Morphometric variability of Arctodiaptomus salinus(Copepoda) in the Mediterranean-Black Sea region
Elena V. ANUFRIIEVA, Nickolai V. SHADRIN
The AO Kovalevsky Institute of Marine Biological Research, Russian Academy of Sciences, Sevastopol 299011, Russia
INTRODUCTION
Intra-species variability, an inherent property of living organisms allowing species to exist in a changing environment, is primary raw material for the evolutionary process. This variability,however, creates the need to know the range of variability of important morphological characteristics of the species (Elgmork& Halvorsen, 1998; Gaviria & Forró, 2000). The size of copepods is affected by a number of environmental factors and varies widely (Deevey, 1948). The length and other linear body dimensions of them therefore, are not always a good identification of the copepod species; the proportions between the body parameters may play a more important role in taxonomy (Elgmork & Halvorsen, 1998; Isinibilir et al, 2009).Phenotypic variability and intra-species diversification of copepods were shown (Matthews et al, 2011) to modulate the availability of resources to other species (ecosystem engineering) and shape selection pressures on other organisms(niche construction). There is growing recognition that both inter-population and intra-population variation can have significant effects on population, community, and ecosystem dynamics (Matthews et al, 2014). However, these issues are poorly understood yet. Changes in intra-population diversity may indicate a destabilization of populations (Knyazeva, 2010;Scheffer et al, 2009; Shadrin, 2012; Williamson, 1981). For some copepod species discordance between the rates of morphological differentiation, molecular evolution, and reproductive isolation has been shown (Burton, 1998; Lee &Frost, 2002). Therefore, the studies of a morphological variability for taxonomic, ecological and evolutionary tasks continue to be useful.1
The eurythermal and euryhaline copepod speciesArctodiaptomus salinusDaday, 1885, which has resting eggs and inhabits the water bodies across Eurasia and North Africa,often plays a dominant role in plankton in different types of water bodies (Folijan, 1966; Krupa et al, 2008; Marrone, 2006;Rokneddine, 2004). The population size structures ofA. salinusin different types of water bodies varies; the average size of females varies from 1.00 mm to 2.38 mm (Folijan, 1966;Rokneddine, 2004; Anufriieva & Shadrin, 2014). Average body mass ofA. salinusin different populations varies at 11.4 times and consequently individual metabolic activity at 6.2 times(Anufriieva & Shadrin, 2014). Two size groups ofA. salinusin the Crimean and the Siberian lakes were observed, the presence of which do not relate to temperature, salinity, and pH(Anufriieva & Shadrin, 2014). The high ecological and morphological plasticity of A. salinus allowed the authors to use it as a good model subject to study different aspects of phenotypic variability in populations of copepods.
The aim of this work is to assess the intra- and interpopulation morphometric variability ofA. salinusin the Mediterranean-Black Sea region, the factors influencing it, and to discuss some observed regularities. The authors suppose that there are significant inter-population differences in morphometric traits, which cannot be explained by environmental peculiarities only.
MATERIALS AND METHODS
Study area and sample collection
Zooplankton samples were collected in the salt waters of the Crimea (largest peninsula in the Black Sea) from 2009 to 2012.One more sample was taken in Tambukan salt lake (July 2012)in the North Caucasus (Russia). On each sampling occasion 50-100 L of water was filtered through 110 μm mesh-size plankton net and the resulting sample was immediately preserved with a 4% buffered formalin solution.In situsalinity,temperature and pH were measured at the time of sampling using a portable hand-held salinity refractometer (Kelilong WZ212) and a portable temperature/pH meter (PHH-830).Copepods abundance was determined by direct counting using an Olympus SZ-ST stereo microscope with subsequent conversion to volumetric values based on the volume of filtered water. Several samples of zooplankton from various places in Italy, Spain, and Tunisia with accompanying information were kindly provided to us by Dr. F Marrone. As a rule, 30 adult individuals of each sex from every sample were measured under a light Carl Zeiss Axio Scope A1 microscope at 20-40×magnifications; the measured linear parameters: TL-total length;WC-width of cephalothorax; LA-length of the abdomen; LC-length of the cephalothorax.
