ZrOCl2·8H2O: An Efficient and Cheap Catalyst for Esterification of Free Fatty Acids to Methyl Esters
Cai Jie; Zhang Qiuyun; Huang Congmin; Zhou Kaizhi; Ma Peihua
(1. School of Science, Guizhou University, Guiyang 550025; 2. School of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025)
ZrOCl2·8H2O: An Efficient and Cheap Catalyst for Esterification of Free Fatty Acids to Methyl Esters
Cai Jie1; Zhang Qiuyun2; Huang Congmin2; Zhou Kaizhi2; Ma Peihua2
(1. School of Science, Guizhou University, Guiyang 550025; 2. School of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025)
The esterification of lauric acid with methanol could be efficiently catalyzed by ZrOCl2·8H2O, and this reaction was studied to develop a green method for biodiesel production. The influencing factors, such as amount of catalyst, reaction time and molar ratio of acid to methanol, were investigated. The results indicated that the ZrOCl2·8H2O catalyst showed high catalytic activity, and gave a 97.0% methyl laurate conversion rate under the following optimized conditions, viz.: a lauric acid/methanol molar ratio of 1:10, a catalyst dosage of 4%, and a reaction duration of 2 h at methanol refluxing temperature. The catalyst could be easily recovered while its activity could be well retained after three cycles. The ZrOCl2·8H2O catalyst also exhibited excellent catalytic activity for the esterification of different free long-chain fatty acids (including nonedible oils with high acid value) with different short carbon chain alcohols. Therefore, the ZrOCl2·8H2O catalyst has good potential for the synthesis of biodiesel from low-cost feedstocks such as waste vegetable oils and non-edible oils.
lauric acid; inorganic salt; esterification; biodiesel
1 Introduction
Nowadays, depleting reserves of fossil fuels and global environmental concerns arising from overconsumption of fossil fuels have prompted an urgent demand for alternative fuels[1]. Biodiesel is considered as an interesting green energy resource since it is a renewable, less polluting, biodegradable and non-toxic fuel[2-3]. At present, most biodiesel is prepared from free fatty acids (FFAs), vegetable oils and animal fats via acid (or base) catalyzed esterification (or transesterification) with short-chain alcohols. However, the high cost of feedstock could account for up to 75% of the biodiesel production cost[4]. There are large amounts of low-grade feedstocks, such as non-edible oils, fried waste oils and animal fats which could be converted to biodiesel, thus lowering the production costs[5]. But, the low-grade feedstock with a high FFAs content cannot be directly used in a base-catalyzed transesterification reaction due to formation of soaps. Therefore, the low-grade materials should be pretreated before application.
On the other hand, esterification of FFAs with alcohol is another feasible route for biodiesel production. The conversion of free fatty acids to fatty acid methyl esters generally involves the esterification using a homogeneous acid catalyst, such as sulfuric acid, hydrochloric acid or methane-sulfonic acid, etc. Since this system has some disadvantages, i.e. catalyst irrecoverability, equipment corrosion, difficulty in separation and purification of the product[6], there is a limited use to serve as a continuous process. Consequently, the development of an efficient catalytic system for esterification process is still an important and interesting objective in biodiesel production. Since some inorganic salts display strong acid properties and they are cheap and easily separated from the esterification products, they have become interesting candidates of choice as catalysts in recent years, and several types of inorganic salt catalysts have been reported[7-9]. However, to our knowledge, ZrOCl2·8H2O has not yet been explored as a catalyst in esterification reactions of FFAs with alcohols. In this study, the application of ZrOCl2·8H2O as a catalyst in the esterification reaction of lauric acid with methanol was performed, as it is relatively cheap, can be easily recovered and, as one of importantfactors, it delivers less toxic emissions. Influence of various reaction parameters (such as catalyst amount, reaction time and fatty acid/alcohol molar ratio) on catalytic performance was studied, and the reusablility performance of the ZrOCl2·8H2O was examined. Furthermore, the catalytic performance of the esterification reaction of different free long-chain fatty acids and high-acid-value non-edible oils upon different low-molecular weight alcohols was elucidated. The ZrOCl2·8H2O catalytic activity was also compared to other inorganic salt catalysts as previously reported.
2 Experimental
2.1 Materials
Lauric acid (AR, 98%), stearic acid (AR, 98%), myristic acid (AR, 98%), palmitic acid (AR, 98%), oleic acid (AR), methanol (AR, >99%), ethanol (AR),n-propanol (AR),n-butanol (AR), zirconium chloride (ZrCl4, AR, >99%) and zirconium oxychloride octahydrate (ZrOCl2·8H2O, AR, 98%) were purchased from the Sinopharm Chemical Regent Co., Ltd. All other chemicals were of analytical grade and used as received, unless otherwise noted.
2.2 Catalytic reaction
The esterification reaction was carried out in a single-necked flask equipped with a reflux condenser and a magnetic stirring apparatus. Certain amounts of free fatty acids (FFAs), short-chain alcohols and catalyst were added to the flask, and the resulting mixture was heated to reflux temperature for 2 h. Upon completion of the reaction, the catalytic system was cooled down to room temperature. The catalyst was separated from the reaction mixture by simple decantation, and the excess methanol was removed under reduced pressure prior to the subsequent analyses. The acid value of the product was determined according to the standard method ISO 660—1996, and the conversion could be calculated using a previously reported technique[10-12]by the following equation:

