of the as-produced PdNFs.
3.6.Formation mechanism of the PdNFs
To predict the possible evolution process of the bio-PdNFs,TEM images at different periods during the reaction were monitored.Fig.6 shows the evolution process of Pd nanostructures.15 min after the addition of AA,the color of the solution changed from yellowish to darkbrown,and the TEM image(Fig.6(a))shows numerous Pd particles with corresponding average particle sizes of 4.0 nm(Fig.S3(a)).As the reaction progressed to time 30 min,most of the nanoparticles aggregated connecting to form bio-PdNFs coupled with other Pd structures under the direction of CTAC with the average particle sizes increasing to 20.6±13.0 nm Fig.S3(B).It can be also be seen from Fig.S3(particle sizes of the bio-PdNFs evolution process)that,the building nanoparticles began to slow down after 3 h of reaction and the average particle diameter of the bio-PdNFs became 43.7±6.0 nm.After which no obvious change in both the shape and size of the bio-PdNF was observed.Meanwhile,the Pd flower like shape slowly emerged under the directional role of CTAC and the well-defined shape was achieved at about 10 h of reaction.
The formation process of the PdNFs can be summarized as follows.Firstly,the interaction between the microorganism(PPCs)and the Pd ions,prior to the addition of AA resulted in the bioreduction of the Pd ions to form Pd(I)ions both on the surface of the PPCs with some