Statistical analysis
Variability of parameters in the samples was characterized by the coefficient of variability-CV (CV=standard deviation of a parameter in a sample divided by the mean parameter value).For each sample the average coefficients of variation were calculated separately for the linear parameters (CVl) and proportions of the body (CVp) as follows: the sum of the coefficients of variation for different traits divided by the number of the particular coefficients of variation. The level of sexual dimorphism (for studied morphometrical parameters) was evaluated as female/male ratio. All the data were subjected to a statistical processing (STATISTICA software package, version 6.0, Statsoft, Inc.). The significance of the differences of the average values was evaluated using the Student′st-test. To test the homogeneity and normality of the general data sets of the studied parameters we used the probability paper method in the analysis of size frequency distributions (Cassie, 1954), and then tested it in STATISTICA. STATISTICA was also utilized to calculate Euclidean distances between stations and to make tree clustering. Selection of the best approximated equations was made from those available in Excel, according to the highest R2. Information on sampling sites and samples is presented in Table 1.
RESULTS
Variability of the linear parameters
From sample to sample, average values of all linear parameters varied over a wide range, and a level of their variability (CV)was also high (Table 2). Changes of all linear parameters correlated with each other; the correlation coefficients were significant (P=0.001) in all cases. Previous work (Anufriieva &Shadrin, 2014) had analysed the influence of different factors on body length, so did not need to repeat that analysis. For this reason only the variability of different linear parameters was analysed in this study.
CV of total body length of males ranged from 3.42% to 12.23% in different populations; of females it ranged from 3.32% to 17.96%. The variability of other linear dimensions was slightly higher (Table 2). The correlation coefficients (R)between CV of different linear parameters of the females ranged from 0.686 to 0.810, and each of them was significant (P=0.005-0.001). In males CV of the different characteristics also correlated;the correlation coefficients ranged from 0.366 to 0.895 (P=0.05-0.001). The average CV of linear parameters (CVl) was calculated for all samples (Table 3). CVl in males and females correlated significantly with each other (R=0.612;P=0.05).
The environmental factors affected CV of the studied traits.The average CVl of females in the general data set increased with temperature (R=0.35;P=0.06). In males the trend was not significant. The trend for the Crimean population was not clear and was not significant for males or females. The average CVl significantly increased with increasing salinity. The dependence for males of the general sample set can be approximated by the equation (R=0.441; P=0.02):
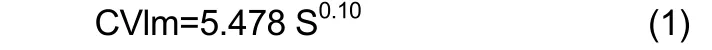
