Mechanistic study on the cellulose dissolution in ionic liquids by density functional theory☆
Yingying Yao ,Yao Li,Xiaomin Liu ,Xiaochun Zhang ,Jianji Wang ,Xiaoqian Yao ,*,Suojiang Zhang ,*
1 Beijing Key Laboratory of Ionic Liquids Clean Process,Key Laboratory of Green Process and Engineering Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
2 School of Chemistry and Chemical Engineering,University of Chinese Academy of Sciences,Beijing 100049,China
3 Key Laboratory of Green Chemical Media and Reactions,Ministry of Education,School of Chemistry and Environmental Science,Henan Normal University,Xinxiang,Henan 453007,China
Keywords:Ionic liquids Cellulose Dissolution mechanism
ABSTRACT Ionic liquids(ILs)have attracted many attentions in the dissolution of cellulose due to their unique physicochemical properties as green solvents.However,the mechanism of dissolution is still under debate.In this work,computational investigation for the mechanisms of dissolution of cellulose in[Bmim]Cl,[Emim]Cl and[Emim]OAc ILs was performed,and it was focused on the process of breakage of cellulose chain and ring opening using cellobiose as a model molecule.The detailed mechanism and reaction energy barriers were computed for various possible pathways by density functional theoretical method.The key finding was that ILs catalyze the dissolution process by synergistic effect of anion and cation,which led to the cleavage of cellulose chain and formation of derivatives of cellulose.The investigation on ring opening process of cellobiose suggested that carbene formed in ILs played an important role in the side reaction of cellulose,and it facilitated the formation of a covalent bond between cellulose and imidazolium core.These computation results may provide new perspective to understand and apply ILs for pretreatment of cellulose.
1.Introduction
Cellulose is an abundant renewable compound on earth and has very attractive properties,such as biocompatibility,biodegradability,and thermal and chemical stability[1].It consists of linear β-(1 → 4)-linked glucose chains which contain numerous intermolecular and intramolecular hydrogen bonds.The highly ordered structure of cellulose makes it a challenge to find appropriate solvents for dissolution and follow-up use[2].
Ionic liquids(ILs)have attracted much attention in cellulose investigation since they show great potential to dissolve cellulose compared to transitional cellulose solvents[3-8].ILs are regarded as a new class of stable,green organic solvents which offer a variety of remarkable physical properties,such as high thermal stability,low melting points,negligible vapor pressure,non flammability,reusability,and designability[9-13].ILs consisting of imidazolium,pyridinium cations and OAc-,HCOO-,(MeO)2PO2-,Cl-anions have the high ability to dissolve cellulose,and were widely investigated[14].However,the mechanism of dissolution of cellulose in ILs remains elusive and needs more detailed analysis.
Experimental and molecular simulation studies have shown that both anions and cations were involved in the dissolution process.Numerous reports have proposed that anions formed hydrogen bonds with cellulose,which may be the major reason for dissolving cellulose in ILs[15-17].On the other hand,cation was suggested to play an indirect role in dissolution process instead of forming strong direct hydrogen bonds with cellulose[18].A proposed dissolution mechanism of cellulose in ILs was supposed that the anion and cation of ILs formed hydrogen bonds with the hydrogen and oxygen atoms of the cellulose respectively[19],which primarily occurred between C6 and C3 hydroxyl groups of neighbored cellulose chains[20].The interaction between ILs and cellulose which resulted in the separation of hydroxyl groups of different chains,may be one possibility leading to the dissolution of cellulose in ILs[19,21].However,little derivative reactions in the process of dissolution of cellulose in ILs were taken into account.
Heinze etal.[22]also studied the mechanism of cellulose dissolution in ILs.They investigated the interaction between cellooligomers and ILs by NMR spectroscope and found that the C1 signal of glucose unit was not present if the cellooligomers were dissolved in[Emim]OAc.In accordance with these results,authors gave an explanation that the C1 carbon of the reducing end of cellooligomer may be covalently bonded with C2 carbon of the imidazolium core,forming a carbon-carbon bond.The proposed structure for the covalent product is shown in Fig.1.
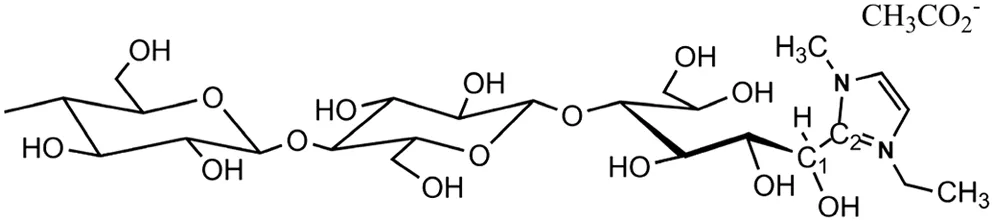
Fig.1.Proposed structure for a covalent binding of[Emim]OAc to cellooligomer(DP 6-10).
Antje Potthast et al.[23]observed that carbonyl groups were present after cellulose dissolution in imidazolium-based ILs.Further,imidazolium-based ILs deprotonated at C2 under basic condition were suggested to be able to react with electrophiles,for instance,benzaldehyde[24].Ebner et al.[25]combined above,and gave a series of13C-isotopic labeling and fluorescence labeling experiments.The experimental evidence verified the hypothesis that the C2 carbon of 1-alkyl-3-methylimidazolium cation may form covalent bond with reducing end of cellulose[25].Surprisingly,this was not the case when cellooligomer was dissolved in[Emim]Cl,[22]which was subsequently supported by experiments of Matthew et al.[26]A series of experiments were then conducted to investigate cellulose dissolution in 1-ethyl-3-methyllimidazolium chloride,[Emim]Cl[27-29].It was reported that[Emim]Cl penetrated into wood and liquefied wood components by depolymerizing them[27].Subsequent studies investigated the reaction behavior of cellulose in[Emim]Cl at 373,393,and 413 K.They showed that the solubilized cellulose in[Emim]Cl was depolymerized into various low molecular weight compounds including cellobiose,cellobioam,glucose,and levoglucosan,but the carbon-carbon covalent bond mentioned above still cannot be detected[28].These results not only suggested that the dissolution of cellulose in ILs may lead to complex reactions but also highlighted the role ILs may play in the dissolution process.
Some studies used quantum mechanical calculations to understand the interaction between cellulose and ILs.The solution of cellulose in[Emim]OAc was studied by density functional theory(DFT)calculation to evaluate the ionic influence on the dissolution mechanism[30].Their results showed that the interaction between ILs and cellulose was stronger than the intermolecular interaction of cellulose.Further,the acetate anion formed strong hydrogen bond with cellulose.R.S.Payal and coworkers investigated the dissolution mechanism of cellulose in explicit solvent([Emim]OAc)by DFT analysis,chosen cellobiose as model[31].The calculation data suggested that both cation and anion were responsible for dissolution process of cellulose,and the results demonstrated the interaction between ion(anion or cation)and cellulose.Du et al.[32]explored the reaction of ring opening by ab initio calculations with an implicit solvent model,and showed that breakage of the C-O bond on the glucose ring was the critical step[32].Wei et al.[33]also investigated the reactivity of cellulose with ILs using glucose as a model by DFT calculation.It was found that TEA facilitated the ring opening of glucose.The theoretical analyzed above mainly focused on the investigation of the interaction between cellulose and ILs,few effects were made to study cleavage of cellulose chain and ring opening of side reaction during the dissolution of cellulose in ILs.Thus,our work was aimed to explore the detailed paths about the cleavage of cellulose chain and the formation of covalent bond between reducing end of cellulose and cation in ILs.The roles that cations and anions played in the process of dissolution were also investigated.
In this work,reaction path calculations were performed to study the key steps in cellulose depolymerization to form levoglucosan and glucose through cleavage of glycosidic bond and side reaction in ILs by DFT method.The cellobiose(shown in Fig.2)was used as a model molecule for cellulose.[Bmim]Cl,[Emim]Cl and[Emim]OAc would be used to investigate how cations and anions influence the reaction of cleavage of cellobiose and ring opening.These ILs have been widely studied in the promising field of cellulose processing.Potential energy surface(PES)scan and the intrinsic reaction coordinate(IRC)theory were carried out to search and construct the minimum-energy path.The key finding was that the anions and cations of ILs would promote the cleavage of cellulose chain.Further,ILs with acetate anion would be reactive to reducing end of the cellulose,whereas not with chloride anion.The similarities and differences between the role of cations and anions played in the reaction process were discussed.Such fundamental information may provide new perspective to understand and apply ILs for pretreatment of cellulose.