slipping into the solution due to the weak interaction between them and the PPCs.It is evident in Fig.5(a),where several nanoparticles were observed even without the addition of AA that,PPC has the ability to bio-reduce the Pd ions.However,compared to PPCs,AA is more likely to totally reduce the Pd ions to Pd(0)than the PPCs.The PPCs therefore might have acted as a preferred nucleation site in this case and the crystal nucleus was probably formed on it.Next,15 min after the addition of AA resulted in the formation of numerous free Pd clusters in the solution with the color of the solution changing from light yellow to black showing the availability of many Pd nanoparticles.These nanoparticles spontaneous accumulated to from Pd structures consisting of both well defined and unwell-defined bio-PdNFs and other Pd clusters(30 min).In addition CTA+absorbed onto the crystal nucleus reducing the growth rate in favor of anisotropic growth to guide the shape evolution process.Then under the direction of CTAC,the flower like morphology gradually emerged as a result of the Ostwald ripening process,with the smaller Pd ions dissolving in solution in favor of the larger ones.A possible formation mechanism of the bio-PdNFs is illustrated in Fig.7 based on the TEM samples taken at the different reaction times.
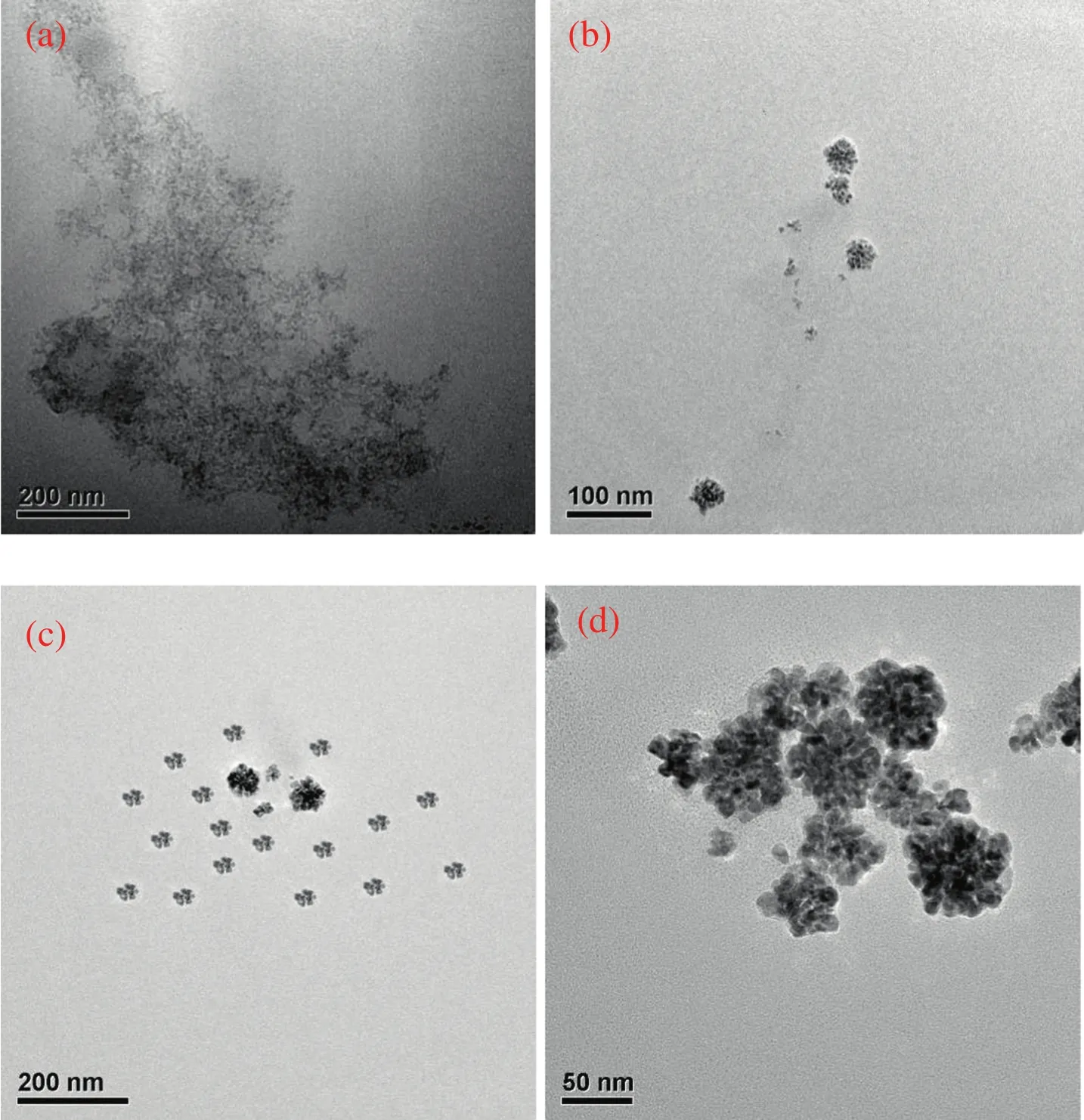
Fig.6.TEM images of Pd nanostructures taken at different reaction times.(a)t=15 min;(b)t=30 min;(c)t=3 h;(d)t=10 h.