where CVlm-the average CVl in males, S-salinity, ppt.
For females the dependence is approximated by the equation(R=0.705;P=0.001):

where CVlf-the average CVl in females.
Separately for the Crimean population we observed an insignificant positive relationship in females. In males it is significant (R=0.602;P=0.05) and is given by:

At salinities of 15-20 ppt there is a maximum of variability of linear parameters. CV of body size of females in the Crimean lakes demonstrates the significant positive linear dependence on the population density (R=0.82;P=0.025). This dependence for males does not demonstrate such a relationship, and was closer to a dome-shaped dependence. There was no pH influence on CV.

?
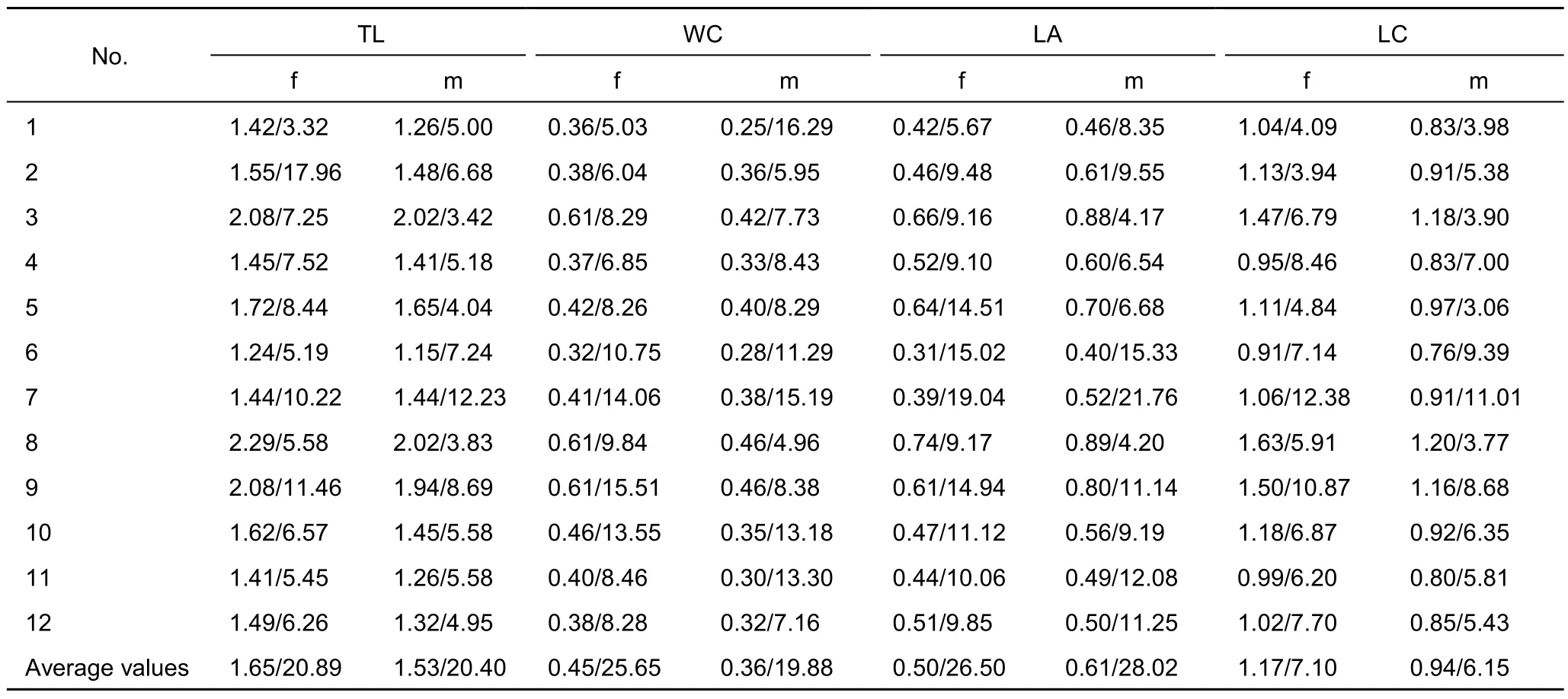
Table 2 Average values of the linear parameters of A. salinus and their variability in the studied samples (average value/CV)

Table 3 Average variability in different samples of A. salinus
Variability in body proportions
The probability paper was used to analyze the general sets of the different body proportions. Distributions of all proportions were unimodal and very close to a normal distribution,indicating that the studied proportions of “big” and “small” forms(Anufriieva & Shadrin, 2014) were practically identical. Mean proportions and their CVp are given in Table 4. CVp values were close to that for the linear parameters, and ranged from 5.27% to 13.79%. TL/LC was the least changeable on average proportion in populations. A significant effect of salinity, pH and temperature on the body proportions was not found. The levels of their intra-population variability are dependent on temperature and salinity. A significant increase of CVp was observed with increasing temperature (P=0.05-0.001). In males,this dependence is stronger. Increasing salinity also leads to significant changes in CVp of some proportions. For example,in females CVp of TL/WC increased with increasing salinity in the range from 2.2 to 74 ppt (R=0.646; P=0.005); dependence can be approximated by the equation:

where CVpf-CVp of TL/WC proportion in females.
In males, the dependence of the variability of this proportion on salinity is expressed even more strongly (R=0.898;P=0.001),and the dependence can be approximated by the equation:

where CVpm-CVp of TL/WC proportion in males.
The increase in variability of TL/LA was not observed in the entire range of salinity in males and females. Using Crimean samples the dependence of the proportions and their variability levels on the density of the population were analyzed; the dependence was absent. CVp of the different proportions were significantly correlated with each other (R=0.450-0.700; P=0.05-0.0005) in males and females separately, and the average CVp for males and females correlated also (R=0.758; P=0.001). CVl positively related with CVp (for females R=0.716;P=0.0005; for males R=0.634;P=0.005). The dependence of the average CVp with salinity in males can be approximated for the general totality of samples by the equation (R=0.589;P=0.001):

where CVpm-the average CVp in males.