2.Computational Methods
All QM calculations were carried out by using Gaussian 09 program packages[34].Ground-and transition-state geometries of the investigated species were optimized by density functional theory(DFT)using B3LYP/6-311+G(d,p)(Becke's three-parameter nonlocal-exchange functional with the nonlocal correlation of Lee-Yang-Parr method)[35].The B3LYP/6-311+G(d,p)and similar level methods were found to be reliable for the calculation of geometry and energy of ionic liquids[36-39].The potential energy surface(PES)scan was performed to search for corresponding minima or saddle points.The number of imaginary frequency indicates whether a minimum ora transition state was located:all positive frequencies for a minimumand one imaginary frequency for a transition state.The connectivity between a given TS and the reaction/product minima,was verified in each case by performing intrinsic reaction coordinate(IRC)[40,41]calculations at the same level of theory.
3.Results and Discussion
[Bmim]Cl,[Emim]Cl and[Emim]OAc were used to investigate the influence of different kinds of cations and anions on the reaction of cleavage of cellobiose and ring opening.The structures of intermediates and transition states involved in these processes were determined,and the relative energies of these species were calculated.PES scan and IRC theory were carried out to search and construct the minimum-energy path.
3.1.The cleavage of cellobiose
To compare with the reaction catalyzed by ILs quantitatively,the reaction which formed levoglucosan and glucose by cleavage of the glycosidic bond in absence of ILs was investigated firstly.The schematic for cleavage of cellobiose in ILs is shown in Fig.3[29].
In our computational exploration,the reaction in the absence of ILs proceeds through the cleavage of a O-H bond of hydroxyl and a C-O bond connecting two rings of cellobiose,and finally leads to the formation of a 5-C ring of levoglucosan and glucose.Geometries of reactant compound(cellobiose),transition state and products(glucose and levoglucosan)for this reaction are optimized at the B3LYP/6-311+G(d,p)level and plotted in Fig.4.The energy diagram of the possible pathway is also shown in Fig.4.
The reaction progresses with the formation of a transition state(cello-ts),which further decomposed into glucose and levoglucosan,in which C-O bond connecting two rings of cellobiose and an O-H bond of hydroxyl will cleavage,and a 5-C ring of levoglucosan will form.In transition state(cello-ts),the distances of C1-O20,C1-O44 and O44-H45 are 0.243,0.267 and 0.140 nm,respectively.The energy barrier of this step is about 207.9 kJ·mol-1.The calculations show that the formation of glucose and levoglucosan from cellobiose is endothermic with reaction energy of 32.2 kJ·mol-1.The total energy of glucose and levoglucosan was 15.0 kJ·mol-1higher than the energy of the product in which glucose and levoglucosan are hydrogen bonded.Previous literature proposed a similar mechanism of cellulose thermal decomposition using DFT method and implicit THF solvent[42].The result reveals that the energy barriers for the reaction of cleavage of glycosidic bond to form glucose and levoglucosan are affected by solvents.
Therefore,our investigation will focus on the influence of different anions and cations on the reaction of cleavage of cellobiose.There are several possible interaction sites for ions that probably lead to cleavage of the C-O bond connecting two rings of cellobiose in each process.The candidates for active sites are selected through optimizations of geometries and performances of the PES scan,before studying bond cleavage barrier.
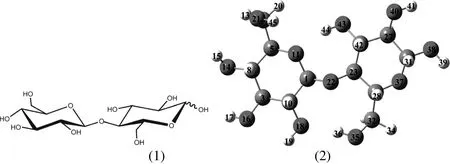
Fig.2.(1)Chemical structure of cellobiose;(2)molecular geometry of cellobiose.
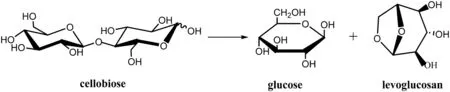
Fig.3.Proposed mechanism for the reaction of the cleavage of cellobiose in ILs.
3.1.1.The cleavage of cellobiose to form glucose and levoglucosan catalyzed by Cl-anion at different sites
To examine the influence of Cl-on the cleavage of cellobiose,we probed the mechanism at different sites,and here will give optimal active sites and paths that are chosen by comparison of energies and steric factor.The energy diagrams of the cleavage of cellobiose catalyzed by Clanion at a-Cl and b-Cl sites are shown in Fig.5.Geometries of reactant obtained by the interaction of cellobiose with Cl-,transition states and final product calculated at the B3LYP/6-311+G(d,p)level are shown in Fig.6.
In path a,the reaction progresses with forming a reactant a1,where the Cl-may facilitate the cleavage reaction at a-Cl site.In reactant a1,Cl-interacts with cellobiose through hydrogen bond(Cl-…H44-O43),and the distance is 0.2187 nm.The reaction then forms a transition state(TSa).In transition state(TSa),an O-H bond and C-O bond connecting two rings of cellobiose will cleavage,and a 5-C ring of levoglucosan will form.The Cl-is located about 0.2186 nm from H44 and forms a hydrogen bond with H44-O43 in a similar orientation with reactant(a1).Almost 214.3 kJ·mol-1is needed to overcome the energy barrier.Reaction then proceeds via the formation of a product(a2)which includes glucose and levoglucosan.This reaction is exothermic by about23.6 kJ·mol-1.The existence of hydrogen bond ata-Clsite appears to have small influence on facilitating the cleavage reaction.
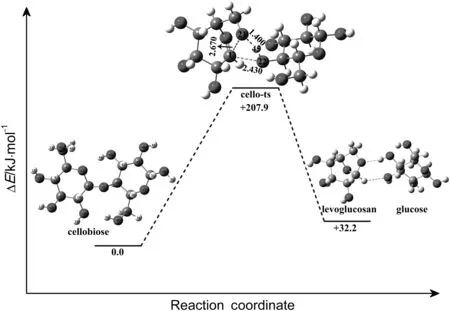
Fig.4.Relative energy diagram for the cleavage of cellobiose to form glucose and levoglucosan.The inset shows the optimized geometries for reactants,transition states and products(in kJ·mol-1).In this diagram,energy reactant complex is set to zero scale.Distances are shown in angstroms.The unit of the chemical bond length is ?,1? =0.1 nm.
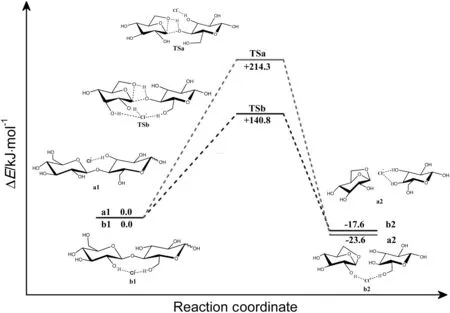
Fig.5.Relative energy and structure diagrams for the cleavage of cellobiose catalyzed by Cl-anion at different sites to form glucose and levoglucosan(in kJ·mol-1).
Assuming that the reaction is catalyzed by Cl-at b-Cl site,it will be initiated with forming a reactant b1 between cellobiose and Cl-.In reactant b1,Cl-is hydrogen bonded with the hydroxyl group(O18-H19,O35-H36)of cellobiose.The product b2 is formed through a transition state(TSb)where the corresponding distances of hydrogen bonds(Cl-…H19-O18,Cl-…H36-O35)are elongated to 0.2309 and 0.2125 nm respectively.A new hydrogen bond(Cl-…H17-O16)was then formed.Only about140.8 kJ·mol-1is required to cross the transition-state energy barrier.The reaction catalyzed by Cl-atb-Clsite is an exothermic process by about 17.6 kJ·mol-1.It can be found that the energy barrier of path b(Cl-at b-Cl site)is much lower than that of path a(Cl-at a-Cl site).The calculation suggests that the catalytic site of Cl-plays a non-negligible role in the reaction of cleavage of cellobiose.
