Recent advances on synchronization of ovulation in goats, out of season, for a more sustainable production
Sim?es J.
Department of veterinary Science. University of Trás-os-Montes e Alto Douro. 5000-811Vila Real, Portugal
Recent advances on synchronization of ovulation in goats, out of season, for a more sustainable production
Sim?es J.
Department of veterinary Science. University of Trás-os-Montes e Alto Douro. 5000-811Vila Real, Portugal
ARTICLE INFO
Article history:
Received 12 February 2015
Received in revised form 15 March 2015
Accepted 20 March 2015
Available online 20 June 2015
Anoestrous
eCG
Goats
Ovulation
Progesterone
Socio-sexual cues
Goats show marked progressive seasonal reproduction at latitude > 25oand reproductive management should be adapted to market demands. The present review aimed to discuss the synchronization of ovulation for timed artificial insemination concerning new insights regarding a clean, green and ethical meat and milk production. Today, the induction of ovulation during breeding season or transitions periods is mainly based on progestagens/ progesterone (P4) devices intravaginally inserted on females, at least during 11 days, plus equine chorionic gonadotropin (eCG) and prostaglandin F2 alfa administration. In last years a reduction to 20 mg of fluorogestone acetate was made and the successful reutilization of devices containing 0.3 g of P4 indicates a possible reduction of their levels. Shortening the period of exogenous progestagens/P4 priming (5 to 7 days) is critical for a rational use of hormones. Moreover, the eCG exchange by socio-sexual cues (male effect) seems to see a great advance, even if a previous photoperiod treatment, or equivalent method, being necessary in high latitudes. Research trends on these subjects are expected in future using different goats breeds in distinct regions of world.
1. Introduction
Goats, as well ewes, are spontaneously ovulating and commonly considered as seasonally polyestrous animals under temperate climatic conditions[1]. The photoperiod is one of the major factors that influence the reproductive activity in small ruminants[2,3]. Gradually, from subtropical regions to higher latitudes, most of local breeds show successive alternated breeding and non-breeding (anoestrous) seasons. This particularity have a great impact on reproductive and production management of flocks[1] and can imply different approaches between regions from different latitudes, breeds and seasons according meat and milk market demands during whole year.
The artificial insemination is a major vehicle for genetic improvement of animal breeds and a reproductive management tool for farmers[4]. Females are normally inseminated following the hormonal synchronization of ovulation in flocks[5,6]. In the last decades, synchronization of ovulation protocols, out of season, are commonly based on controlled internal drug release (CIDR) or intravaginal polyurethane sponges impregnated with progesterone (P4), or their synthetic analogues (progestogens) mainly medroxyprogesterone, melengestrol and fluorogestone acetate forms, plus equine chorionic gonadotropin (eCG) and prostaglandin F2 alfa (PGF2 ) or even estrogenic pharmacologic active substances[6-12]. These protocols are dependent of country availability of licensed hormones [12,13].
The kidding rate can reach 65% after timed artificial insemination with frozen straws (100 × 106spermatozoids/0.2 mL) in goats presenting estrus following a 11-day progestagen priming + eCG + PGF2 protocol and inseminated 43 to 46 hours after sponge withdrawal[14,15]. This fertility rate can be obtained during anoestrous season in regions with high latitude and mainly onintensive dairy herds. Moreover, the possibility to use of sex-sorted spermatozoa in goats was also recently reported[16]. However, other worldwide extensive and semi-extensive production systems, concerning milk and/or meat products[17], also can profit with these reproductive tools, which should be adapted to the different local realities.
Today, the redesigning animal production systems for sustainable agriculture with a lower environmental impact and the adaptation to new hazards, such as the global climate changes, are significant challenges[18]. Animal welfare focused in reproductive management, should be also improved[19].
The use of hormones in animal production was strictly regulated, from last two decades, in several countries. For example, in European Union (directives 96/22/EC, 2003/74/EC and 2008/97/EC) the use of oestradiol 17β(E2) in food-producing animals was banned and P4 utilization was limited, reducing hormones residues on food chain and environment, with potential benefices for public health. Consequently, a decrease from 45 mg to 20 mg of fluorogestone acetate in each sponge was approved, without apparent negative impact on goat fertility[20].
Due to the advent of the ultrasonography and molecular endocrinology as tools, animal welfare improvement and fertility increment of flocks, minimizing economic expanses, several researches were focused to shorten the duration period of intravaginal progestagen/ P4 device exposition from 11 or more days to 5-7 days in females[21], reutilize the intravaginal devices[22,23] or reduce the oxidative stress due to the device contact with the vaginal mucosa[24]. However, recently, a great attention was done to knowledge concerning the natural stimulations of ovulation, especially socio-sexual cues such as the male effect t[25] and even the female effect[26].
In the present paper, we discussed more significant recent advances concerning the synchronization of ovulation with potential impact on reproduction management systems, during the anoestrous season, at the specific goat reproduction contexts. The ultimate purpose was demonstrate that the lucid use of P4 or progestogens and male effect as a tool can achieve good practices of reproductive management in goats, compatible with a sustainable production.
2. Reproductive seasonality and anoestrous season
Goats and ewes are species presenting a reproductive seasonality, mainly according genotypes[27,28] and photoperiod stimulation[3,27,28]. Most of breeds originated from Latitude > 35° North or South and someone’s in subtropical region, located between Latitude 35° and 25°, show a breeding season[15]. Toward to tropical regions, the reproductive seasonally of local small ruminants tends to disappear[29,30] and other factors, such as nutrition and environmental thermic stress (or other stressors), take place[31]. However, Delgadillo et al[25] recently observed that the continuous presence of sexually active bucks can prevent the display of seasonal anestrus of goats in Mexico (L 26° N). According these researchers, further studies are need in order to clarify the degree of the photoperiodic influence and other non-photoperiodic environmental factors, especially sociosexual cues, on seasonality of goats.
At latitude > 45° N (temperate and polar regions), the onset of the breeding and non-breeding seasons of local breeds occur at the end of January/February/early Mars and late August/ September/early October presenting a transition period between seasons[1,32]. From Mars to September all goats remain without ovulatory activity[27]. In hemisphere south, anoestrous season occurs between October and January, like the reported by Rivera et al[33] in Argentina (L 30° S).