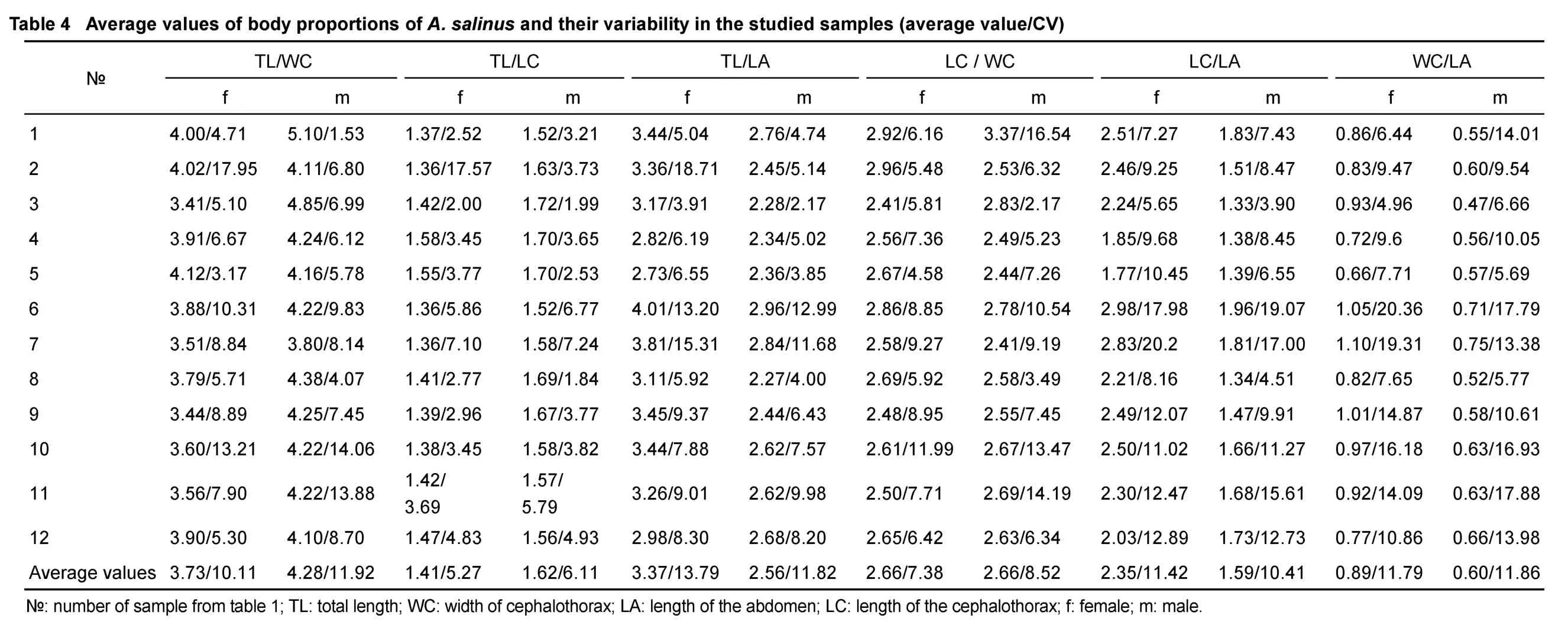
?
In females this index is slightly but significantly positively correlated also with salinity (R=0.418;P=0.01); the dependence may be approximated by the equation:

where CVpf-the average CVp in females.
In the Crimean population a significant correlation of CVp with salinity in females was not observed; but in the males it was significant (R=0.771; P=0.005) and is approximated by the equation:

Temperature also significantly affected CVp in all samples.Dependence in males can be approximated by the following equation (R=0.664; P=0.0005):

where T- temperature (°C).
In the Crimean population the dependence in males can be approximated (R=0.903;P=0.0001):

Population density does not affect CVp of males, but in females CVp significantly increased with an increase of population density (R=0.694;P=0.005); the dependence is not strong linear.
Sexual dimorphism in body proportions was expressed in the same degree as in the linear parameters (Table 5). The exception is the LC/WC, which is almost identical in males and females. The level of inter-population variability in the expression of sexual dimorphism in body proportions was rather high; the coefficients of variation for different proportions varied from 4.8% to 15.4%. There were no effects of temperature, pH and salinity on the female/male parameter ratio. The significant differences in the influence of salinity and temperature on variability in the proportions of males and females that were revealed are as above.

Table 5 Sexual dimorphism of A. salinus in body proportions in different samples
Inter-population differences in proportions
All samples were compared with each other in pairs for body proportions; in most cases significant differences were found.For example, Table 6 shows the level of significance of differences between the samples for TL/WC in females. For TL/WC of females differences were observed in 53% of the compared pairs and 50% of the male pairs; for TL/LA ? in females was 85%, and 73% in males. For TL/WC 26% of pairwise comparisons of samples revealed significant differences for males and females, and in TL/LA it was significantly different in 64% of comparisons. In 35% of the pairwise comparisons males were significantly different in both proportions, and females were significantly different in 42%. In 14% of the cases both females and males were significantly different in both proportions.