3.1.2.The cleavage of cellobiose to form glucose and levoglucosan catalyzed by OAc-anion at different sites
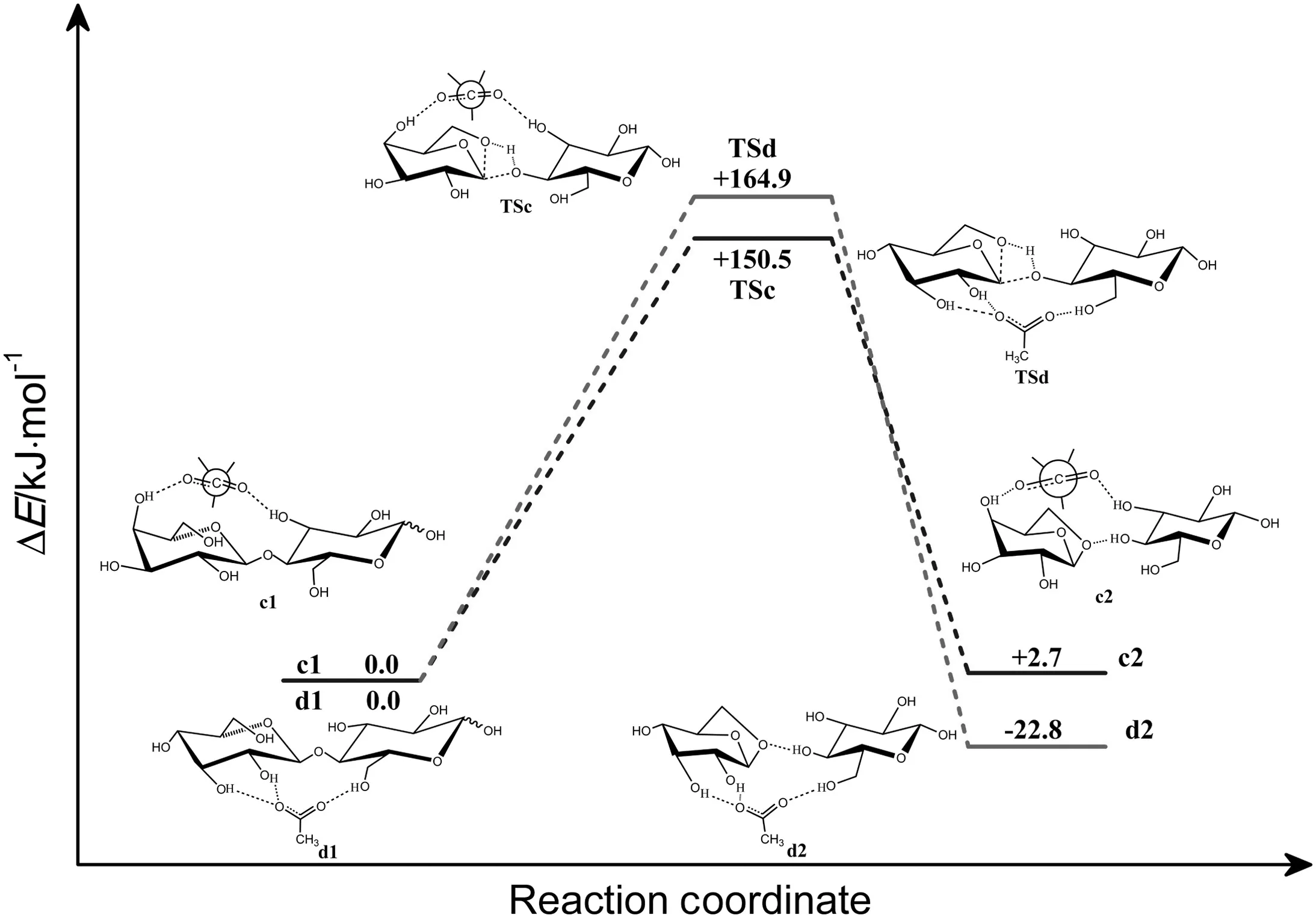
Fig.7.Relative energy and structure diagrams for the cleavage of cellobiose catalyzed by OAc-anion at different sites to form glucose and levoglucosan(in kJ·mol-1).
The mechanisms of cleavage of cellobiose to form glucose and levoglucosan catalyzed by OAc-at c-oac and d-oac sites were investigated.The energy diagrams are shown in Fig.7.Geometries of reactants obtained by the interaction of cellobiose with OAc-,transition states and final product at the B3LYP/6-311+G(d,p)level are shown in Fig.8.When the reaction is catalyzed by OAc-atc-oac site,it is initiated with forming a bimolecular complex(c1)with OAc-anion and cellobiose.In complex(c1),OAc-forms hydrogen bond with two OH bonds and a CH bond of cellobiose(O14-H15,O43-H44 and C5-H9).The corresponding distances of hydrogen bond above are 0.1700,0.1995,0.2350 nm respectively.In transition state(TSc),C-O bond connecting two rings of cellobiose will cleavage,and a C-O bond of ring in levoglucosan will form.The calculated energy barrier is about 150.5 kJ·mol-1.The reaction progresses with forming a product complex(c2)consisted of glucose and levoglucosan.This reaction is slightly endothermic by 2.7 kJ·mol-1.The existence of OAc-atc-oac site has a positive influence on the cleavage reaction kJ·mol-1.The energy barrier of this process is 57.3 kJ·mol-1lower than that without ILs.
When the reaction is catalyzed by OAc-at d-oac site,it progresses with forming a reactant complex(d1)where OAc-forms hydrogen bonds with various OH groups(O16-H17,O18-H19 and O35-H36)of cellobiose.The product(d2)is formed via a transition state(TSd)where the distances of the hydrogen bond(C51…H17-O16,C52…H19-O18)are shortened to 0.1664 and 0.1634 nm,and hydrogen bond C52…H36-O35 is elongated to 1.764 ?.About 164.9 kJ·mol-1energy is needed to cross the height of energy barrier.The cleavage reaction catalyzed by OAc-anion at d-oac site is an exothermic process by about 22.8 kJ·mol-1.The calculation results show that the energy barriers of the processes catalyzed by OAc-are lower than the one without ILs.But the catalytic site of OAc-appears to have slight influence on this process.OAc-is supposed to have superior ability to dissolve cellulose than the corresponding Cl-anion[20].As to the cleavage process,OAc-may be also better than Cl-.Compared with the process catalyzed by Cl-anion at different sites where the catalytic site does really matter,the process catalyzed by OAc-at both sites will facilitate the reaction.
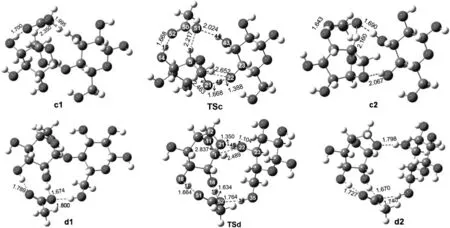
Fig.8.Optimized geometries for reactants,transition states and products involved in path(c)and path(d).Distances are in angstroms.The unit of the chemical bond length is ?,1?=0.1 nm.
3.1.3.The cleavage of cellobiose to form glucose and levoglucosan catalyzed by[Bmim]+and[Emim]+cation at different sites
It was reported that cation can play a non-negligible role in dissolution process[43].In order to explore the role that cations played in this reaction,we firstly investigated the mechanism of cleavage of cellobiose only catalyzed by cations at various sites.The cations at those sites,which are similar to the locations of OAc-and Cl-,were computationally studied.We considered detailed routes for cleavage of cellobiose when they were catalyzed by[Bmim]+cation at e-bmim site,and by[Emim]+cation at f-emim site.The corresponding energy diagrams are shown in Fig.9.Geometries of reactant complexes,transition states and final product obtained are plotted in Fig.10.
When the cleavage reaction is catalyzed by[Bmim]+cation at ebmim site,it is initiated to form a reactant complex(e1)between cellobiose and[Bmim]+.In this complex,[Bmim]+is located at 0.2521 nm from O21,0.2018 nm from O43 and 0.2237 nm from O40,and it forms hydrogen bonds with various oxygen atoms of cellobiose.The reaction progresses by forming a transition state(TSe)in which a C-O bond of cellobiose will cleavage and a C-O bond of levoglucosan will form.About 191.2 kJ·mol-1energy is required to overcome the energy barrier.The reaction then proceeds with forming the final product(e2)which consists of glucose and levoglucosan.The whole process is only slightly endothermic by about 14.6 kJ·mol-1.When the cleavage reaction is catalyzed by[Emim]+at f-emim site,the reaction progresses with forming a reactant complex(f1)between[Emim]+and cellobiose.In reactant complex(f1),orientation of the[Emim]+will facilitate the cleavage process.[Emim]+is located at 0.2010,0.2355 and 0.2421 nm from O43,O45 and O21 respectively,with hydrogen bonded to them.A product complex(f2)is formed via a transition state.In transition state(TSf),similar changes for bonds will happen.Approximately 181.4 kJ·mol-1energy is needed to cross the energy barrier.This reaction is endothermic by only 11.1 kJ·mol-1.