The annual patterns of reproduction activity is related with spring and winter solicits causing a progressive variation of daily light/dark duration. Light signals were detected by retina and processed by suprachiasmatic nucleus; signals arrives by sympathetic neurons via to pineal gland which produces melatonin, a key hormone, during short days/ darkness periods[35-37]. A neuronal network mediated by neurotransmission (dopamine, serotonin and other amino acids) is stimulated by melatonin in order to modulate the hypothalamic secretion of gonadotropin-releasing hormone (GnRH)[37]. There are several evidences that these photoperiod variations entraining an endogenous circannual rhythm, and the end and onset of breeding season were due to refractoriness to short and long days, respectively, entraining a circannual endogenous rhyming[38-40]. Is necessary approximately 40 days for the (re)stimulation of luteinizing hormone (LH) pulse activity by melatonin[41], but can reach approximately 66 days according breeds[42].
Chemineau et al[41] observed an increase of frequency and amplitude of LH secretion toward the breeding season, in Saanen goats, but the low plasmatic E2 levels remained constants suggesting a decrease of hypothalamic/pituitary sensibility to their inhibitory effects. The GnRH secretory neurons represents the output of the neural network responding to homeostatic and environmental stimulus which regulate the pituitary LH and follicle-stimulating hormone (FSH) secretion and both P4 and E2 hormones are also related with this system[43].
Above Latitude < 45° N, the anoestrous intensity, i.e. the degree of hypothalamic-pituitary-gonadal axis inhibition, indirectly measured during the non-breeding season according the percentage of females presenting spontaneous ovulations, gradually decrease[44]. The percentage of spontaneous ovulations were very well characterized in Blanca Andaluza goats by Gallego-Calvo et al[45] in Spain (L 37° N; Figure 1) and contrast with the 0% of spontaneous ovulations observed in France[27]. The anoestrous season is shortened, approximately from February/Mars to August like the reported in goats and ewes by some researchers[46,47], but a variable percentage of goats can ovulate before (June and July)[47], including during whole non-breeding season[45].
The body condition score (BCS) was based on a 1 to 5 scale (1 = emaciated and 5 = extremely fat). A considerable percentage of goats presented ovulations in non-breeding season. Seven females showed regular cyclic ovulations over the entire experimental period[45].
The anoestrous intensity have practical implications in order to induce ovulation during the deep anestrus. For example, a photoperiodic treatment (16 h light and 8 h darkness during December to April) was necessary for the improvement of the females response to the male effect in France (L 46oN)[48], or lower dose of eCG can be used in local breeds of regions with lower latitude than 45°[49-51].
Breeds originated from a high latitude maintain the seasonality under a tropical photoperiod treatment (11-13 hour of light per day), but the ovulatory pattern can be influenced like the observed by Chemineau et al[27] in Alpine goats at the final phase of a 3 years study. Inversely, non-seasonal creole goats, from Guadalupe, presented ovulatory inactivity when subjected to a temperate photoperiod treatment (8-16 hour of light per day)[2].
All of these evidences indicates, that different reproductive management of goats, out of season, are necessary according animal breeds and geographic localization.
The anoestrous season is characterized by anestrus and anovulatory activities, but a follicular wave-like (modified) pattern on ovaries persist, in absence of corpora lutea[52]. Following the nonbreeding season, throughout the transition period to breeding season, a great percentages of females shows silence ovulations (mainly in ewes) or 5 -11 days short oestrus cycles (mainly in goats) due to prematurely regressing corpora lutea[53]. The occurrence of short oestrus cycles in goats could be due to an inadequate luteotropic support after ovulation, when LH pulses are essential for CL or premature activation of the luteolytic mechanism[11,54,55].
During the breeding season, the length of oestrus cycle is, in average, 21 days[1] until conception or the end of season, when another similar transition period occurs. A new wave emerge each 5-7 days and the last follicular wave of oestrus cycle origin one or more ovulations accompanied by estrus behavior[56,57].
3. Improving the progestagen + eCG + PGF2α protocols
3.1. Conventional protocols
Today, the principal hormonal protocols used in goats industry are based on devices contained progestogens/P4. Intravaginal polyurethane sponges or CIRD, an inert silicone elastomer, are impregnated with 20-40 mg of fluorogestone acetate, 50-60 mg of medroxyprogesterone or 0.3 g of P4[12]. These devices are inserted intravaginally, in elective goats, for 11 days or more (up to 21 days).
Studies about P4 were firstly reported for ewes[58] and subsequently adapted to goats[59-61]. Progesterone modulates the pituitary LH secretion, inducing a negative feedback, modifying the hypothalamic GnRH activity[62], followed by a pre-ovulatory LH surge after device withdrawal, if eCG is administered in order to improve the development of follicles, including the pre-ovulatory follicle(s), in anoestrous females. Contrarily to LH, P4 don’t influence basal (pulsatile) and wave-like FSH secretion, at least in early oestrus cycle phase of goats[63]. The FSH is modulated by inhibin A and E2 produced mainly by large (dominant) follicle(s)[64].
At time device withdrawal, 24 or 48 h before[65], PGF2 or their synthetic analogues, and eCG are administered i.m. in order to promote the luteolysis of potential corpora lutea (if present in some goats) and the development of antral (preovulatory) follicle(s), respectively. The eCG have a primordial FSH effect and secondarily a LH effect, and acts directly at ovaries level. However, this glycoprotein can develop antibodies when two time successively administered with adverse effect on fertility of the same goat when treatment is repeated in next anoestrous season[5,66]. Their substitution by E2 was tested seems to showing poor results[49].
The eCG, normally administrated from 250 to 600 IU i.m. according breeds, latitude, milk yield (<3.5kg vs. 3.5 kg/day), parity of female, post-partum delay and season, is the key for the induction of ovulation in anestrus goats[4,19,67]. However, Leyva et al[68] observed that the progestagen priming increased the number of follicles stimulated by eCG and consequently the ovulation rate in anestrous ewes. Moreover, the progestagen priming can synchronize the ovulatory wave[11].
These devices impregnated with P4 or progestogens should be excreted the exogenous hormone below the residues limit in milk approved by official authorities. The P4 levels in milk, detected after device use (0.3 g of P4) were lower than that endogenously produced during diestrus or pregnancy[69,70]. However, according Lopez-Sebastian et al[71], a high residue concentration in milk can be observed in the first few days after the sponge insertion impregnated with 20 mg of fluorogestone acetate. This last aspect requires attention and more researches are necessary to determine if the fertility rate remain unaltered with a lower fluorogestone dose, even if a previous photoperiod (or melatonin implant) treatment is necessary.