The greatest number of differences with the other samples was observed in samples from Lake Takilskoe in the Crimea(56% comparisons), from Lake Banyoles in Spain (50%) and from Sebkha El Ariana in Tunis (48%).
Clustering was used to identify similarity between different samples separately for males and females as well as for both sexes together, taking into account all linear traits and proportions (Figure 1). Resulting graphs show that there are different grouping pictures for male and females. The distance between the studied lakes varies from less than 50 km to more than 3 000 km. Euclidean distance (Figure 1) did not significantly correlate with geographical distance between sampling sites and differences in salinity.
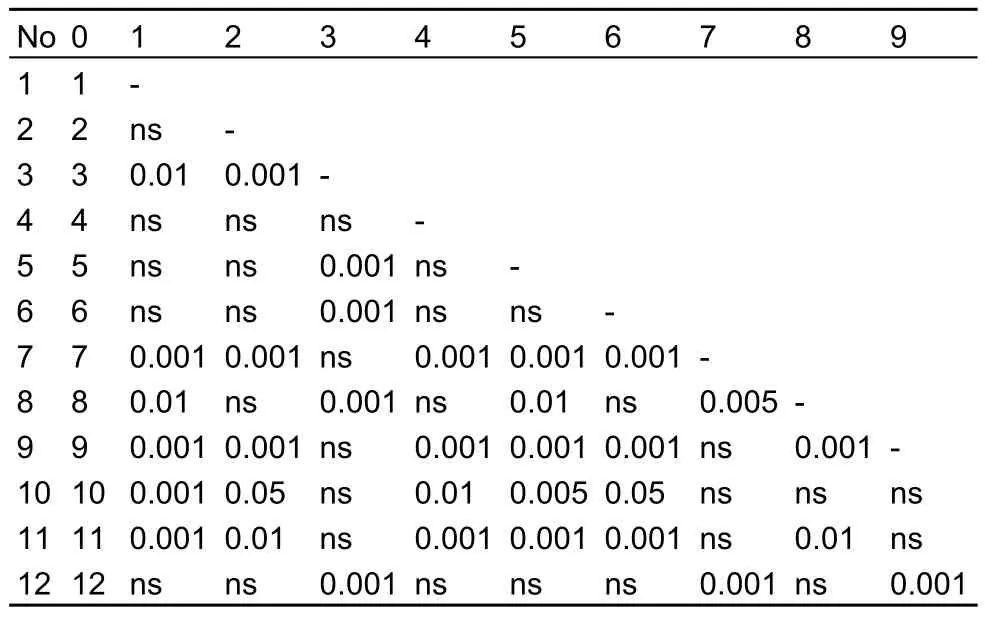
Table 6 Significance level of inter-sample differences for the proportion of total length to width of cephalothorax of A. salinus females
DISCUSSION
Intra- and inter-population variability
As seen from the above data, the linear dimensions and the level of their variation inA. salinuspopulations were not constant; to a certain extent they depended on temperature,salinity, and density of population. The results lead to the general conclusion that the impacts of factors on linear morphological characteristics and their variability can manifest itself in different ways at intra-population and inter-population levels. For example, it was shown that about 85% of total variability in body length ofA. salinuscan be explained by temperature changes in local populations (Crimean and Sicilian), but only 28% in the total of samples collected in Mediterranean-Black Sea region (Anufriieva & Shadrin, 2014).There is a negative linear correlation between body size and altitudes ofA. salinushabitats; 22% of total body size variability might be explained by a water body altitude above sea level(Anufriieva & Shadrin, 2014). Similar trend was shown for other diaptomid copepods (Hausch et al, 2013). Temperature, salinity,and population density do not have an influence on body proportions of A. salinus, because changes of linear traits correlate each other; these factors may have an influence on intra-population variability of proportions. The presented data show that a level of morphological variability in populations is not constant and reflects on different factors. In copepods,including A. salinus, variability can increase when animals are near limit values of factors, such as temperature, salinity or increased population density (Devreker et al, 2007; Jimenez-Melero et al, 2007; Whitehouse & Levis, 1973; Zelikman &Geinrikh, 1959). Those experimental data are consistent with our field data on the impact of the different factors on the morphometrical variability inA. salinuspopulations. Interpopulation differences of A. salinus cannot be explained by only studied factors, and it is assumed that there are some overlooked factors as well as differences in the genetic architecture of populations.