It can be found that the corresponding geometries of reactant complexes,transition states and product complexes for the cleavage reaction catalyzed by[Emim]+cation atf-emim site do not show significant variation compared to the one catalyzed by[Bmim]+cation at ebmim site.However,energy barrier of the reaction catalyzed by[Emim]+at f-emim site is a bit lower than the one catalyzed by[Bmim]+at e-bmim site.It was reported that alkyl chain length had effect on the interaction between cellulose and the imidazolium ring[44].According to our calculations,[Bmim]+and[Emim]+have the similar feature of electronic structures[37].And for this process,[Emim]+has better catalysis than[Bmim]+.This reaction may be slightly affected by alkyl chain length.
3.1.4.The cleavage of cellobiose to form glucose and levoglucosan catalyzed by cations and anions of ILs
It is considered that routes for cleavage of cellobiose are catalyzed by[Bmim]Cl at g-bcl site,[Emim]Cl at h-ecl site and[Emim]OAc at i-eoac site.The sites are based on the calculation evidences above.The corresponding energy diagrams are shown in Fig.11.Geometries of reactant complexes,transition states and final product obtained are shown in Fig.12.
When the reaction is catalyzed by[Bmim]Clatg-bclsite,the reaction progresses with forming a reactant complex(g1)with[Bmim]Cl and cellobiose.In reactant complex(g1),cellobiose is hydrogen bonded with Cl-and various C-H bonds of[Bmim]+.The reaction proceeds through a transition state(TSg),forming product complex g2.In transition state(TSg),a C-O bond of cellobiose will cleavage and a C-O bond of levoglucosan will form.About 150.4 kJ·mol-1energy is needed to cross the energy barrier.The total process is exothermic by about 51.6 kJ·mol-1.When the reaction is catalyzed by[Emim]Cl at h-ecl site,the process is initiated with formation of a reactant complex(h1),where cellobiose also forms numerous hydrogen bonds with Clanion and C-H bonds of[Emim]+cation.Transition state(TSh)energy barrier is about 148.6 kJ·mol-1.The final product(h2)is formed then.The whole process is only slightly exothermic by about 3.8 kJ·mol-1.When the reaction is catalyzed by[Emim]OAc ati-eoac site,the reaction progresses with forming a reactant complex(i1),where cellobiose form various hydrogen bonds with OAc-anion and C-H bonds of[Emim]+cation.About 145.2 kJ·mol-1energy is required to overcome the barrier.This process is only slightly endothermic by about 0.4 kJ·mol-1.
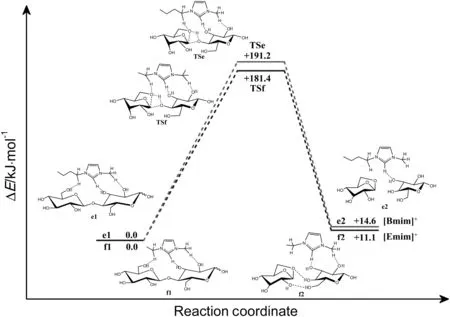
Fig.9.Relative energy and structure diagrams for the cleavage of cellobiose catalyzed by[Bmim]+and[Emim]+cations at different sites to form glucose and levoglucosan(in kJ·mol-1).
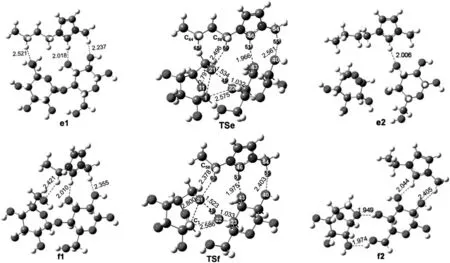
Fig.10.Optimized geometries for reactants,transition states and products involved in path(e),path(f).Distances are in angstroms.The unit of the chemical bond length is?,1?=0.1 nm.
Table 1 shows the total relative energies for the reaction of cleavage of the cellobiose catalyzed by anion and cation at different sites to form glucose and levoglucosan.Making comparison among relative energy diagrams of the reaction catalyzed by Cl-at b-clsite and[Bmim]Clat gbcl,[Emim]Cl at h-ecl site,we found that the alkyl chain length of the cation does not have good effect on catalysis of the cleavage reaction for[Bmim]+and[Emim]+.The result is consistent with the lower solubility of cellulose in ILs containing longer chain length cation[44,45].According to the relative energy in Fig.9,in general,the reaction catalyzed by anion and cation is more favorable than that catalyzed separately by anion or cation.The results above may be due to a synergistic effect of the cation-anion catalysis.In solution process of cellulose,[Emim]OAc are supposed to be better than[Emim]Cl[20].We also stated above that OAc-at both site facilitates the reaction.However,in the process which is catalyzed by anion and cation,we found that at these sites(h-ecl,ieoac),the reaction catalyzed by[Emim]OAc is a little more favorable than the one catalyzed by[Emim]Cl,with a lower barrier of energy.Both of anions and cations play non-negligible roles in this process.
3.2.The possible mechanism for ring opening of cellobiose
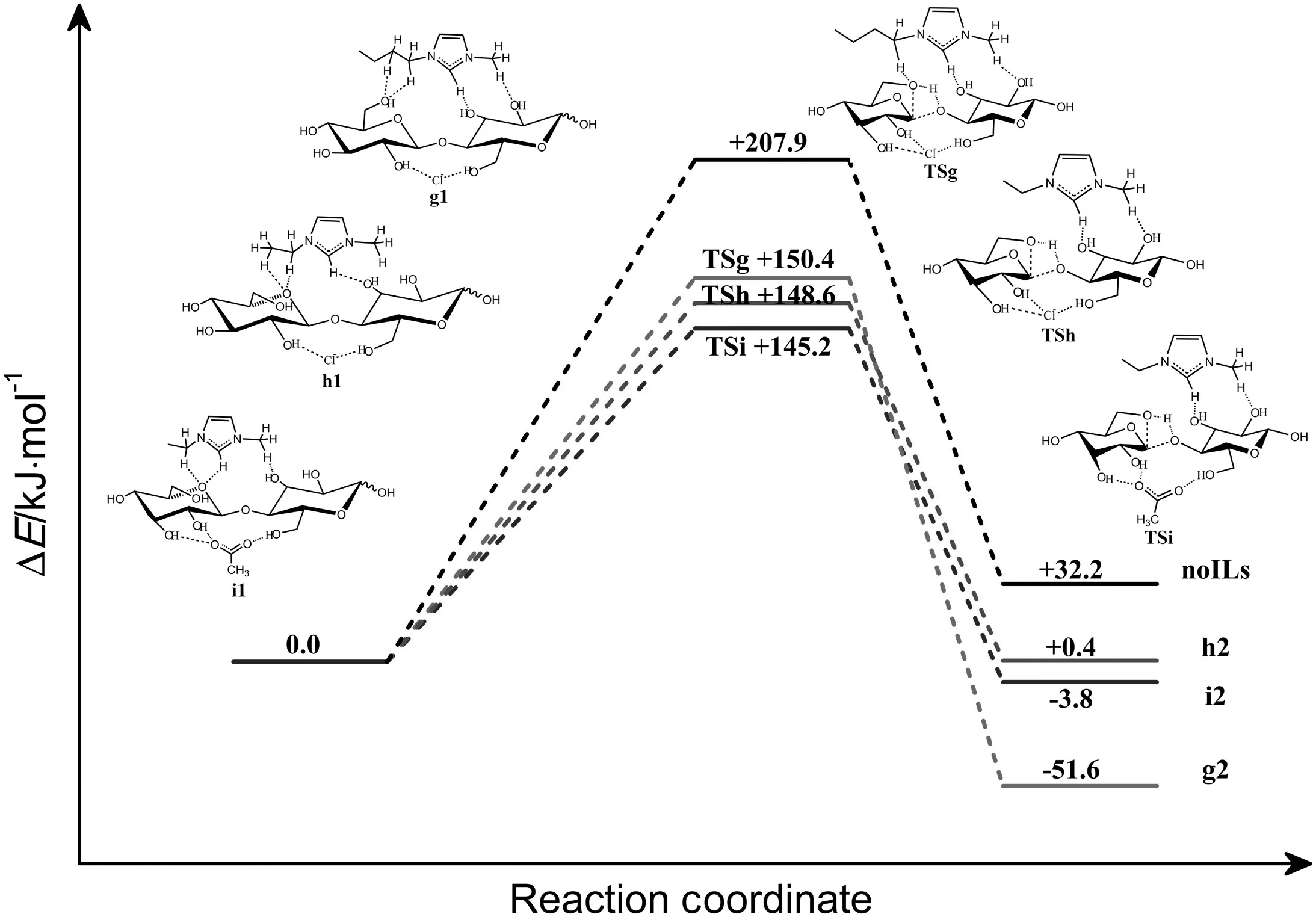
Fig.11.Relative energy and structure diagrams for the reaction of the cleavage of the cellulose chain with and without ILs to form glucose and levoglucosan(in kJ·mol-1).