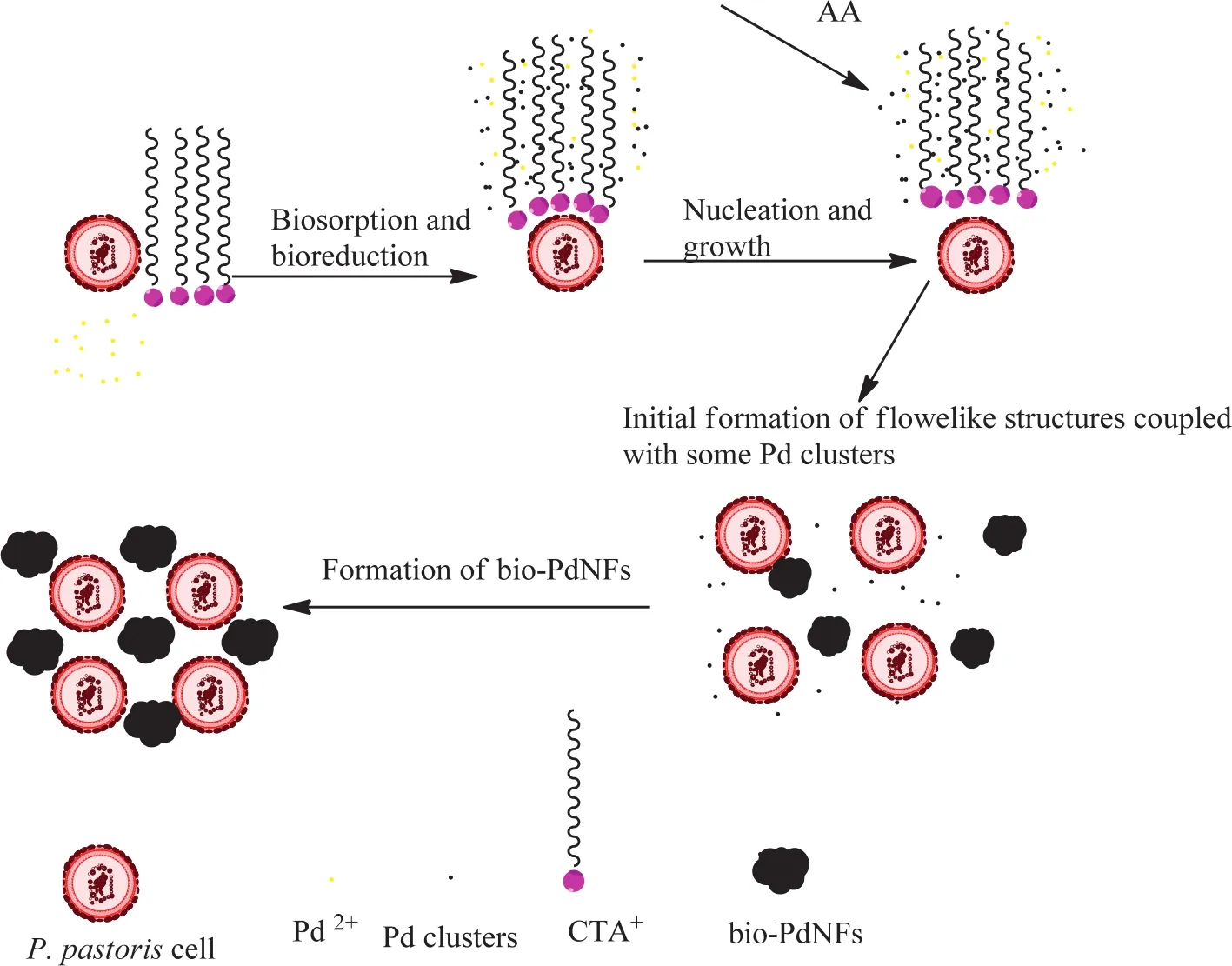
Fig.7.Schematic diagram of the formation of the bio-PdNFs through the reduction of aqueous Pd(NO3)2 with AA(0.5 mmol·L-1)in the presence of CTAC and PPCs.
3.7.Catalytic performance of the bio-PdNFs for CO oxidation
The catalytic test was done by placing the as-produced bio-PdNFs on TiO2supports to form bio-PdNF/TiO2catalyst using the solimmobilization process.These catalysts were then employed in the oxidation ofCO.The test was carried out using 0.2 g of catalyst packed in a glass fixed-bed reactor with the reaction temperature controlled by a tubular furnace.The feed gas consisted of N2,O2,and CO in the composition of 98/1/1,and the outlet gas velocity was analyzed online using a gas chromatogram analyzer equipped with a thermal conductivity detector(TCD).The three gas components were absolutely separated in a 5 ? molecular sieve column and inert nitrogen was used as internal standard for calculation.Calcination has been reported in literature to significantly improve the performance of catalyst by exposing their active sites.
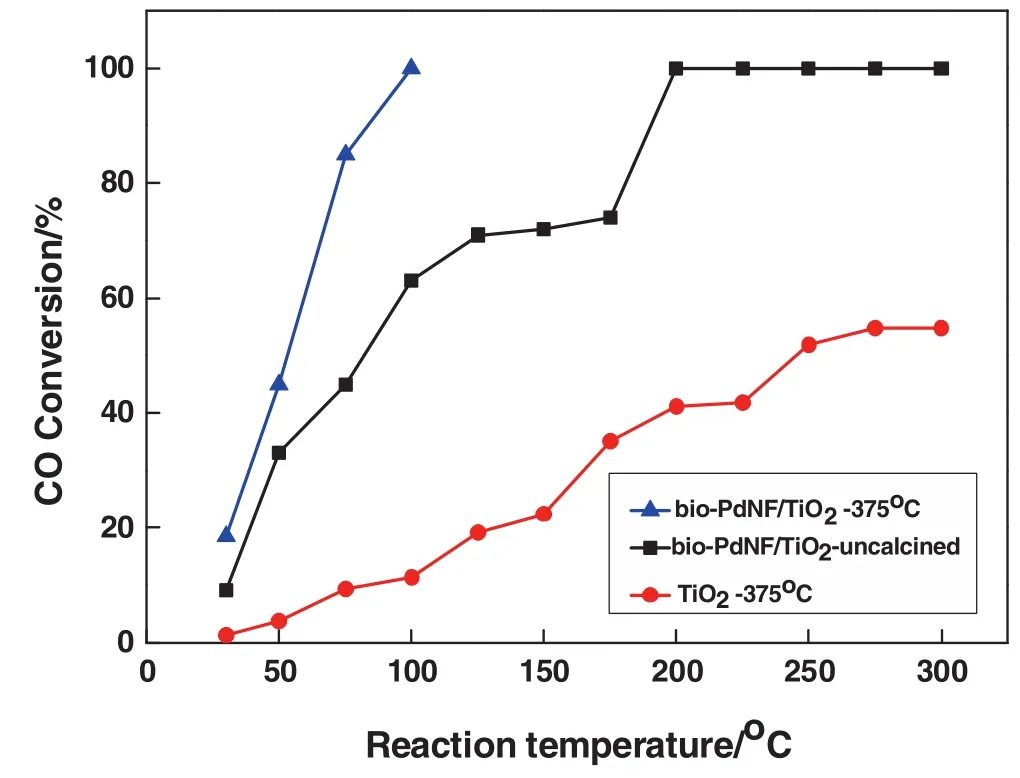
Fig.8.CO oxidation over different conditons.▲bio-PdNF/TiO2 calcinated at 375 °C;■bio-PdNF/TiO2,untreated;●TiO2 calcined at375°C.Reaction conditions:0.2 g ofcatalyst,volume concentration of feed gas CO/O2/N2 is 1%/1%/98%;space velocity:18000 ml·h-1·(g cat)-1.
