Isolation and identification of phytoestrogens and flavonoids in an Ayurvedic proprietary medicine using chromatographic and Mass Spectroscopic analysis
Sulaiman CT, Arun A, Anandan EM, Sandhya CR, Indira Balachandran
1Phytochemistry Division, Centre for Medicinal Plants Research, Arya Vaidya sala, Kottakkal, India
2Product Development Department, Arya Vaidya Sala, Kottakkal, India
Isolation and identification of phytoestrogens and flavonoids in an Ayurvedic proprietary medicine using chromatographic and Mass Spectroscopic analysis
Sulaiman CT1*, Arun A2, Anandan EM2, Sandhya CR2, Indira Balachandran1
1Phytochemistry Division, Centre for Medicinal Plants Research, Arya Vaidya sala, Kottakkal, India
2Product Development Department, Arya Vaidya Sala, Kottakkal, India
ARTICLE INFO
Article history:
Received 10 August 2014
Received in revised form 12 February 2015
Accepted 21 March 2015
Available online 20 June 2015
Phytoestrogens
Herbal medicine
Isoflavones
LC/MS
Objective:To develop analytical methods for the isolation and structural identification of poly phenols including phytoestrogens in Mensokot tablet, a herbal proprietary medicine.Methods:Isolation consisted of an ultrasound-assisted extraction, followed by acid hydrolysis and a final liquid-liquid extraction step in diethyl ether. Identification and structural characterisation was done by liquid chromatography coupled with Q-TOF-ESI-MS/MS analysis.Results:Phytoestrogens such as Coumestrol, Genistein and Glycitein have been identified in Mensokot tablet along with several other flavonoids.
Conclusion:In the present research, a rapid HPLC-MS/MS method has been developed for the identification of phytoestrogens and other flavonoids from an Ayurvedic proprietary medicine. Phytoestrogens are considered to play an important role in the prevention of cancers, heart disease, menopausal symptoms and osteoporosis.
1. Introduction
Phytoestrogens are non-steroidal polyphenolic plant metabolites that, because of their structural similarities to 17β-estradiol, have the ability to bind to estrogen receptors and so they may exert estrogenic effects. The major phytoestrogen groups are isoflavones, coumestans and lignans. There is growing evidence that dietary phytoestrogens could have a role in prevention of estrogen-related cancers like breast cancer, prostate cancer etc., and also some beneficial effects regarding postmenopausal symptoms, osteoporosis and cardiovascular diseases[1]. Many women turn to phytoestrogens as an alternative to hormone replacement therapy and estrogen replacement therapy because of their undesirable side effects[2].
Natural products extracts of therapeutic relevance are of paramount importance as reservoirs of structural and chemical diversity. A recent review on National Pharmacopoeias from several countries reveals at least 120 distinct chemical substances from different plants that have utility as lifesaving drugs[3]. Numerous drugs have entered the international market through exploration of ethnopharmacology and traditional medicine. Although scientific studies have been done on a large number of Indian botanicals, a considerably smaller number of marketable drugs or phytochemical entities have entered the evidence-based therapeutics[4].
Mensokot tablet is prepared out of specific parts of eight medicinal plants such as Saraca asoka, Moringa oleifera, Aloe barbadensis, Zingiber officinale, Piper longum, Piper nigrum, Sesamum indicum and Boerhavia diffusa. Chemical and instrumental analyses are routinely used for analyzing synthetic drugs to confirm its authenticity. In the case of herbal drugs, however the scene is different especially for polyherbal formulation, as there are no chemical or analytical methods available. The current study describes a rapid and convenient analytical method for the identification of bioactive compounds from a finished poly herbal product using reversed phase high-performance liquid chromatography coupled to tandem mass spectrometry.
2. Materials and methods
2.1. Preparation of sample
Mensokot tablets prepared in the Product Development department of Arya Vaidya Sala, Kottakkal were ground into powdered form using mortar and pestle. 10 g of this powdered sample was taken for the isolation of phytoestrogens.
2.2. Chemicals
Formic acid and acetonitrile (LC MS grade) were obtained from Fluka chemicals, Bangalore, India. All other chemicals employed were of standard analytical grade from Merck India.