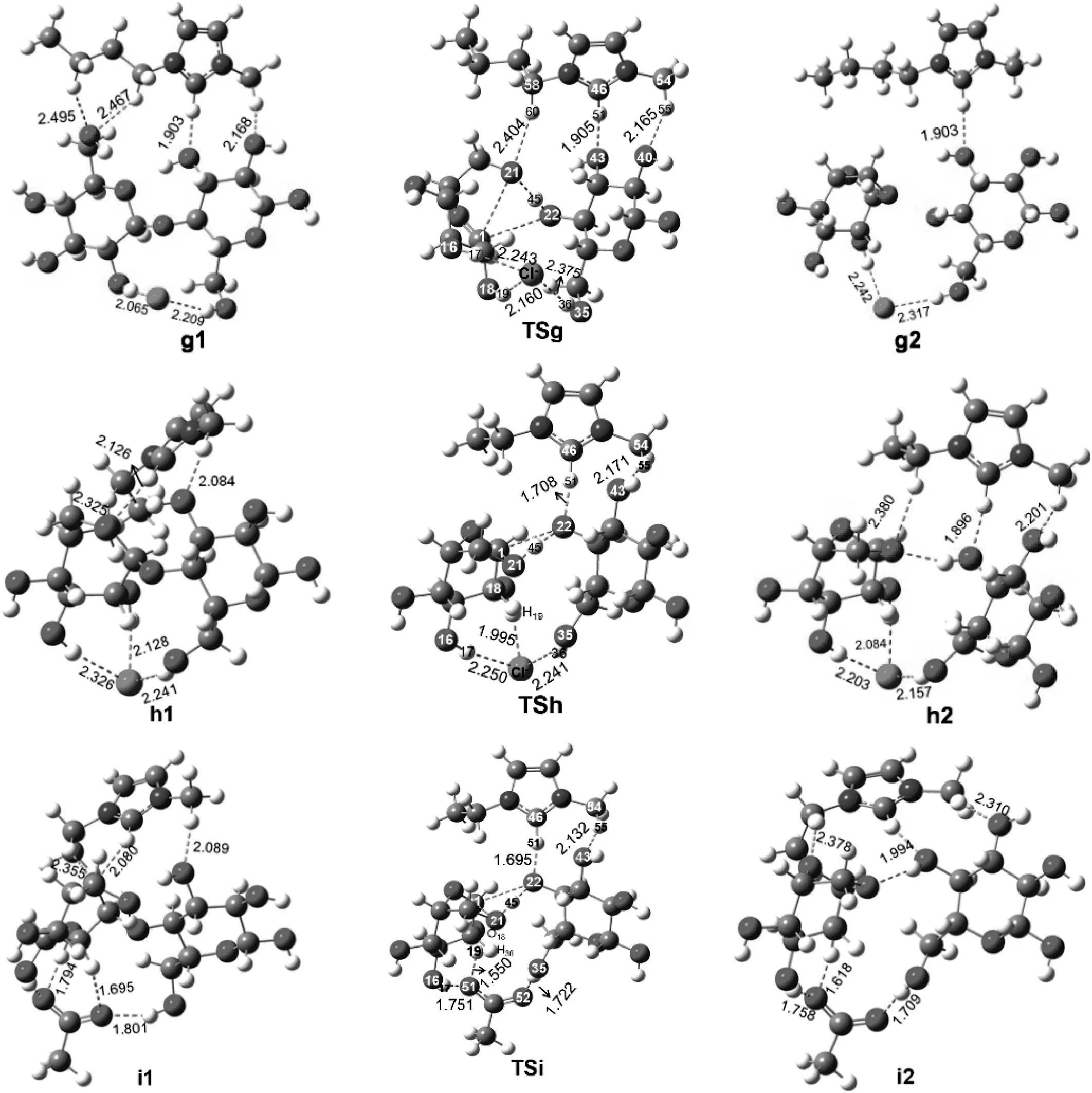
Fig.12.Optimized geometries for reactants,transition states and products involved in path(g),path(h)and path(i).Distances are in angstroms.The unit of the chemicalbond length is?,1?=0.1 nm.
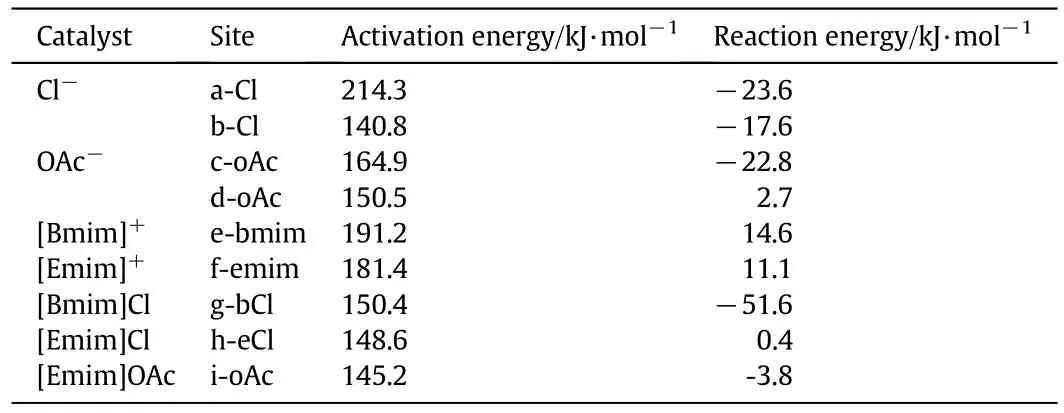
Table 1 Computed relative energies(kJ·mol-1)for the reaction of cleavage of cellobiose catalyzed by different catalysts at different sites to form glucose and levoglucosan
During the dissolution of cellulose in[Emim]OAc,it was found that the C2 carbon of 1-alkyl-3-methylimidazolium IL may be covalently bonded with the reducing end of cellulose in the experiment[22,25].Such adduct-forming reactions are not found in ILs when the anion is Cl-[46].[Emim]Cl was demonstrated to be inert solvent to cellulose[26].
As for this adduct-forming reaction between[Emim]OAc and glucose(cellulose model compound),Du et al.[32]hypothesized a mechanism that N-heterocyclic carbene and acetic acid were generated in ionic liquid[Emim]OAc via transfer of proton from imidazolium cation to acetate anion.Then the formed carbene reacted directly with the reducing end of cellulose,meanwhile the acetic acid in ILs donated a proton to O atom on the ring,leading to the cleavage of glucose ring.
An N-heterocyclic carbene has a lone pairofelectrons and is chemically very reactive[47-49].The ability of[Emim]OAc to form N-heterocyclic carbene was discussed in Matthew's investigation[26].It was thought to be well established and supported by calculations and experiments[50-57].Transfer of the proton on C2 position of cation to anion is facile,and this proton plays an important role in the reaction between acetate based ILs and solvated cellulose[26].The formed Carbene in this ILs is able to react with aldehydes to give nucleophilic product[58].In order to explore detailed paths for ring opening reaction,the PES scan and IRC were carried out to study the mechanism.