These conventional protocols were developed following several studies concerning the pre-ovulatory events evaluation, such as the LH preovulatory peak and ovulatory follicle disappearance observed mainly by invasive laparoscopy[53] and the fertility rate results. Therefore, the follicular dynamics before ovulation was not sufficiently evaluated. With the advent of the ultrasonograhic tool, a noninvasive technique, and their association with molecular endocrinology represented an important advance. Several studies concerning the follicular and corpus luteum dynamics were also performed in goats [11,56]. Consequently, the ovarian morphologic dynamics can be more easily related with hormonal events[56,57], minimizing potential adverse stressors due to successive animal manipulations. A potential important impulse on this subject was done by Uruguayan researchers.
3.2. 5 to 7-days short progestogen priming protocols
Rubianes et al[73] observed that the plasmatic P4 levels remained higher (>5 ng/mL) for 3 or 4 days after a device insertion on anoestrous goats than those observed during the mid-late luteal of cyclic females, but decreased to subluteal levels (2 ng/mL) until the end of treatment (device withdrawn). It was suggested that the conventional 11-days progesterone priming induce low plasmatic P4 concentrations toward the end of treatment affecting LH secretion pattern and consequently follicular development (oocyte health and ovulation) and fertility[73-76].
The follicular status at the time of progestogen device insertion is also very important. Using cyclic ewes, No?l et al[77] observed that fluorogestone acetate accelerated the mechanisms of follicular growth, reducing the number of large follicles during luteal and increasing the atresia rate in luteal phase and was detrimental to both the number of large follicles and the ovulation rate during follicular phase. When sponges impregnated with medroxyprogesterone acetate were inserted on Day 6 (toward middle luteal phase), the time of the preovulatory LH surge and ovulation were delayed compared with ewes in which sponges were inserted on Days 0 and 12 of estrous cycle.
Vi?oles and Rubianes[78] observed in ewes that the dominant follicles in the growing or plateau phase at the time of luteolysis became ovulatory. Inversely, if it was already in the regressing phase, the dominant follicle of wave 2 became the ovulatory.
Menchaca and Rubianes[79] observed that the device P4 insertion affected the lifespan of the largest follicle of wave 1 and advanced the emergence of wave 2 in early phase of the oestrus cycle. After several studies in cycling dairy goats submitted to short P4 priming (5-7 days), Rubianes and Menchaca[11] hypothesized their effect on follicular dynamics (Figure 2), claimed an unjustified long progestogen/P4 priming and suggested that this protocol short can be used successfully in both anoestrous and cycling goats.
Menchaca and Rubianes[49] reported the short P4 priming plus 200-300 IU of eCG and PGF2 use during breeding and anoestrous seasons in Uruguayan small dairy goat farmer (L 34° S). Globally, estrus behavior rate reaching 90 % and pregnancy rate reaching 60% after timed artificial insemination, without significant differences between seasons. In non-breeding season (April; L 40° N), the oestrus behavior rate (50%) and pregnancy rate (62.5%) after a 6-days short-term progestogen (20 mg of fluorgestone acetate) priming followed by male effect treatment (in substitution to eCG) and AI was also acceptable in Serrana goats[80].
1)Constraints on NP:if something such as articles and novels etc uoods,its meaning is realized or known.
So, the short-term progestogen/P4 priming (and reduction on device P4 concentration) seems to be a rational protocol and should be widely tested in different breeds and latitudes (Table 1) in both cyclic and anoestrous goats.
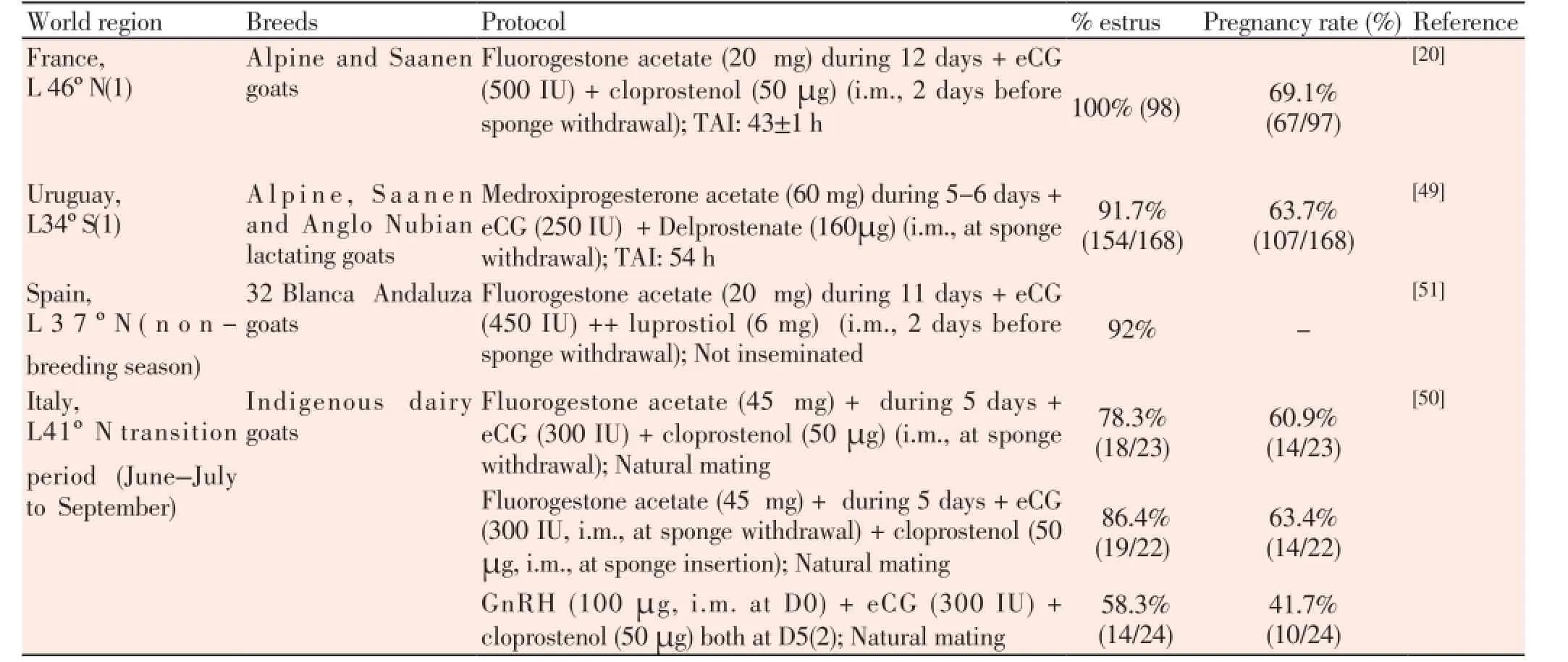
Table 1 Hormonal protocols tested in goats in anoestrous season or transition period.