2.3. Isolation of phytoestrogens
The isolation of phytoestrogen was done using the method of Rostagno et al[5], briefly; 10 g of powdered material was subjected to extraction with 100 mL of 50% ethanol. In order to release the aglycone form of the isoflavones, the extract was treated with 2M hydrochloric acid (1/1, v/v) and maintained on a heated water bath at 70 ℃ for 2h. After cooling, 5 mL of the medium was extracted 2 times with 10 mL diethyl ether in a Multi Pulse vortexer (100 rpm) for 5 min and centrifuged at 3 000 rpm for 6 minutes until the complete separation of the two phases. The organic phase was evaporated on a heated water bath. The residue was dissolved in 5 ml of LC/MS grade methanol.
2.4. Isolation of polyphenols
The polyphenolic compounds were extracted as per the method of Sulaiman et al[6]. In brief, 100 mg of the powdered tablets was dissolved in 200 mL of deionized water. The solution was washed with 50 mL petroleum ether (2 × 25 mL). 20 mL of absolute ethanol (2 × 10 mL) was used to precipitate protein from the solution. After centrifugation at 10 000 rpm for 20 min., the supernatant was concentrated by rotary evaporator at 48 ℃. 10 mg of this extract was dissolved in methanol and used for LC MS analysis.
2.5. HPLC analysis
HPLC profiling of estrogenic extract was done using a Shimadzu High Performance Liquid Chromatographic system equipped with LC-10ATVP pump, SPD M10AVP Photo Diode Array Detector in combination with CLASS-VP 6.12 SP5 integration software. The mobile phase used for the separation was HPLC grade Methanol ( A) and Acetonitrile (B) in the ratio of 60: 40 ( V/V). The column used was C18 - ODS (Octadecylsilane), Lichrospher RP 18e (5 μm) (Merck) with a Phenomenex guard column (4 mm × 2 mm i.d: 5 μm). The samples were injected using a 20 μL loop (Rheodyne Rohnet Park, CA, USA). The flow rate was maintained to 1 mL/min for a total run time of 15 minutes. The PDA signal was recorded at 320 nm.
2.6. LC-MS analysis
LC-ESI-MS analysis was conducted on Agilent 6520 accurate mass Q-TOF LC/MS coupled with Agilent LC 1200 equipped with Agilent Eclipse XDB -C18 column of 5 μm, 4.6 × 150 mm. Gradient elution was performed with methanol (solvent A) and water/0.1% formic acid (solvent B) in a ratio of 70:30 at a constant flow rate of 0.9 mL/ min. Column temperature was maintained at 30 ℃. The MS analysis was performed using ESI in the negative mode. The conditions for mass spectrometry were: drying gas (nitrogen) flow 5 L/min; nebulizer pressure 40 psig; drying gas temperature 325 ℃; capillary voltage - 3000 V; fragmentor volt 125V; Oct RF Vpp 750 V. The mass fragmentation was performed by auto MS/MS mode with varying collision energy 3-4 V/ 100 DA with an offset of 8V. The consistency of fragmentation pattern was further confirmed by targeted MS/MS analysis.
3. Results
3.1. HPLC analysis
The HPLC chromatogram of phytoestrogenic extracts showed three major peaks at Rt 2.83, 3.84 and 4.86 minutes (Figure 1). The online UV spectrum of each peak was also recorded. The peak at 2.83 minute showed maximum absorption at 248 nm. The peak at 3.84 minute showed maximum absorption at 230 nm and 340 nm. The compound at Rt4.86 showed maximum absorption at 230 nm and 280 nm. The UV λmaxindicated the poly phenolic nature of the separated compounds.
3.2. LC-MS analysis of estrogenic extract
The estrogenic fraction was dissolved in LC-MS grade acetonitrile and used for further MS and MS/MS analysis. The analysis was performed by LC/MS in negative polarity mode. The mass fragmentation was performed by Collision Induced Dissociation (CID) in hexapole collision cell by varying the collision energy. The structural identification was done by comparing the mass fragments with the previously reported mass fragmentation patterns. Phytoestrogens such as Coumestrol (Mol. wt 268.22), Genistein (Mol.wt 270.24) and Glycitein (Mol.wt 284.26) have been identified in Mensokot tablet using LC/MS analysis (Figure 2). The structural identification was done by comparing the same with the previously reported fragmentation pattern[1].