The mechanism for generation of carbene and acetate anion is investigated firstly(shown in Fig.13).The reaction proceeds initially with forming a reactant complex[Emim]OAc,in which OAc-forms hydrogen bonds with hydrogen on C2 of imidazolium cation.The hydrogen(H)on C2 is located at 0.1135 nm from C2 and 0.1643 nm from oxygen(O)of OAc-.Reaction progresses through forming an intermediate state intercar-acetic,where about 3.8 kJ·mol-1is needed to cross the energy barrier.The geometrical parameters of the intermediate state are gotten.In intermediate state inter-car-acetic,the C2-H bond is elongated to 0.1728 nm and the O-H bond shortens to 0.1041 nm.After inter-caracetic complex is formed,the proton transfers from C2 to the O atom on the acetate, finally forming acetic acid and carbene.The reaction energy of this process is about 55.2 kJ·mol-1due to separation of hydrogen bond C2…H-O.The reaction is endothermic by about 59.0 kJ·mol-1.
Fig.14 shows a plot on the PES scan as a function of H-C2 bond length.As can be seen from Fig.14,the relative energies have the same trend along the elongation of the distances of H-C2 bond.Although there is a little barrier,the activation energy is so small that we think basically that there is no transition state in this process.According to experimental results,the concentration of free carbene in ILs was small and cannot be observed directly[48,59],but the presence of carbene was indirectly demonstrated through providing observable catalytic activity for reactions catalyzed by[Emim]OAc[46,60].
For investigating the mechanism of generation of carbene in ILs,we performed the PES scan to find out the detailed pathway for the ring opening attacked by carbene and acetic acid.Different possible pathways were investigated as followed:carbene attacks C1 on the reducing end of cellobiose,and then hydrogen on hydroxyl of acetic acid forms O-H bond with O neighboring C1 of the ring;hydrogen atom on acetic acid coordinates with O neighboring C1 in cellobiose firstly,and then carbene attack C1 to open the ring of cellobiose;carbene and acetic acid attack the reducing end of cellobiose simultaneously.
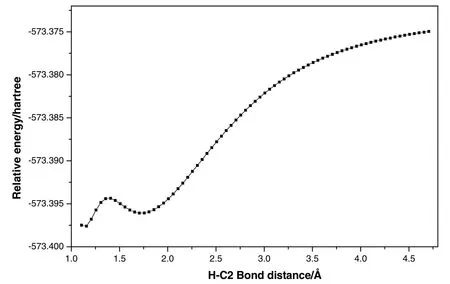
Fig.14.Potential energy curve for the formation of carbene via H-C2 bond rupture along the reaction coordinate with H-C2 bond.1 Hartree=2625.5 kJ·mol-1,1? =0.1 nm.
According to the computational exploration,the path attacked by carbene is unfavorable process as carbene is very active.It is very easy to interact with the hydroxyl on C1 to form a stable hydrogen bond.Even carbene can interact with C1 directly without disturbance of hydrogen bond,it's more likely to proceed with an insertion reaction instead of ring opening.So,there is little possibility for carbene attacking reaction to happen.
Then,we investigated the path where cellobiose is attacked by acetic acid firstly.In order to further analyze and understand this process,frontier molecular orbital(FMO)analyses[61,62]were performed for carbene and three possible complexes(cello-hac,cello-hac-1 and inter-cello-h-ac)with acetic acid and cellobiose,shown in Fig.15.These three complexes show three possibilities how acetic acid attached to cellobiose.In Fig.15,the energy gap between the HOMO of carbene and the LUMO of complexes was compared.It can be seen that the energy gap between LUMO of inter-cello-h-ac and HOMO of carbene is 4.65 eV,which is the smallest among energy gaps of three.Further,the HOMO of carbene is symmetrically matched with the LUMO of complex inter-cello-h-ac.The complex inter-cello-h-ac is with obvious advantage to react with carbene.Our testing PES scan calculation also shows similar results.
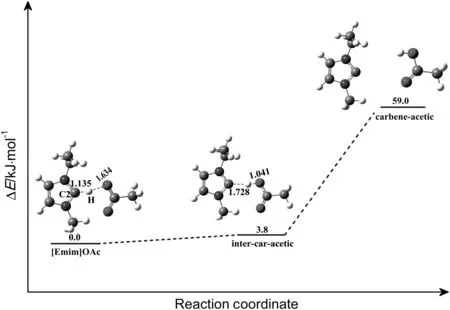
Fig.13.Relative energy diagram for the reaction between imidazolium cation and acetate anion to generate a carbene(in kJ·mol-1).The unit of the chemical bond length is ?,1?=0.1 nm.
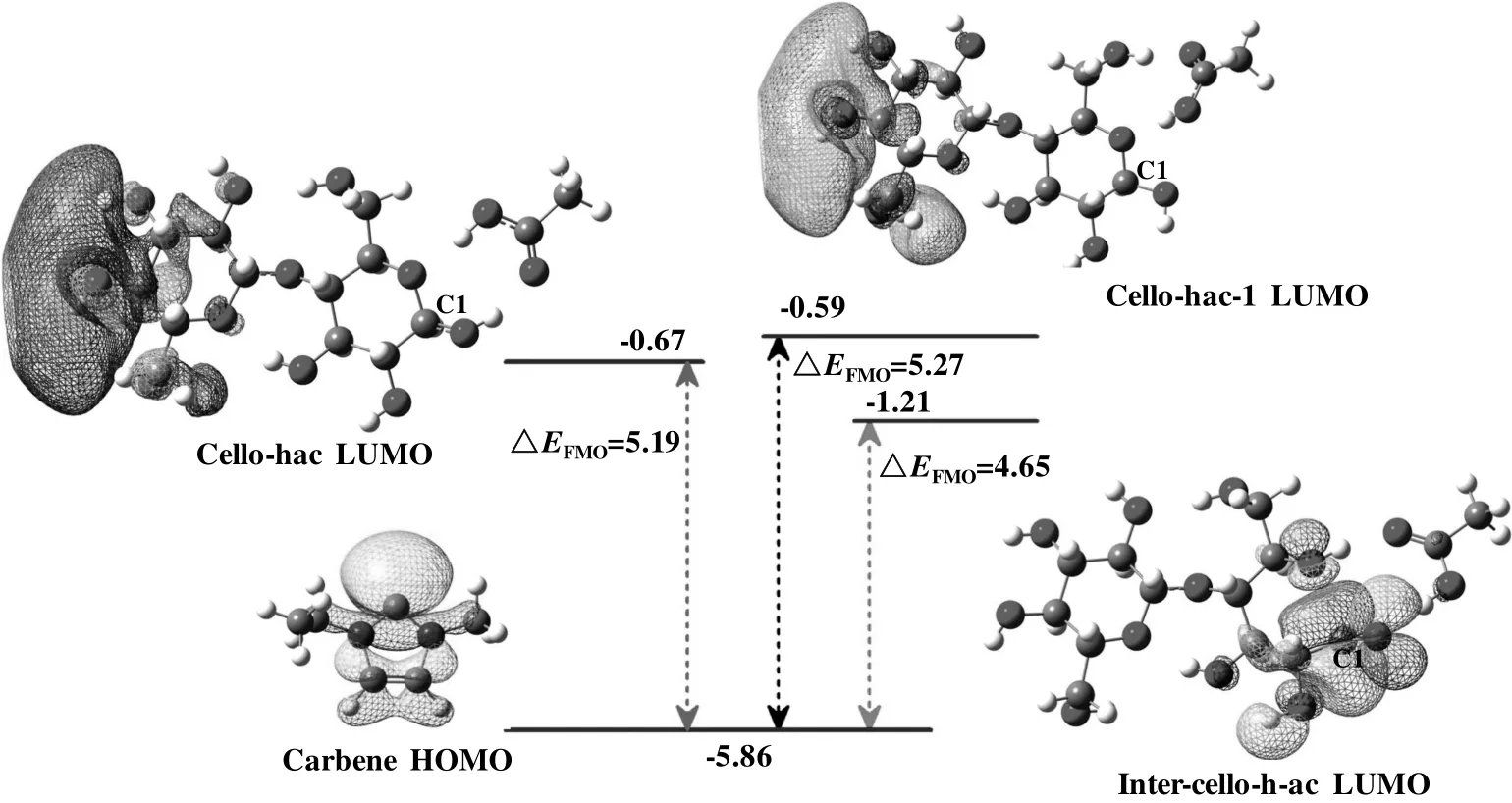
Fig.15.Energy levels(in eV)of HOMO for carbene and LUMO for cello-hac,cello-hac-1 and inter-cello-hac-ac.HOMO represents highest occupiedmolecular orbital;LUMO represents lowest unoccupiedmolecular orbital.