Finally, one of the inconvenient of intravaginal devices is the vaginal bacteria contamination and inflammation, even when a 6-d short protocol is applied[81,82]. In fact, Manes et al[83], observed a decrease of fertility rate in ewes after a 13-days intravaginal permanence of polyurethane sponge impregnated or not with 60 mg of medroxyprogesterone acetate when compared with females with natural estrus and probably due to the vaginal contamination provoked by devices. Oliveira et al[81], observed an increase of colony-forming units after a 6-day short-term protocol using intravaginal sponges in goats reaching the highest value at the time of sponge withdrawal, but also a rapid re-establishment of the normal vaginal microbiota. These contaminations seems to be time-based and is more one rational argument for short-term protocols use. The use of local antimicrobial applied to device is a common practice for mitigating vaginal flora[84], but probably the short-term protocols can minimize the degree of vaginal contamination/inflammation if the duration of contact between device and vaginal mucosa is a significant risk factor.
4. Socio-sexual cues: male effect
The male effect is an interaction male-females, which promote the induction of ovulation in anoestrous females after male introduction in flock (proportion of one buck per 12 goats)[85-87]. Traditionally, females should be isolated from males at least 40 days before male introduction[88]. However, a complete isolation could be not necessary due female interaction regarding not only male sexual activity but also male novelty[87] and novel bucks can induce the male effect even familiar males remained in flocks[45] . Immediately after sexually active male introduction in flock, an increase of plasma LH pulse occurs[89] from anterior pituitary of females, due to hypothalamic GnRH discharges[90]. Bedos et al [3] observed an increase of LH pulsatility on anoestrous goats when bucks were introduced in flocks and remained in permanent or intermittent (2 hours per days) contact with females during 5 days, and a decrease of LH pulsatility was observed once the male was removed in the intermittent group. These researchers also observed, in another experiment of the same study, that a similar proportion of goats ovulated when the contact with a sexual active male was 1, 2, 4 or 24 hour of contact per day.
The pre-ovulatory LH peak occurs normally between 1 and 3 days after male introduction and goats ovulate approximately 22 hours after LH peak[91-94]. An intense sexual behavior by male goats is necessary to induce LH preovulatory surge and ovulation[95].
Stimulatory factors are multisensorial and can be classified as fero-hormones, behavior (male-females interactions), and stress factors[96,97]. Probably, all the olfactory, visual, tactile and hearing pathways are involved [86,92,98]. The intensity of male-female interaction is great related with sexual active males presence[99,100]. The previous photoperiod treatment of buck (other than melatonin implant), at least during 30 days, is one natural form to increase the sexual activity of bucks[101]. The previous male sexual experience, recent sexual stimulation with females, novelty of the stimulus are described also as factors that improve the ovulation response[86]. However, social dominance within females[102] and their sexual behavior (ex.: tail wagging)[103], but apparently not their parity[104],can play an important role.
More than substitute the eCG effect for induction of ovulation in goats without ovulatory activity throughout a single estrus period[92], the male effect also can re-initiate oestrus cycles, anticipating the breeding season[86]. However, male effect is on dependence of both anestrous intensity[86,92] and stimulatory factors[97]. The previous photoperiodic stimulation of females (and males), or the use of melatonin implants, are two effective methods to minimize the anestrus intensity in flocks, and sexually stimulate bucks at latitude 46° N[48] or 37° N[105].
Although a silent ovulation can occurs at first time, inversely to ewes, goats mainly presents estrus behavior. However, normally this first ovulation in goats is followed by a short estrous cycle and a fertile second ovulation event occurs 5 to 7 days after the first one, accompanied by estrus behavior[86,106]. In fact, Delgadillo et al [107] observed estrus behavior in 94.7% (18/19) of goats after buck introduction and Lassouet et al [108] observed the occurrence of short estrous cycles with a mean duration of (5.6 ± 1.2) days in 100% (20/20) of goats.
In order to increase the synchronization of fertile ovulations, and even to reduce the occurrence of short estrous cycles, a progestogen priming before or at the same time of male introduction can be applied[109]. Véliz et al [110] observed that the use of P4 priming can accelerate the response of goats to the buck stimulus. During the first 5 days exhibition of estrus was 70% higher in P4 treated than untreated groups. At 10th day, similar percentages of estrus were observed as well the pregnancy rate after mating. The interval between male introduction and onset of estrus was shorten from (115.0 ± 10.4) h to (64.8 ± 6.1) h using a progestogen priming protocol[111].
At latitude 20° N (Mexico), a 5-day short (0.3g) P4 priming plus male effect was tested with success in 126 dairy goats (French Alpine, Saanen and Toggenburg breeds) during anoestrous season (April and May) even when devices were reused two or three times. Estrous rate (97.5%, 100% and 100%), intervals to estrus (33.8±1.5, 35.2±2.1 and 29.7±1.1 h) and pregnancy rate (62.5%, 79.5% and 69.5%) were not different between groups that used the device for first, second and third time, respectively[102].
López-Sebastian et al [113] observed higher pregnancy rate (64.6%), at 40th day, using the IMA.PRO2.1? method when compared with the classical hormonal treatment (pregnancy rate = 46.8%; 45 mg of fluorogestone acetate during 11 days plus 350 IU eCG I.M. and 75 μg cloprostenol I.M. 2 days before sponge removal; AI 46h after), from April to June in Murcia-Spain (L 38° N). The IMA.PRO2.1? method was based in the male effect and a single 25 mg dose of progesterone, I.M., given at the time of buck introduction, for the first ovulation induction. A single 75 μg dose of cloprostenol was administered i.m. 9 days later in order to induce early luteolysis and a new ovulation period. Females were inseminated (dose of 200 × 106spermatozoa in 0.25 mL straws cooled to 5 °C) 50 h after PGF2 administration.
According to López-Sebastián et al [71], other new protocol was tested (Flock-Reprod trademarked progestagen-free protocols) with similar results to the obtained with a classical hormonal treatment. This protocol was based on the application of PGF2 17 days after the buck introduction in flock, i.e. after the short cycle occurrence. However, to our knowledge, results were not yet published.
In conclusion, the use of P4 or progestagens remain crucial for synchronization of fertile ovulation if we want to maximize the fertility during the anoestrous season. However, several recent studies suggest that is possible to reduce the exogenous P4/progestagens exposition on females using short progesterone priming protocols, previous photoperiod treatments in high and middle latitudes, combinations with male effect or even the lower P4 concentration in some devices, including their reutilization. So, endeavors are necessary in order to apply and deepen widely this knowledge.