In this study the authors evaluated total phenotypic variability in the populations. It is known that differences in phenotypes can be caused by genes, environmental factors, or a combination of both. The idea, which originated with Schmalhausen (1941, 1949) and Waddington (1942, 1957),suggested that genetic variation may get canalized under stabilizing selection and released under directional selection or under stress. A decrease in comfort of living conditions to a certain limit leads to destabilization of ontogeny in a population and as a result, to an increase of variability in the population.Upon reaching a higher level of environmental discomfort there is a dramatic increase in the selection pressure against the individuals with a low degree of canalization of ontogeny; a decrease in variability in the populations has been observed.Populations may realize a random search for the better individuals to survive in an extreme environment by increasing intra-population diversity. However, populations need to spend more energy to support their additional diversity. Resources of energy in populations are very limited in extreme environments; populations have not enough energy to support high diversity in a high extreme environment(Anufriieva & Shadrin, 2014). The diversity in the population begins to decrease; the result is that only individuals with the most stable ontogeny are left. Populations switch from one to another survival strategy. When data on female was examined it was seen that highest variability was featured in the unpredictably changing and ephemeral ponds of Tunisia,Sicily, and twice in the lakes of the Crimea, which have the most unstable environment. This suggests that the selection pressure (or intra-population regulation?) also may drive a variability level in populations.
Sexual dimorphism ofA. salinusmanifests not only in linear dimensions and proportions of the body, but also in variability level and reactions to the fluctuations of environmental conditions. Sexual differences in morphological variability were also observed in other animal species (Elgmork & Halvorsen,1998; Gaviria & Forró, 2000; Istomin, 2008).
Connections between populations in the region and some sources of their variability
The distance between the Crimean closed lakes does not exceed 50 km, i.e. gene flow between them cannot be limited due to isolation-by-distance. It has been shown that genetic isolating barriers (isolation-by-distance) amongEudiaptomusgraciloides Lilljeborg, 1888, living in different reservoirs located within 100 km of each other, are non-existent (Zeller et al, 2006).Therefore, in our opinion, all the samples from the Crimean lakes were taken from one local population. However, our data also show that there are significant differences between samples in some parameters, although these differences on average are less than in the inter-population comparisons. This is, in our opinion, valid also for three samples taken from the Sicilian water bodies. The presence of significant differences in the body proportions of males and females in different populations suggests that some local populations are in the initial stages of differentiation. This does not exclude that all the samples could have been taken from a single metapopulation of A. salinus. It may be concluded that variations in body proportions were thus related to environmental or genetic factors rather than to geographic distance. It is known that the resting eggs of crustaceans can be carried long distances very successfully by wind, birds, or insects (Caceres & Soluk, 2002;Frisch et al, 2007; Green & Figuerola, 2005; Khomenko &Shadrin, 2009; Van de Meutter et al, 2008). Based on this fact,complete isolation of A. salinus local populations may be excluded within the studied area. Episodic transportation of resting eggs between local populations may have occurred; it may be one of the reasons causing fluctuations in individual morphometry and the levels of its variability within populations.This may also explain why Euclidean distance between morphological traits of different populations does not correlate with geographical distance and why there are high morphological differences in samples taken in one single lake at different time. The question of why samples from two close Crimean lakes and one sample from Tunis are in one group cannot be answered by analysing clustering, or why samples taken in Lake Yanyshskoe at different times demonstrate such high Euclidean distance (Figure 1A-C).
The presence of small genetic differences even in adjacent generations was shown for different groups of animals(Altukhov, 2003). Given the ability of the resting eggs to remain dormant in bottom sediments, at least from tens to hundreds of years (Hairston et al, 1995; Marcus et al, 1994), it is easy to imagine how the genetic diversity of the resting egg bank is larger than in the active part of a population. This is shown in particular for Onychodiaptomus sanguineus Forbes, 1876 with a long-lived egg bank (Hairston et al, 1996). The wind regime largely determines the output of nauplii from dormant eggs buried in the sediment. Strong wind, which mixes bottom sediments, leads to a resuspension of a thicker layer of sediments, so this can also lead to outbreaks of diversity in populations. Of course, the role of the wind factor depends on the depth of a water body. The wind factor is least pronounced in Lake Banyoles, the deepest lake of those studied here. The wind factor would be highest in ephemeral ponds with a depth of less than 1 m, and a majority of the studied habitats of Crimea are as such.