Although there was little possibility for carbene attacking reaction to happen firstly,the detailed pathway for this process was investigated as a comparison.The computed mechanism of this reaction in which the cellobiose is attacked by HOAc and carbene simultaneously is shown in Fig.16.About 175.3 kJ·mol-1energy is required to cross the activation barrier.In the transition state(cello-hac-car-ts-1),C2 on carbene is located 0.2561 nm.from C1 on cellobiose.And the C1-O11 bond is elongated to 0.2169 nm.A product complex(C-C product)is formed via carbene covalent binding with cellobiose.The reaction energy of this process is about 20.9 kJ·mol-1.
Ring opening in[Emim]OAc is more likely to happen when the HOAc attacks firstly,then nucleophilic is attacked by carbene on C1 atom,leading to forming a covalent bond.The proposed mechanism for this reaction and the corresponding structures are shown in Figs.17-18.
The attachment of HOAc to cellobiose takes place firstly to form an aldehyde-like intermediate.Carbene then attacks subsequently at the C1 site leading to breaking of the C1-O11 bond of the ring.Such reaction in[Emim]OAc starts with forming a reactant complex cello-hac(Fig.17).It is evident from the geometrical parameters of the reactant that HOAc interacts with cellobiose through hydrogen bonds(O29-H28…O11,O31…H23-O19),the distances of which are 0.1806 and 0.1841 nm,respectively.About 54.0 kJ·mol-1energy is needed to overcome the hydrogen transformation activation barrier.Then the reaction formed an aldehyde-like intermediate(inter-cello-h-ac),which is active to get electron from C1.The formed aldehyde-like group is a nucleophilic active site.This step is endothermic by 32.2 kJ·mol-1.Then,carbene with a lone pair of electron attacks the C1 carbon to form a covalent bond by electron transfer,which finally leads to the breakage of C1-O11 bond of the ring(Fig.18).Fig.19 shows a plot of the PES scan as a function of their bond length.It can be seen that the relative energies have the same trend along the elongation of the distances of C2-C1.About 92.0 kJ·mol-1energy is released due to the C-C product formation(Fig.18).
Two possible paths with different transition states for ring opening with the formation of covalent compounds are shown in Fig.20.Compared with the reaction initially attacked by HOAc,the pathway accomplished by attacking of HOAc and carbene simultaneously is less favorable with higher activation energy.
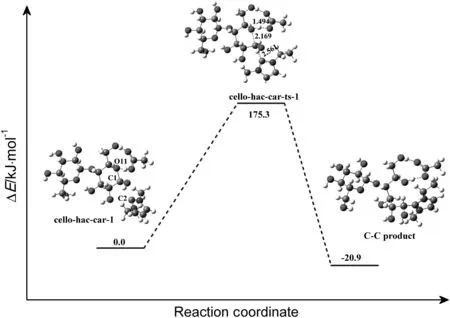
Fig.16.Proposed mechanism for the reaction of[Emim]OAc with the reducing end of cellulose by one step.The unit of the chemical bond length is ?,1? =0.1 nm.
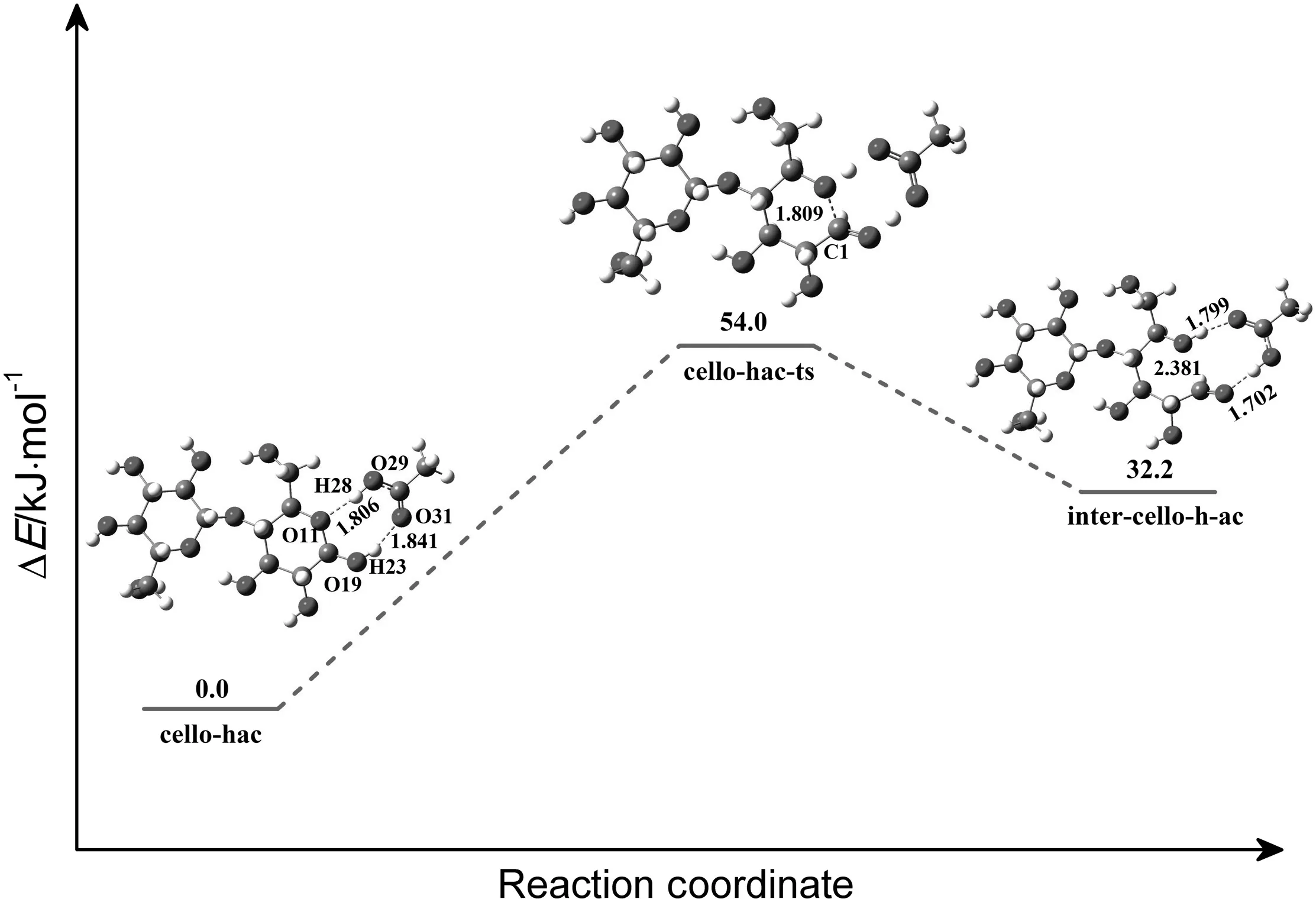
Fig.17.Relative energy diagram for the reaction of HOAc with methyl-glucose forming the aldehyde-like intermediate(in kJ·mol-1).The unit of the chemical bond length is ?,1? =0.1 nm.
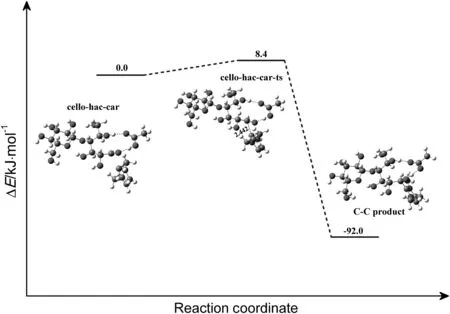
Fig.18.Relative energy diagram for the reaction of carbene with aldehyde-like intermediate,forming a carbon-carbon bond(in kJ·mol-1).The unit of the chemicalbond length is?,1?=0.1 nm.