Conflict of interest statement
Author declare that we have no conflict of interest.
[1] Fatet A, Pellicer-Rubio MT, Leboeuf B. Reproductive cycle of goats. Anim Reprod Sci 2011; 124: 211-219.
[2] Chemineau P, Daveau A, Cognié Y, Aumont G, Chesneau D. Seasonal ovulatory activity exists in tropical Creole female goats and Black Belly ewes subjected to a temperate photoperiod. BMC Physiology 2004; 4: 12.
[3] Bedos M, Duarte G, Flores JA, Fitz-Rodríguez G, Hernández H, Vielma J, et al. Two or 24 h of daily contact with sexually active males results in different profiles of LH secretion that both lead to ovulation in anestrous goats. Domest Anim Endocrinol 2014; 48: 93-99.
[4] Leboeuf B, Restall B, Salamon S. Production and storage of goat semen for artificial insemination. Anim Reprod Sci 2000 18; 62: 113-141.
[5] Drion PV, Furtoss V, Baril G, Manfredi E, Bouvier F, Beckers JF, et al. Four years of induction/synchronization of estrus in dairy goats: effects on the evolution of eCG binding rate in relation with the parameters of reproduction. Reprod Nutr Dev 2001; 31: 401-412.
[6] Whitley NC, Jackson DJ. An update on estrus synchronization in goats: a minor species. J Anim Sci 2004; 82: 270-276.
[7] Bretzlaff KN, Madrid N. Clinical use of norgestomet ear implants or intravaginal pessaries for synchronization of estrus in anestrous dairy goats. Theriogenolgy 1989; 31: 419-424.
[8] Ritar AJ, Ball PD, O’May PJ. Artificial insemination of Cashmere goats: effects on fertility and fecundity of intravaginal treatment, method and time of insemination, semen freezing process, number of motile spermatozoa and age of females. Reprod Fertil Dev 1990; 2: 377-384.
[9] Wheaton JE, Carlson KM, Windels HFLJ. CIDR: A new progesteronereleasing intravaginal device for induction of estrus and cycle control in sheep and goats. Anim Reprod Sci 1993; 33: 127-141.
[10] Freitas VJ, Baril G, Saumande J. Estrus synchronization in dairy goats: use of fluorogestone acetate vaginal sponges or norgestomet ear implants. Anim Reprod Sci 1997; 46: 237-244.
[11] Rubianes E, Menchaca A. The pattern and manipulation of ovarianfollicular growth in goats. Anim Reprod Sci 2003; 78: 271-287.
[12] Abecia JA, Forcada F, González-Bulnes A. Pharmaceutical control of reproduction in sheep and goats. Vet Clin North Am Food Anim Pract 2011; 27: 67-79.
[13] Baldassarre H, Karatzas CN. Advanced assisted reproduction technologies (ART) in goats. Anim Reprod Sci 2004; 82-83: 255-266.
[14] Leboeuf B, Manfredi E, Boué P, Piacére A, Brice G, Baril G, et al. Artificial insemination of dairy goats in France. Livestock Prod Sci 1998; 55: 193-203.
[15] Leboeuf B, Delgadillo JA, Manfredi E, Piacère A, Clément V, Martin P, et al. Management of goat reproduction and insemination for genetic improvement in France. Reprod Domest Anim 2008; 43 (Suppl 2): 379-385.
[16] Bathgate R, Mace N, Heasman K, Evans G, Maxwell WM, de Graaf SP. Birth of kids after artificial insemination with sex-sorted, frozen-thawed goat spermatozoa. Reprod Domest Anim 2013; 48: 893-898.
[17] Abecia JA, Forcada F, González-Bulnes A. Hormonal control of reproduction in small ruminants. Anim Reprod Sci 2012; 130: 173-179.
[18] Dumont B, González-García E, Thomas M, Fortun-Lamothe L, Ducrot C, Dourmad JY, et al. Forty research issues for the redesign of animal production systems in the 21st century. Animal 2014; 29: 1-12.
[19] Roger PA. Welfare issues in the reproductive management of small ruminants. Anim Reprod Sci 2012; 130: 141-146.
[20] Leboeuf B, Forgerit Y, Bernelas D, Pougnard JL, Senty E, Driancourt MA. Efficacy of two types of vaginal sponges to control onset of oestrus, time of preovulatory LH peak and kidding rate in goats inseminated with variable numbers of spermatozoa. Theriogenology 2003; 60: 1371-1378.
[21] Menchaca A, Miller V, Salveraglio V, Rubianes E. Endocrine, luteal and follicular responses after the use of the short-term protocol to synchronize ovulation in goats. Anim Reprod Sci 2007; 102: 76-87.
[22] Souza JM, Torres CA, Maia AL, Brand?o FZ, Bruschi JH, Viana JH, et al. Autoclaved, previously used intravaginal progesterone devices induces estrus and ovulation in anestrous Toggenburg goats. Anim Reprod Sci 2011; 129: 50-55.
[23] Vilari?o M, Rubianes E, Menchaca A. Re-use of intravaginal progesterone devices associated with the short-term protocol for timed artificial insemination in goats. Theriogenology 2011; 75: 1195-1200.
[24] S?nmez M, Bozkurt T, Türk G, Gür S, Kizil M, Yüce A. The effect of vitamin E treatment during preovulatory period on reproductive performance of goats following estrous synchronization using intravaginal sponges. Anim Reprod Sci 2009; 114: 183-192.
[25] Delgadillo JA, Flores JA, Hernández H, Poindron P, Keller M, Fitz-Rodríguez G, et al. Sexually active males prevent the display of seasonal anestrus in female goats. Horm Behav 2015; 69: 8-15.
[26] Rodríguez-Martínez R, ángel-García O, Guillén-Mu?oz JM, Robles-Trillo PA, De Santiago-Miramontes Mde L, Meza-Herrera CA, et al. Estrus induction in anestrous mixed-breed goats using the “female-tofemale effect”. Trop Anim Health Prod 2013; 45: 911-915.
[27] Chemineau P, Daveau A, Maurice F, Delgadillo JA. Seasonality of estrus and ovulation is not modified by subjecting female Alpine goats to a tropical photoperiod. Small Rum Res 1992; 8: 299-312.
[28] Santiago-Moreno J, Lopez-Sebastian A, González-Bulnes A, Gomez-Brunet A, Chemineau P. Seasonal changes in ovulatory activity, plasma prolactin, and melatonin concentrations, in Mouflon (Ovis gmelini musimon) e Manchega (Ovis aries) ewes. Reprod Nutr Dev 2000; 40: 421-430.