3.3. LC-MS/MS analysis of flavonoid extract
The total ion chromatogram obtained was extracted into Base Peak Chromatogram (BPC) using Agilent molecular featured extraction algorithm (Figure 3). The base peak ions and fragments obtained on CID were presented in Table 1. The ion with m/z [M-H]- 191 yielded a fragment with m/z 173 which was identified as Quinic acid on the basis of mass fragmentation pattern[7]. The peak with m/z 301 yielded two fragments with m/z 227 and 151 and the same was identified as Quercetin as reported earlier[6]. The ion with m/z 329.26 presented two major fragments 163 and 123 upon CID and it was identified as vanillic acid-4-glucoside. The fragment 163 is due to the loss of sugar moiety and the other fragment m/z 123 is consistent with the fragmentation due to decarboxylation[7]. The ion found at m/z 137 yielded a fragment with m/z 93. This is inconsistent with the fragmentation pattern of hydroxybenzoic acid, characterized by the loss of a CO2(44 u) from the carboxylic acid group, providing an anion of [M-HCOO]-[8] and it was tentatively identified as 4-hydroxy benzoic acid. The ion at m/z 325 presented a fragment with m/z 145 which corresponded to the fragmentation of coumaric acid derivative. The fragment 145u due to the loss of M-1-162-H2O confirmed the strucutre as p-coumaric acid glucoside[9]. The ion at m/z 179 corresponded to the deprotonated of caffeic acid. The major fragment ions produced were m/z 161 and m/z 135corresponding to loss of water and carbon dioxide molecules respectively[10]. The ion found at m/z 269 fragmented as m/z 158 and was identified as apigenin as compared with previously reported fragmentation pattern[11]. The peak with m/z 341 was identified as Caffeoyl glucose as it presented two fragments with m/z 179 and 135[12]. The fragmentation of ion found with m/z 311 was in good agreement with the fragmentation of caftaric acid [7]. The ion found at m/ z 299 presented a major fragment with m/z 137 and the structure was assigned as hydroxy benzoic acid -O-hexoside in comparison with previously reported MS/Ms data[8]. The molecular ion found at m/z 345 was fragmented with two fragments, m/z 153 and m/ z 109, which was reported earlier as the fragmentation pattern of protocatechuic acid glucoside. The same was tentatively assigned as protocatechuic acid -4-glucoside[7].
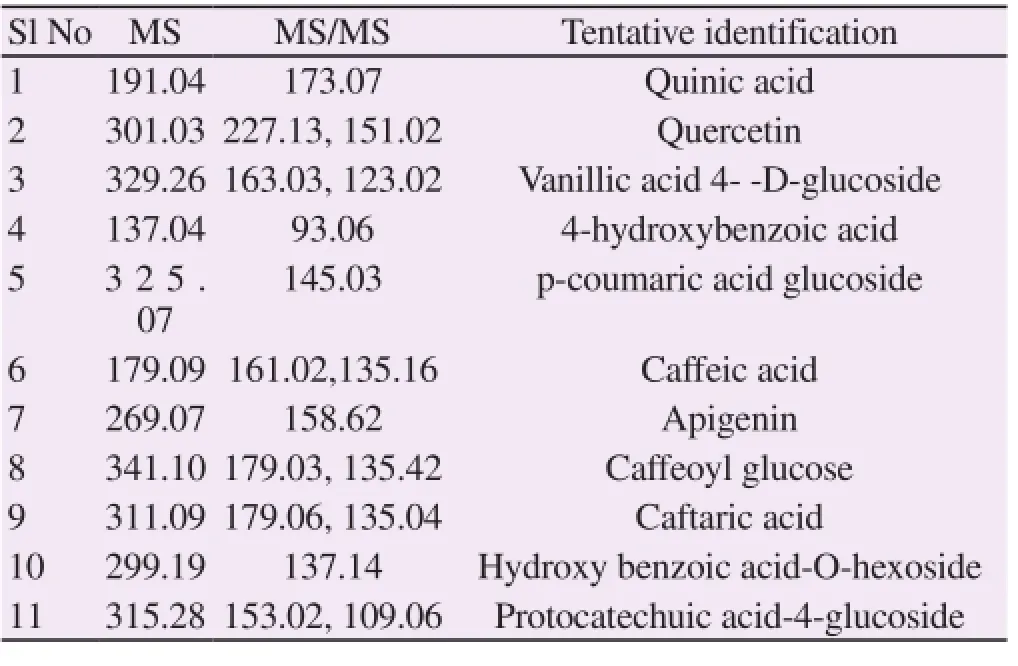
Table 1 Flavonoids/Phenolics identified in mensokot tablet by LC-MS/MS analysis.