CONCLUSION
The authors have not been able to quantitatively assess causes of fluctuation in the level of variability in populations of copepods having resting eggs and living in shallow ephemeral ponds. Indicators of phenotypic or genotypic diversity in populations of shallow ephemeral pond copepods cannot be used to assess population sustainability now. It can be concluded that the significant inter-population differences in morphometric traits cannot be explained by only environmental peculiarities or distance between sites. More field and experimental studies are needed to understand the roles of different factors causing variability on different scales. Studied environmental factors had no effect on the body proportions and their female/male ratio; the body proportions and their female/male ratio may be used as species descriptors.
ACKNOWLEDGEMENTS
The authors are grateful to Mr. Oleg Eryomin (Russia) who passed away on November 18, 2014 for his help in all expeditions and to Mrs. Ekaterina Galagovets (Russia) for sample processing. Special thanks to Dr. Federico Marrone (Italy) who kindly provided theA. salinussamples from Spain, Italy,Tunisia and Dr. Bindy Datson (Australia) for her English editing of our manuscript.
Altukhov YP. 2003. Genetic Processes in Populations. Moscow:Akademkniga. (in Russian)
Anufriieva EV, Shadrin NV. 2014. Factors determining the average body size of geographically separatedArctodiaptomus salinus(Daday, 1885)populations.Zoological Research, 35(2): 132-141.
Burton RS. 1998. Intraspecific phylogeography across the point conception biogeographic boundary.Evolution, 52(3): 734-745.
Caceres CE, Soluk DA. 2002. Blowing in the wind: A field test of overland dispersal and colonization by aquatic invertebrates.Oecologia, 131(3):402-408.
Cassie RM. 1954. Some uses of probability paper in the analysis of size frequency distributions.Australian Journal of Marine & Freshwater Research, 5(3): 513-522.
Deevey GB. 1948. The zooplankton of Tisbury Great Pond.Bulletin of the Bingham Oceanographic Collection, 12(1): 1-44.
Devreker D, Souissi S, Forget-Leray J, Leboulenger F. 2007. Effects of salinity and temperature on the post embryonic development ofEurytemora affinis(Copepoda; Calanoida) of the Seine estuary: a laboratory study.Journal of Plankton Research, 29(Suppl 1): i117-i133.
Elgmork K, Halvorsen G. 1998. Intraspecific morphological variation in a freshwater copepod (Crustacea) in relation to geographic distribution and environment.Canadian Journal of Zoology, 76(4): 751-762.
Folijan LA. 1966. Quantitative development of zooplankton in Lake Issyk-Kul. In: Biological Basis of Fishing Industry in the Water Reservoirs of the Central Asia and Kazakhstan. Alma-Ata: Nauka, 172-175. (in Russian)
Frisch D, Green AJ, Figuerola J. 2007. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds.Aquatic Sciences, 69(4):568-574.
Gaviria S, Forró L. 2000. Morphological characterization of new populations of the copepodEurytemora velox(Lilljeborg, 1853) (Calanoida, Temoridae)found in Austria and Hungary.Hydrobiologia, 438(1-3): 205-216.
Green AJ, Figuerola J. 2005. Recent advances in the study of longdistance dispersal of aquatic invertebrates via birds.Diversity and Distributions, 11(2): 149-156.
Hairston NG, Kearns CM, Ellner SP. 1996. Phenotypic variation in a zooplankton egg bank.Ecology, 77(8): 2382-2392.
Hairston NG, Van Brunt RA, Kearns CM, Engstrom DR. 1995. Age and survivorship of diapausing eggs in a sediment egg bank.Ecology, 76(6): 1706-1711.
Hausch S, Shurin JB, Matthews B. 2013. Variation in body shape across species and populations in a radiation of Diaptomid Copepods.PLoS ONE,8: e68272.
Isinibilir M, Svetlichny L, Hubareva E, Ustun F, Yilmaz IN, Kideys AE, Bat L.2009. Population dynamics and morphological variability ofCalanus euxinusin the Black and Marmara Seas.Italian Journal of Zoology, 76(4): 403-414.
Istomin AV. 2008. An influence of environmental destabilization of the variability and correlation of the morphological traits.Vestnik Pskovskogo PedagogicheskogoUniversiteta.Seriaestestvennieifizikomatematicheskie nauki, 4: 13-23. (in Russian)
Jimenez-Melero R, Parra G, Souissi S, Guerrero F. 2007. Post-embryonic developmental plasticity ofArctodiaptomus salinus(Copepoda: Calanoida)at different temperatures.Journal of Plankton Research, 29(6): 553-567.