[29] Greyling JP. Reproduction traits in the Boer goat doe. Small Rumin Res 2000; 36: 171-177.
[30] Arroyo LJ, Gallegos-Sánchez J, Villa-Godoy A, Berruecos JM, Perera G, Valencia J. Reproductive activity of Pelibuey and Suffolk ewes at 19 degrees north latitude. Anim Reprod Sci 2007; 102: 24-30.
[31] Clarke IJ, Tilbrook AJ. Influence of non-photoperiodic environmental factors on reproduction in domestic animals. Anim Reprod Sci 1992; 28: 219-228.
[32] Baril G, Brebion P, Chesne P. Manuel de formation pratique pour la transplantation embryonnaire chez la brebis et la chèvre. In: Etude FAO Production et Santé Animales, no. 115. Rome: FAO;1993.
[33] Rivera GM, Alanis GA, Chaves MA, Ferrero SB, Morello HH. Seasonality of estrus and ovulation in Creole goats of Argentina. Small Rumin Res 2003; 48: 109-117.
[35] Ebling FJP, Hasting MH. The neural basis of seasonal reproduction. Ann Zootech 1992; 41: 239-246.
[36] Malpaux B. Thiery JC, Chemineau P. Melatonin and the seasonal control of reproduction. Reprod Nutr Dev 1999; 39: 355-366.
[37] Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms 2001; 16: 336-347.
[38] Gebbie FE, Forsyth IA, Arendt J. Effects of maintaining solstice light and temperature on reproductive activity, coat growth, plasma prolactin and melatonin in goats. J Reprod Fertil 1999; 116: 25-23.
[39] Gómez-Brunet A, Santiago-Moreno J, Toledano-Díaz A, López Sebastián A. Evidence that refractoriness to long and short day lengths regulates seasonal reproductive transitions in Mediterranean goats. Reprod Domest Anim 2010; 45: 338-343.
[40] Delgadillo JA, De La Torre-Villegas S, Arellano-Solis V, Duarte G, Malpaux B. Refractoriness to short and long days determines the end and onset of the breeding season in subtropical goats. Theriogenology 2011; 76: 1146-1151.
[41] Chemineau P, Martin GB, Saumande J, Normant E. Seasonal and hormonal control of pulsatile LH secretion in the dairy goat (Capra hircus). J Reprod Fertil 1988; 83: 91-98.
[42] Zarazaga LA, Celi I, Guzmán JL, Malpaux B. The response of luteinizing hormone secretion to photoperiod is modified by the level of nutrition in female Mediterranean goats. Anim Reprod Sci 2011; 126: 83-90.
[43] Smith JM, Jennes J. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction 2001; 122: 1-10.
[44] Walkden-Brown SW, Martin GB, Restall BJ. Role of male-female interaction in regulating reproduction in sheep and goats. J Reprod Fertil (Suppl) 1999; 54: 243-257.
[45] Gallego-Calvo L, Gatica MC, Guzmán JL, Zarazaga LA. Role of body condition score and body weight in the control of seasonal reproduction in Blanca Andaluza goats. Anim Reprod Sci 2014; 151: 157-163.
[46] Zarazaga LA, Guzmán JL, Domínguez C, Pérez MC, Prieto R. Effect of plane of nutrition on seasonality of reproduction in Spanish Payoya goats. Anim Reprod Sci 2005; 87: 253-267.
[47] Duarte G, Flores JA, Malpaux B, Delgadillo JA. Reproductive seasonality in female goats adapted to a subtropical environment persists independently of food availability. Domest Anim Endocrinol 2008; 35: 362-370.
[48] Pellicer-Rubio MT, Leboeuf B, Bernelas D, Forgerit Y, Pougnard JL,Bonné JL, et al. High fertility using artificial insemination during deep anoestrus after induction and synchronisation of ovulatory activity by the“male effect” in lactating goats subjected to treatment with artificial long days and progestagens. Anim Reprod Sci 2008; 109: 172-188.
[49] Menchaca A, Rubianes E. Pregnancy rate obtained with short-term protocol for timed artificial insemination in goats. Reprod Domest Anim 2007; 42: 590-593.
[50] Martemucci G, D’Alessandro AG. Induction/synchronization of oestrus and ovulation in dairy goats with different short term treatments and fixed time intrauterine or exocervical insemination system. Anim Reprod Sci 2011; 126: 187-194.
[51] Zarazaga LA, Gatica MC, Gallego-Calvo L, Celi I, Guzmán JL. The timing of oestrus, the preovulatory LH surge and ovulation in Blanca Andaluza goats synchronised by intravaginal progestagen sponge treatment is modified by season but not by body condition score. Anim Reprod Sci 2014; 146: 170-175.
[52] Schwarz T, Wierzchos E. Ovarian follicles in goats during anoestrus. Medycyna Weterynaryjna 2002; 58: 620-622.
[53] Camp JC, Wildt DE, Howard PK, Stuart LD, Chakraborty PK. Ovarian activity during normal and abnormal length estrous cycles in the goat. Biol Reprod 1983; 28: 673-681.
[54] Baird DT. Luteotropic control of the corpus luteum. Anim Reprod Sci 1992; 228: 937-944.
[55] Menchaca A, Rubianes E. Effect of high progesterone levels during the early luteal phase on the length of the ovulatory cycle of the goat. Anim Reprod Sci 2001; 68: 69-76.
[56] de Castro T, Rubianes E, Menchaca A, Rivero A. Ovarian dynamics, serum estradiol and progesterone concentrations during the inte rovulatory interval in goats. Theriogenology 1999; 52: 399-411.
[57] Sim?es J, Almeida JC, Valentim R, Baril G, Azevedo J, Fontes P, et al. Follicular dynamics in Serrana goats. Anim Reprod Sci 2006; 95: 16-26.
[58] Dutt RH, Casida LE. Alteration of the estrual cycle in sheep by use of progesterone and its effect upon subsequent ovulation and fertility. Endocrinology 1948; 43: 208-217.
[59] Corteel JM, Mauleon P, Thimonier J, Ortavant R. Essais d′obtention de gestations synchrones avant le début de la saison sexuelle de la chévre a l′aide de 17 -acétoxy, 9- fluoro, II -hydro-xyprèn-4-ene 3, 20 dione, administré par la voie vaginale. Ann Zootech 1967; 18: 343-350.
[60] Ritar AJ, Maxwell WMC, Salamon S. Ovulation and LH secretion in the goat after intravaginal sponge-PMSG treatment. J Reprod Fertil 1984; 72: 559-563.