4. Discussion
The diverse biological activity of phytoestrogens is due in part to their ability to act estrogenically as estrogen agonists and antiestrogenically as antagonists. As estrogen agonists, phytoestrogens mimic endogenous estrogens and cause estrogenic effects. Phytoestrogens such as Coumestrol, Genistein and Glycitein have been identified in Mensokot tablet. Coumestrol was first reported in 1957 by Bickoff and coworkers as a new phytoestrogen that was isolated from ladino clover (Trifolium repens), strawberry clover (Trifolium fragiferum) and alfalfa (Medicago sativa)[13]. The phytoestrogenic effects of Coumestrol and 4’-methoxy Coumestrol has been reported earlier[2]. The isoflavone, Genistein has been reported for their estrogenic activity and antioxidant activities[14,15]. Glycitein has also been reported for its estrogenic activity[16].
Medicinal efficacy of flavonoids identified in Mensokot tablet as antibacterial, hepatoprotective, anti-inflammatory, anticancer,and antiviral agents is well established[17]. Higher consumption of phytoestrogens, including isoflavones and other flavonoids, has been shown to provide protection against prostate cancer risk[18]. Flavonoids such as apigenin, quercetin are reported for their hepatoprotective activities [19]. Several flavonoids including apigenin, galangin, flavone and flavonol glycosides, isoflavones, flavanones, and chalcones have been shown to possess potent antibacterial activity[20]. A number of flavonoids such as protocatechuic acid, apigenin and quercetin are reported to possess anti-inflammatory and analgesic effects[21]. Flavonoids like quercetin have been reported as cancer chemo preventive agents[22]. The antiviral activity of various flavonoids is well established. Various combinations of flavones and flavonols have been shown to exhibit synergism[23].
The physiological role of biologically active compounds present in plants has attracted more consideration over the last decade, particularly the phytoestrogens. In the present research, a rapid HPLC-MS/MS method has been developed for the identification of phytoestrogens and other flavonoids from an Ayurvedic proprietary medicine. The extraction and isolation of such active components from finished herbal products are important analytical aspects due to the strong evidence of their physiological action. Traditional herbal medicines have been used in the treatment of menopausal symptoms for several thousands of years, but many mysteries remain about the relationship between the active component and their function owing to the complexity of the mixtures. The current phytochemical studies can be used as an attempt for the scientific validation of polyherbal formulations.
Declare of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are thankful to the authorities of Arya Vaidya Sala Kottakkal for extending the facilities and TATA Trust, Mumbai for financial assistance. Authors also would like to thank Dr. Geetha S Pillai, Senior Scientist, Biotechnology division, CMPR for the intellectual support and motivations.
[1] Kiss B, Popa DS, Popa DHA, Loghina F. Ultra-performance liquid chromatography method for the quantification of some phytoestrogens in plant material. Rev Roum Chim 2010; 55: 459-465.
[2] Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res 2003; 17: 845-869.
[3] Goswami A, Barooch PK, Sandhu JS. Prospect of herbal drugs in the age of globalization-Indian scinario. J Sci Ind Res 2002; 61: 423-443.
[4] Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional chinese medicine: A comparative overview. Evid Based Complement Alternat Med 2005; 2: 465-473.
[5] Rostagno MA, Palma M, Barroso CG. Ultrasound-assisted extraction of soy isoflavones. J Chromatogr A 2003; 1012: 119-128.
[6] Sulaiman CT, George S, Balachandran I. Characterization of phenolic compounds in fruit extracts of three medicinal plants using liquid chromatography-negative electrospray ionization tandem mass spectroscopy. Ana chem Lett 2013; 3: 322-329.
[7] Rodríguez-Medina IC, Segura-Carretero A, Fernández-Gutiérrez A. Use of high-performance liquid chromatography with diode array detection coupled to electrospray-Qq-time-of-flight mass spectrometry for the direct characterization of the phenolic fraction in organic commercial juices. J Chromatogr A 2009; 1216: 4736-4744.