From Fig.8,it can be seen that the bio-PdNF/TiO2without calcination reached 100%conversion at 200°C,however,when it was calcined at 375°C for 6 h the performance improved significantly to 100%conversion at100°C.Calcination treatment of the bio-Pd catalyst helped to expose the active sites of the catalyst for better performance by the removal of significant amount of CTAC on the catalyst surface.
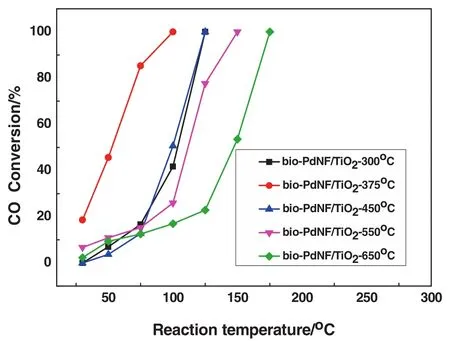
Fig.9.CO oxidation overbio-PdNF/TiO2 catalyst with different calcination temperatures.■bio-PdNF/TiO2—300 °C,● bio-PdNF/TiO2—375 °C,▲ bio-PdNF/TiO2—450 °C,▼ bio-PdNF/TiO2—550°C,◆bio-PdNF/TiO2—650°C.Reaction conditions:0.2 g of catalyst,volume concentration of feed gas CO/O2/N2 is 1%/1%/98%;space velocity:18000 ml·h-1(g·cat)-1.
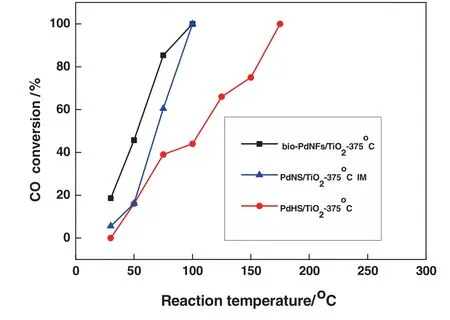
Fig.10.CO oxidation comparison test,over different Pd nanostructures.■bio-PdNF/TiO2 at375 °C,▲ PdNS/TiO2 at375 °C,● PdHS/TiO2 at 375 °C Reaction conditions:0.2 g of catalyst,volume concentration of feed gas CO/O2/N2 is 1%/1%/98%;space velocity:18000 ml·h-1(g·cat)-1.
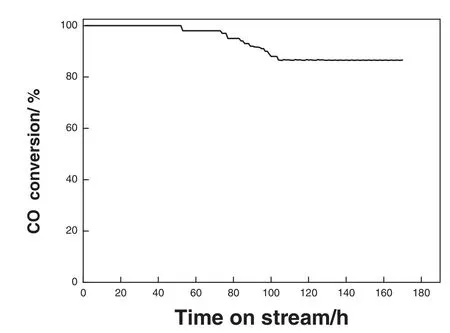
Fig.11.Stability test of the bio-PdNFs/TiO2.volume concentration of feed gas CO/O2/N2 is 1%/1%/98%;space velocity:18000 ml·h-1(g·cat)-1.
In addition,it was not possible to achieve 100%conversion employing only the TiO2support for the reaction even at a higher temperature of 300°C.The excellent performance of the bio-PdNF/TiO2catalyst can therefore be attributed to synergistic interaction between the bio-PdNFs and that of the support,enhanced by the calcination treatment.In order to further ascertain the effect of calcination on the catalytic performance,different bio-PdNF/TiO2catalysts were calcined at 300 °C,375 °C,450 °C,550 °C,and 650 °C and denoted as bio-PdNF/TiO2-375 °C in the case of calcination temperature at 375 °C etc.Fig.9 shows the results of the catalytic performance of the bio-PdNF/TiO2at different calcination temperatures.The performance of the bio-PdNF/TiO2calcined at 375 °C outperformed the catalyst calcined at 300 °C even though they had the same initial size before calcination.In addition it showed the best catalytic performance.Increasing the temperature from 375 °C to 650 °C resulted in a reduction in activity.The decrease in activity could be attributed to the increase in the size of the particles due to calcination.