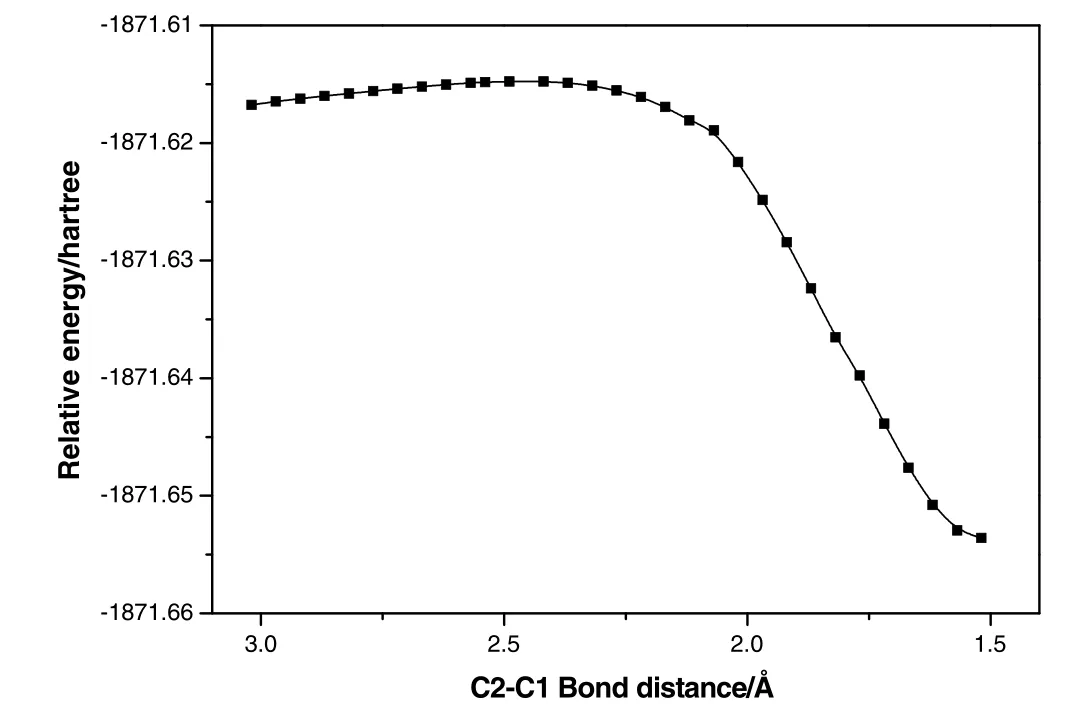
Fig.19.Potential energy curve for the reaction of carbene to form C1-C2 bond via migration of C2 along the reaction coordination.1 Hartree=2625.5 kJ·mol-1,1? =0.1 nm.
On the basis of calculation results,the ring opening reaction path needs a low energy via formation of carbene and attacking of HOAc.However,the ring opening reaction is easy to be a reversible process when the barrier is only 62.4 kJ·mol-1.And carbene in acetate-ILs is unstable,so actually the ring opening is lesslikely to happen.The experimental results also showed that the final modification of cellulose is minor,and the reaction needs high temperature and long reaction time[25,26].
4.Conclusions
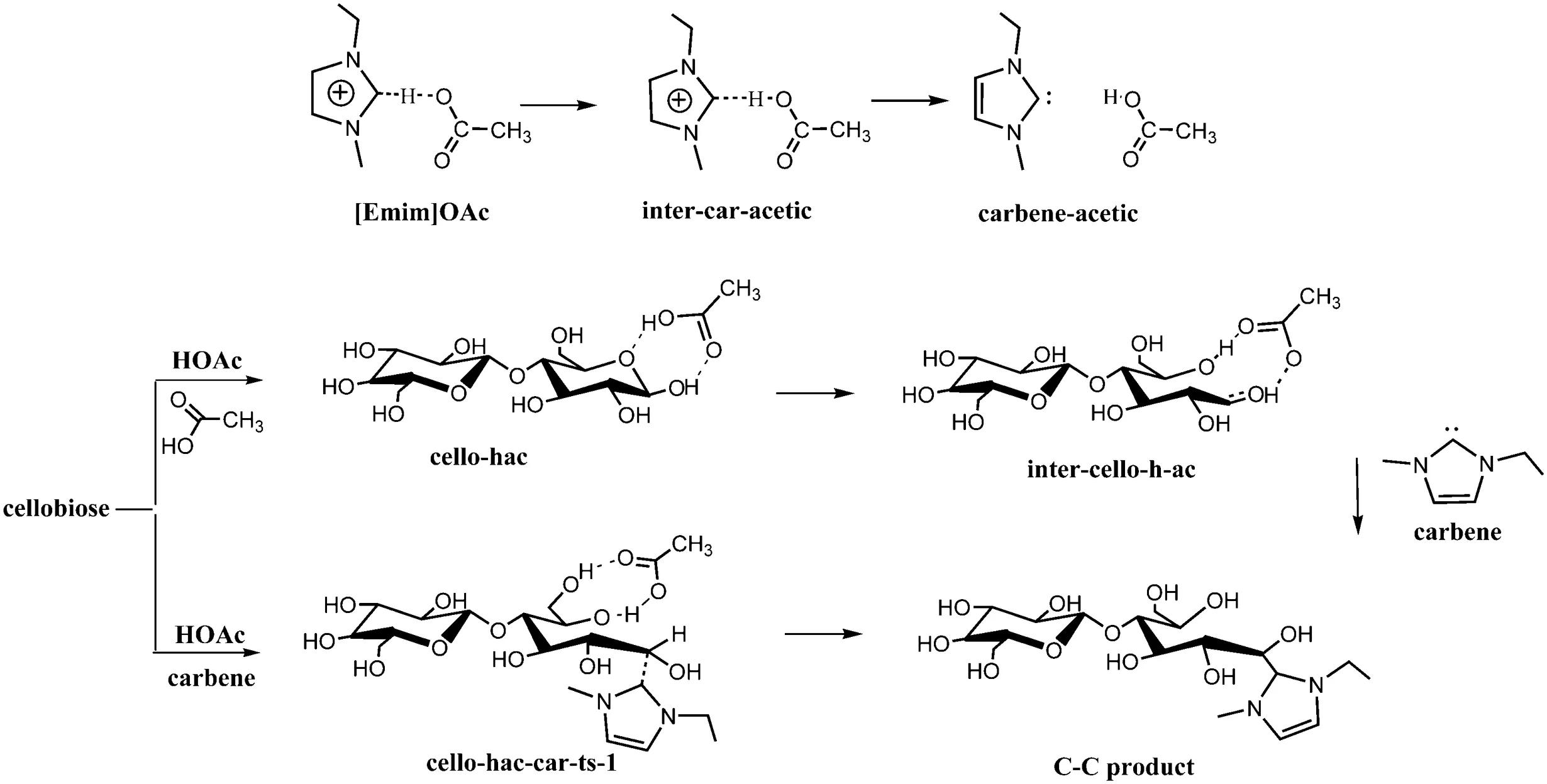
Fig.20.Proposed mechanisms for the reaction of[Emim]OAc with the reducing end of cellulose.
Comprehensive quantum chemical computation on the reactions of the cleavage of cellobiose in[Emim]Cl,[Bmim]Cl and[Emim]OAc,was systematically performed.The side reaction of ring opening of cellobiose was also studied.The results indicated that for the cleavage of cellobiose in ILs,low energy was required to overcome the activation barrier due to a synergistic effect of the cation-anion.The reaction barriers would also be affected by anion or cation solely.For Cl-,the effect of different catalytic sites was obvious while that of OAc-anion was negligible.Meanwhile,we found that alkylchain of cations has little effect on catalysis of the cleavage reaction.These results indicated that anions played an important role in influencing the reaction while cation is also nonnegligible in this process.For the ring opening reaction of cellobiose in acetate ionic liquids,the formation of carbene in ILs would facilitate the opening of the ring according to the computational analysis,which is consistent with experimental results.It was found that the nucleophilic attack by carbene on C atom of the monosaccharide ring led to the formation of covalent bond between cellulose and imidazolium core and ring opening.The calculation information above demonstrates modification of cellulose happening during the dissolution process in ILs.We hope that the theoretical results will give valuable information and be helpful for understanding cellulose processing in ILs.
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- Facile synthesis ofporous Pd nano flowers with excellentcatalytic activity towards CO oxidation☆
- Long-term nitritation performance of ammonium-rich land fill leachate☆
- Analysis of fouling characteristic in enhanced tubes using multiple heat and mass transfer analogies☆
- Removal of elemental mercury by modified bamboo carbon☆
- Simple processing technology of leaching water using CO2 microbubbles☆