Khomenko SV, Shadrin NV. 2009. Iranian endemicArtemia urmianain hypersaline Lake Koyashskoe (Crimea, Ukraine): a preliminary discussion of introduction by birds.Branta: Sbornik nauchnich trudov Azovo-Chernomorskoi ornitilogicheskoi stancii, 12: 81-91. (in Russian)
Knyazeva SG. 2010. Intrapopulation variability of the common juniper.Khvoinye borealnoy zony, 27: 91-96. (in Russian)
Krupa EG, Stuge TS, Lopareva TY, Shaukharbaeva DS. 2008. Distribution of planktonic crustaceans in Lake Balkhash in relation to environmental factors.Inland Water Biology, 1(2): 150-157.
Lee CE, Frost BW. 2002. Morphological stasis in theEurytemora affinisspecies complex (Copepoda: Temoridae).Hydrobiologia, 480(1-3): 111-128.Marcus NH, Lutz R, Burnett W, Cable P. 1994. Age, viability, and vertical distribution of zooplankton resting eggs from an anoxic basin: evidence of an egg bank.Limnology and Oceanography, 39(1): 154-158.
Marrone F. 2006. The microcrustacean fauna of Sicily and the Central Mediterranean Sea area - current knowledge and gaps to be filled.Polish Journal of Ecology, 54(4): 681-686.
Matthews B, De Meester L, Jones CG, Ibelings BW, Bouma TJ, Nuutinen V,van de Koppel J, Odling-Smee J. 2014. Under niche construction: an operational bridge between ecology, evolution, and ecosystem science.Ecological Monographs, 84(2): 245-263.
Matthews B, Hausch S, Winter C, Suttle CA, Shurin JB. 2011. Contrasting ecosystem-effects of morphologically similar Copepods.PLoS ONE, 6:e26700.
Rokneddine A. 2004. The influence of salinity and temperature on the growth ofArctodiaptomus salinus(Daday, 1885) (Copepoda, Calanoida),from the temporary salt marsh, “La Sebkha Zima”, Morocco.Crustaceana,77(9): 1025-1044.
Scheffer M, Bascompte J, Brock WA. 2009. Early-warning signals for critical transitions.Nature, 461(7260): 53-59.
Schmalhausen II. 1941. Stabilizing selection and its role among the factors of evolution.Zhurnal obstchei biologii, 2: 307-354. (in Russian)
Schmalhausen II. 1949. Factors of evolution: the theory of stabilizing selection. Philadelphia: The Blakiston Company. (Translation from Russian by T. Dobzhansky)
Shadrin NV. 2012. Ecosystem dynamics and evolution: multiplicity of steady states and tipping points. Necessity of new understanding.Morskyji ekologichnyji zhurnal, 11(2): 85-95. (in Russian)
Van de Meutter F, Stoks R, de Meester L. 2008. Size-selective dispersal ofDaphniaresting eggs by backswimmers (Notonecta maculata).Biology Letters, 4(5): 494-496.
Waddington CN. 1942. Canalization of development and the inheritance of acquired characters.Nature, 150(3811): 563-565.
Waddington CN. 1957. The Strategy of the Genes. London: Allen and Uniwin.
Whitehouse IV, Levis BG. 1973. The effect of diet and density on development, size and egg production inCyclops abyssorumSars, 1863(Copepoda, Cyclopoida).Crustaceana, 25(3): 225-236.
Williamson PG. 1981. Palaeontological documentation of speciation in Cenozoic mollusks from Turkana Basin.Nature, 293(5832): 437-443.
Zelikman AL, Geinrikh AK. 1959. On the question of the influence of a population density on mortality and its components ofEucyclops serrulatus(Copepoda, Cyclopoida).Bulletin of Moscow Society of Naturalists Biological Series, 64: 125-140. (in Russian)
Zeller M, Reusch TBH, Lampert W. 2006. A comparative population genetic study on calanoid freshwater copepods: Investigation of isolation-bydistance in twoEudiaptomusspecies with a different potential for dispersal.Limnology and Oceanography, 51(1): 17-124.
- Zoological Research的其它文章
- Morphometric studies of genus Placocheilus (Teleostei:Cypriniformes) from Red River, China
- Patterns of reptile and amphibian species richness along elevational gradients in Mt. Kenya
- Stress-relevant social behaviors of middle-class male cynomolgus monkeys (Macaca fascicularis)
- Accelerated evolution of constraint elements for hematophagic adaptation in mosquitoes
- Physiological approaches to understanding molecular actions on dorsolateral prefrontal cortical neurons underlying higher cognitive processing
- Editor’s comments