[61] Welch RAS, Tervit HR. Artificial insemination of goats: comparison of oestrus control with CIDR dispensers and sponges. New Zealand Ministry of Agriculture & Fisheries. Agric Res Div Annu Rep 1984; 83:64-65.
[62] Hansel W, Convey EM. Physiology of the estrous cycle. J Anim Sci 1983; 57(Suppl.): 104-412.
[63] Suganuma C, Kuroiwa T, Tanaka T, Kamomae H. Changes in the ovarian dynamics and endocrine profiles in goats treated with a progesterone antagonist during the early luteal phase of the estrous cycle. Anim Reprod Sci 2007; 101: 285-294.
[64] Gonzalez-Bulnes A, Santiago-Moreno J, Garcia-Garcia RM, Souza CJ, Lopez-Sebastian A, McNeilly AS. Effect of GnRH antagonists treatment on gonadotrophin secretion, follicular development and inhibin A secretion in goats. Theriogenology 2004; 61: 977-985.
[65] Baril G, Saumande J. Hormonal treatments to control time of ovulation and fertility of goats. In: Proceedings of the 7th International Conference on Goats, France, 15-21 May, 2000, p. 400-405.
[66] Baril G, Remy B, Leboeuf B, Beckers JF, Saumande J. Synchronization of estrus in goats: the relationship between eCG binding in plasma, time of occurrence of estrus and fertility following artificial insemination. Theriogenology 1996; 45: 1553-1559.
[67] Karaca F, Tasal I, Alan M. Preliminary report on induction of estrus with multiple eCG injections in Colored Mohair goats during the anestrus season. Anim Reprod Sci 2009; 114: 306-310.
[68] Leyva V, Buckrell BC, Walton JS. Regulation of follicular activity and ovulation in ewes by exogenous progestagen. Theriogenology 1998; 50: 395-416.
[69] Rowe JD, Tell LA, Carlson JL, Griffith RW, Lee K, Kieu H, et al. Progesterone milk residues in goats treated with CIDR-G(?) inserts. J Vet Pharmacol Ther 2010; 33: 605-609.
[70] Reyes JM, Murcia C, Zarco L, Alvarez L. Progesterone concentrations in milk of CIDR-treated goats. Small Ruminant Res 2012; 106: 178-180.
[71] Lopez-Sebastián A1, Coloma MA, Toledano A, Santiago-Moreno J. Hormone-free protocols for the control of reproduction and artificial insemination in goats. Reprod Domest Anim 2014; 49(Suppl 4): 22-29.
[72] Letelier CA, Contreras-Solis I, García-Fernández RA, Ariznavarreta C, Tresguerres JA, Flores JM, et al. Ovarian follicular dynamics and plasma steroid concentrations are not significantly different in ewes given intravaginal sponges containing either 20 or 40 mg of fluorogestone acetate. Theriogenology 2009; 71: 676-682.
[73] Rubianes E, de Castro T, Kmaid S. Estrous response after a short progesterone priming in seasonally anestrous goats. Theriogenology 1998; 49: 356 (abstract).
[74] Scaramuzzi RJ, Downing JA, Campbell BK, Cognie Y. Control of fertility and fecundity of sheep by means of hormonal manipulation. Aust J Biol Sci 1988; 41: 37-45.
[75] Menchaca A, Rubianes E. Effect of high progesterone concentrations during the early luteal phase on the length of the ovulatory cycle of goats. Anim Reprod Sci 2001; 68: 69-76.
[76] Menchaca A, Rubianes E. Relation between progesterone concentrations during the early luteal phase and follicular dynamics in goats. Theriogenology 2002; 57: 1411-1419.
[77] No?l B, Bister JL, Pierquin B, Paquay R. Effects of FGA and PMSG on follicular growth and LH secretion in Suffolk ewes. Theriogenology 1994; 41: 719-727.
[78] Vi?oles C, Rubianes E. Origin of the preovulatory follicle after induced luteolysis during the early luteal phase in ewes. Can J Anim Sci 1998; 78: 429-431.
[79] Menchaca A, Rubianes E. Relation between progesterone concentrations during the early luteal phase and follicular dynamic in goats. Theriogenology 2002; 57: 1411-1419.
[80] Sim?es J, Baril G, Cunha T, Azevedo J, Teixeira V, Ribeiro CF, et al. Oestrus and ovulatory response of goats with different parity to male effect and short-term progestagen treatment. Reprod Domes Anim 2008; 43(Suppl 5): 71 (Abstract P74).
[81] Oliveira JK, Martins G, Esteves LV, Penna B, Hamond C, da Fonseca JF, et al. Changes in the vaginal flora of goats following a short-term protocol of oestrus induction and synchronisation with intravaginal sponges as well as their antimicrobial sensitivity. Small Rumin Res 2013;113: 162-166.
[82] enna B, Libonati H, Director A, Sarzedas AC, Martins G, Brand?o FZ, et al. Progestin-impregnated intravaginal sponges for estrus induction and synchronization influences on goats vaginal flora and antimicrobial susceptibility. Anim Reprod Sci 2013; 142: 71-74.
[83] Manes J, Campero C, Hozbor F, Alberio R, Ungerfeld R. Vaginal histological changes after using intravaginal sponges for oestrous synchronization in anoestrous ewes. Reprod Domest Anim 2015 Jan 21. doi: 10.1111/rda.12482. [Epub ahead of print].
[84] Gatti M, Zunino P, Ungerfeld R. Changes in the aerobic vaginal bacterial mucous load after treatment with intravaginal sponges in anoestrous ewes: effect of medroxiprogesterone acetate and antibiotic treatment use. Reprod Domest Anim 2011; 46: 205-208.
[85] Shelton M. Influence of the presence of a male goat on the initiation of estrous cycling and ovulation of angora does. J Anim Sci 1960; 19: 368-375.
[86] Walken-Brown SW, Martin GB, Restall BJ. Role of male-female interaction in reglating reproduction in sheep and goats. J Reprod Fert 1999; 52(Suppl.): 243-257.
[87] Delgadillo JA, Gelez H, Ungerfeld R, Hawken PAR, Martin GB. The‘male effect’ in sheep and goats - Revisiting the dogmas. Behav Brain Res 2009; 200: 304-314.
[88] Chemineau P, Malpaux B. [Melatonin and reproduction in domestic farm animals]. Therapie 1998; 53: 445-452.
[89] Vielma J, Chemineau P, Poindron P, Malpaux B, Delgadillo JA. Male sexual behavior contributes to the maintenance of high LH pulsatility in anestrous female goats. Horm Behav 2009; 56: 444-449.