[8] Hossain M, Dilip K, Brunton N, Martin-Diana A, Barry-Ryan C. Characterization of phenolic composition in Lamiaceae spices by LCESI-MS/MS. J Agric Food Chem 2010; 58: 10576-10581.
[9] Wulf JS, Ruehmann S, Rego I, Puhl ID, Treutter MZ. Nondestructive application of laser-induced fluorescence spectroscopy for quantitative analyses of phenolic compounds in strawberry fruits. J Agric Food Chem 2008; 56: 2875-2882.
[10] Sulaiman CT, Thushar KV, George S, Balachandran I. Phenolic characterisation of selected Salacia species using LC-ESI-MS/MS analysis. Nat Prod Res 2014a; 28: 1021-1024.
[11] Plazoni A, Bucar F, Male? , Mornar A, Nigovi B, Kujund i N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009; 14: 2466-2490.
[12] Sulaiman C, Sivadasan PG, Balachandran I. Identification of phenolic antioxidants in Ipomoea mauritiana Jacq. using spectrophotometric and mass spectroscopic studies. Avicenna J Phytomed 2014b; 4: 89-96.
[13] Bickoff EM, Booth AN, Lyman RL, Livingston AL, Thompson CR, Deeds F. Coumestrol, a new estrogen isolated from forage crops. Science 1957; 126: 969-970.
[14] Wagner JD, Cefalu WT, Anthony MS, Litwak KN, Zhang L, Clarkson TB. Dietary soy protein and estrogen replacement therapy improve cardiovascular risk factors and decrease aortic cholesteryl ester content in ovariectomized cynomolgus monkeys. Metabolism 1997; 46: 698-705.
[15] Mazur W. Phytoestrogen content in foods. Baillieres Clin Endocrinol Metab 1998; 12: 729-742.
[16] Song TT, Hendrich S, Murphy PA. Estrogenic activity of glycitein, a soy isoflavone. J Agric Food Chem 1999; 47: 1607-1610.
[17] Kumar S, Pandey AK, Chemistry and biological activities of flavonoids: An overview. Sci World J 2013; doi:10.1155/2013/162750.
[18] Siess MH, Mas JP, Canivenc-Lanvier MC, Suschetet M. Time course of induction of rat hepatic drug-metabolising enzyme activities following dietary administration of flavonoids. J Toxicol Environ Health 1996; 49: 481-496.
[19] Tapas AR, Sakarkar DM, Kakde RB. Flavonoids as nutraceuticals: a review. Trop J Pharm Res 2008; 7: 1089-1099.
[20] Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrobial Agents 2005; 26: 343-356.
[21] Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988; 334: 661-665.
[22] Davis WL, Matthew SB. Antioxidants and cancer III: quercetin. Alt Med Rev 2000; 5: 196-208.
[23] Gerdin B, Srensso E. Inhibitory effect of the flavonoid on increased microvascular permeability induced by various agents in rat skin. Int J Microcirc Clin & Exp 1983; 2: 39-46.
ment heading
doi:10.1016/S2305-0500(15)30013-0
*Corresponding author: Sulaiman CT, Phytochemistry Division Centre for Medicinal Plants Research Arya Vaidya Sala, Kottakkal-676503 Malappuram, Kerala, India.
Tel: +914832806209
Mob: +919447382799
E-mail: slmnct@gmail.com
Foundation Project: This project was supported by TATA Trust Mumbai.
 Asian Pacific Journal of Reproduction2015年2期
Asian Pacific Journal of Reproduction2015年2期
- Asian Pacific Journal of Reproduction的其它文章
- Successful triplet pregnancy in an African with pure gonadal dysgenesis: A plus for assisted reproduction
- Recent advances on synchronization of ovulation in goats, out of season, for a more sustainable production
- Apogamous sporophyte development through spore reproduction of a South Asia’s critically endangered fern: Pteris tripartita Sw.
- The relationship between embryo quality assessed using routine embryology or time-lapse videography and serum progesterone concentration on the day of ovulatory trigger in in vitro fertilization cycles
- Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells
- Effects of aqueous and ethanol extract of dried leaves of Pseudocalymma alliaceum (Bignonaceae) on haematological and biochemical parameters of wistar rats