For comparison,two different Pd nanostructures were fabricated and immobilized onto TiO2,one as described in our previous report[35]and denoted as PdNS/TiO2and the other supported PdHexagons as shown in Fig.3(a)and denoted as PdHS/TiO2.The two Pd nanostructures were made to undergo the same pretreatment as the bio-PdNFs.Fig.10,presents the catalytic performance of the catalysts.It can be seen from the figure that even though both the bio-PdNFs/TiO2and that of PdNS/TiO2achieved complete conversion(100%)at 100°C,the bio-PdNFs/TiO2had a better initial performance compared to that of PdNS/TiO2and was highly stable.In contrast it out-performed the PdHS/TiO2.The superior performance of the bio-PdNF/TiO2catalyst compared to the two other catalysts(PdNS/TiO2and PdHS/TiO2)could be attributed to the spaces between the nanoparticles and the shape defects of the particles.It is worth noting that the catalysts were not of the same size,therefore their performance is based on individual integrated particle size and composition.
Catalyst stability is a trait vital for industrial application;as such stability test was performed for the catalyst.Fig.11 shows the time on stream test of the bio-PdNF/TiO2.It achieved 100%conversion at 100°C maintaining this stability over 52 h of reaction before reducing gradually to about 86.5%conversion.After which it maintained the 86%conversion for over a week.The stability could be attributed to CTAC which acted as a stabilizer during the production of the bio-PdNFs.The decline in activity could be assigned to aggregation and particles growth and probably the formation of carbonates on the surface of the catalyst.As a result of the adsorption of gaseous CO2,this has been reported to bind with the surface of catalyst blocking some of the active sites thereby reducing activity[43,44].
Table 1 below compares the catalyst prepared by the microorganismmediated surfactant-directed method in this paper with others in literature.Although its T100is not the lowest,its activity is still satisfactory with regard to the lower Pd loading,oxygen deficiency condition and higher space velocity.Additionally,it had a very commendable stability compared with the others.The synthesis process is also milder,inexpensive and eco-friendlier compared to pure chemical means and without the need of using any sophisticated equipment.
4.Conclusions
In summary, flower-like Pd nanostructures were synthesized using a microorganism-mediated approach,under the direction of the surfactant CTAC.The morphology of the as-produced PdNFs could be tuned by adjusting the amount of precursor solution and microbial biomass used in the reaction.Controlled experiments showed that the formation of the PdNFs was highly dependent on experimental parameters.In the case of the reductant ascorbic acid(AA),the optimal concentration was found to be 1.0 mmol·L-1;lowering orincreasing it was detrimental to the formation of the as-produced PdNFs.In addition,the bio-PdNFs showed commendable catalytic activity for CO oxidation when it was immobilized onto TiO2supports to form bio-PdNF/TiO2catalyst.Catalytic stability test showed that the bio-PdNF/TiO2catalyst was highly stable.
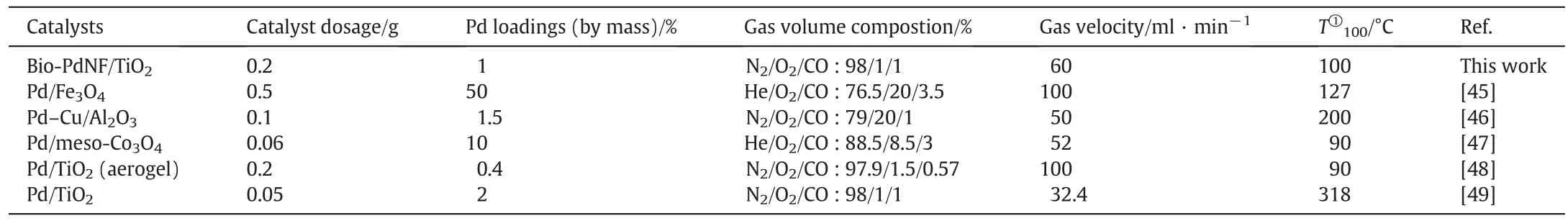
Table 1 Catalytic activity for CO oxidation on various supported Pd catalysts
Appendix A.Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2015.08.009.
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- Mechanistic study on the cellulose dissolution in ionic liquids by density functional theory☆
- Long-term nitritation performance of ammonium-rich land fill leachate☆
- Analysis of fouling characteristic in enhanced tubes using multiple heat and mass transfer analogies☆
- Removal of elemental mercury by modified bamboo carbon☆
- Simple processing technology of leaching water using CO2 microbubbles☆