[90] Ichimaru T, Takeuchi Y, Mori Y. Stimulation of the GnRH pulse generator activity by continuous exposure to the male pheromones in the female goat. J Reprod Dev 1999; 45: 243-248.
[91] Claus R, Over R, Dehnhard M. Effect of male odour on LH secretion and the induction of ovulation in seasonally anoestrous goats. Anim Reprod Sci 1990; 22: 27-38.
[92] Thimonier J, Cognie Y, Lassoued N, Khaldi G. L’effet male chez les ovins: une technique actuelle de ma?trise de la reproduction. INRA Prod Anim 2000; 13: 223-231.
[93] Ungerfeld R, Suárez G, Carbajal B, Silva L, Laca M, Forsberg M, et al. Medroxyprogesterone priming and response to the ram effect in Corriedale ewes during the nonbreeding season. Theriogenology 2003; 60: 35-45.
[94] Fernández IG, Luna-Orozco JR, Vielma J, Duarte G, Hernández H, Flores JA, et al. Lack of sexual experience does not reduce the responses of LH, estrus or fertility in anestrous goats exposed to sexually active males. Horm Behav 2011; 60: 484-488.
[95] Martínez-Alfaro JC, Hernández H, Flores JA, Duarte G, Fitz-Rodríguez G, Fernández IG, et al. Importance of intense male sexual behavior for inducing the preovulatory LH surge and ovulation in seasonally anovulatory female goats. Theriogenology 2014; 82: 1028-1035.
[96] Knight TW, Lynch P. Source of ram pheromones that stimulate ovulation in the ewe. Anim Reprod Sci 1980; 3: 133-136.
[97] Rosa HJD, Bryant MJ. The ‘ram effect’ as a way of modifying the reproductive activity in the ewe. Small Rumin Res 2002; 45: 1-16.
[98] Gelez H, Fabre-Nys C. The “male effect” in sheep and goats: a review of the respective roles of the two olfactory. Horm Behav 2004; 46: 257-271. [99] Flores JA, Veliz FG, Perez-Villanueva JA, Martinez de la Escalera G, Chemineau, P, Poindron P, et al. Male reproductive condition is the limiting factor of efficiency in the male effect during seasonal anestrus in female goats. Biol Reprod 2000; 62: 1409-1414.
[100] Delgadillo JA, Carillo E, Moran J, Duarte G, Chemineau P, Malpaux B. Induction of sexual activity of male Creole goats in subtropical northern Mexico using long days and melatonin. J Anim Sci 2001; 79: 2245-2252.
[101] Ponce JL, Velázquez H, Duarte G, Bedos M, Hernández H, Keller M, et al. Reducing exposure to long days from 75 to 30 days of extra-light treatment does not decrease the capacity of male goats to stimulate ovulatory activity in seasonally anovulatory females. Domest Anim Endocrinol 2014; 48: 119-125.
[102] Alvarez L, Martin GB, Galindo F, Zarco LA. Social dominance of female goats affects their response to the male effect. Appl Anim Behav Sci 2003; 84: 119-126.
[103] Haulenbeek AM, Katz LS. Female tail wagging enhances sexual performance in male goats. Horm Behav 2011; 60: 244-247.
[104] Luna-Orozco JR, Fernández IG, Gelez H, Delgadillo JA. Parity of female goats does not influence their estrous and ovulatory responses to the male effect. Anim Reprod Sci 2008; 106: 352-360.
[105] Zarazaga LA, Celi I, Guzmán JL, Malpaux B. Enhancement of the male effect on reproductive performance in female Mediterranean goats with long day and/or melatonin treatment. Vet J 2012; 192: 441-444.
[106] Chemineau P. Effect on oestrus and ovulation of exposing creole goats to the male at three time of the year. J Reprod Fertil 1983; 67: 65-72.
[107] Delgadillo JA, Flores JA, Véliz FG, Hernández HF, Duarte G, Vielma J, et al. Induction of sexual activity in lactating anovulatory female goats using male goats treated only with artificially long days. J Anim Sci 2002; 80: 2780-2786.
[108] Lassoued N, Khaldi G, Chemineau P, Cognié Y, Thimonier J. Role of the uterus in early regression of corpórea lutea induced by the ram effect in seasonally anoestrous Barbarine ewes. Reprd Nutr Dev 1997; 37: 559-571.
[109] Chemineau P, Pellicer-Rubio MT, Lassoued N, Khaldi G, Monniaux D. Male-induced short oestrous and ovarian cycles in sheep and goats: a working hypothesis. Reprod Nutr Dev 2006; 46: 417-429.
[110] Véliz FG, Meza-Herrera CA, De Santiago-Miramontes MA, Arellano-Rodriguez G, Leyva C, Rivas-Mu?oz R, et al. Effect of parity and progesterone priming on induction of reproductive function in Saanen goats by buck exposure. Livest Sci 2009; 125: 261-265.
[111] Mellado M, Olivas R, Ruiz F. Effect of buck stimulus on mature and pre- pubertal norgestomet-treated goats. Small Rumin Res 2000; 36: 269-274.
[112] López-Sebastian A, González-Bulnes A, Carrizosa JA, Urrutia B, Díaz-Delfa C, Santiago-Moreno J, et al. New estrus synchronization and artificial insemination protocol for goats based on male exposure, progesterone and cloprostenol during the non-breeding season. Theriogenology 2007; 68: 1081-1087.
ment heading
doi:10.1016/S2305-0500(15)30014-2
*Corresponding author: Sim?es J., Department of veterinary Science. University of Trás-os-Montes e Alto Douro. 5000-811Vila Real, Portugal.
Tel.: +351259350666
Fax: +351259350480
E-mail: jsimoes@utad.pt
 Asian Pacific Journal of Reproduction2015年2期
Asian Pacific Journal of Reproduction2015年2期
- Asian Pacific Journal of Reproduction的其它文章
- Successful triplet pregnancy in an African with pure gonadal dysgenesis: A plus for assisted reproduction
- Isolation and identification of phytoestrogens and flavonoids in an Ayurvedic proprietary medicine using chromatographic and Mass Spectroscopic analysis
- Apogamous sporophyte development through spore reproduction of a South Asia’s critically endangered fern: Pteris tripartita Sw.
- The relationship between embryo quality assessed using routine embryology or time-lapse videography and serum progesterone concentration on the day of ovulatory trigger in in vitro fertilization cycles
- Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells
- Effects of aqueous and ethanol extract of dried leaves of Pseudocalymma alliaceum (Bignonaceae) on haematological and biochemical parameters of wistar rats