3 Results and Discussion
3.1 Catalytic activity of ZrOCl2·8H2O for esterification of lauric acid with methanol
3.1.1 Effect of catalyst amount
The effect of catalyst amount on lauric acid conversion is demonstrated in Figure 1. The ZrOCl2·8H2O catalyst amount used for the reaction was studied in the range of between 0—6%. It can be seen from Figure 1 that in the absence of catalyst, the lauric acid conversion rate was low (less than 30%), and it can be also observed that the conversion was raised from 77.9% to a maximum value of 97.0% with catalyst amount increasing from 1% to 4%. However, the conversion slightly dropped to 95.8% as the catalyst amount further increased. Thus, considering the reaction conversion rate and the cost of the catalyst, a catalyst amount of 4% was employed in ZrOCl2·8H2O catalyzed esterification reaction.
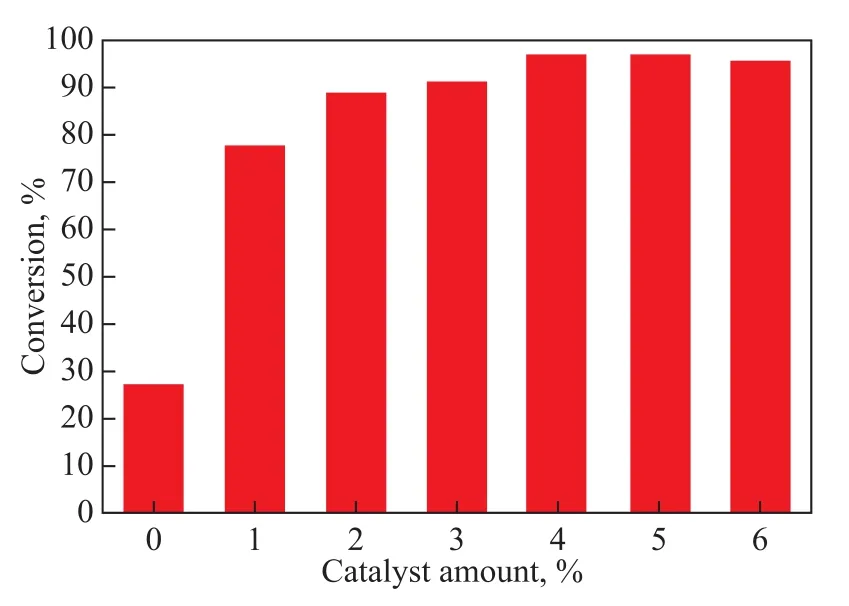
Figure 1 Influence of catalyst amount on conversion
3.1.2 Reaction time
The effect of reaction time on the lauric acid conversion can be seen in Figure 2. The reaction time ranged from 30 min to 180 min. It can be seen from Figure 2 that at a short reaction time of 30 min the insufficient contact between the reactants led to low reaction conversion (62.3%). The conversion increased with an increasing reaction time in the range of between 60 min and 120 min, and reached its maximum level in 120 min, with the highest reaction conversion equating to 97.0%. However, when the reaction time was extended to 180 min, there was no significant increase in the methyl laurate yield. This observation can be explained by the fact that the esterification reaction of lauric acid with methanol is reversible. Based on the above results, the optimum reaction time for the catalyst was 120 min.

Figure 2 Influence of reaction time on conversion
3.1.3 Study on lauric acid/methanol molar ratio
Since esterification is a reversible reaction, an excess of reactant methanol is required in order to increase the rate of methanolysis[13]. Figure 3 depicts the effect of lauric acid to methanol molar ratio on the esterification of lauric acid over zirconium oxychloride octahydrate catalyst. It is observed that with the increase in lauric acid/methanol molar ratio, the conversion increased progressively, and the highest conversion of lauric acid achieved was 97.0% with a lauric acid to methanol mole ratio of 1:10 in a reaction duration of 120 min. With further increase of the lauric acid/methanol molar ratio, the conversion of lauric acid slightly decreased, probably because lauric acid and zirconium oxychloride octahydrate catalyst became excessively diluted with surplus methanol. Therefore, the optimum lauric acid to methanol molar ratio for this system was set at 1:10.

Figure 3 Influence of lauric acid to methanol molarratio on conversion
3.2 Catalyst reusability
To confirm the model prediction of catalyst reusability, this catalyst was investigated in several consecutive runs under the optimum reaction conditions. After the reaction was completed, the product and excess methanol was isolated from the catalytic system by decantation. Subsequently, the catalyst was reused without any further treatment in the next run. When the molar ratio of lauric acid to methanol molar ratio was set at 1:10, and a dosage of 4% catalyst was tested at the methanol refluxing temperature in 120 min of reaction duration, with the performance of recycled catalyst shown in Figure 4. The test results showed that the process was repeated three times, but the conversion of lauric acid changed little, which indicated that the catalyst maintained its catalytic activity after three reaction runs. Although the catalytic activity slightly decreased in subsequent reuse, the lauric acid conversion was still maintained up to 80% after the catalyst was reused for three times. The loss of catalytic activity might be due to the loss of catalyst in the mixing process, and water accumulation could adversely affect the reaction[14]. Such a result suggested that the catalyst could be reused without significant loss in catalytic activity after reuse for three runs.

Figure 4 Reusability of catalysts
3.3 Catalytic activity of ZrOCl2·8H2O for esterification of other short-chain alcohols
In order to further investigate the catalytic activity of ZrOCl2·8H2O, the esterification reaction of lauric acid with different short-chain alcohols (including methanol,ethanol,n-propanol, andn-butanol) was also discussed (Table 1). Excellent conversion rates were obtained in all cases. Notably, the alkyl chains of alcohols had a certain effect on the conversion of fatty acids (entries 1—4). However, our investigation showed that the zirconium oxychloride octahydrate catalyst had an excellent catalytic activity for esterification of various short-chain alcohols, which could produce biodiesel with different characteristics[15].

Table 1 Result of esterification for lauric acid with different alcohols catalyzed by ZrOCl2?8H2O
3.4 Catalytic activity of ZrOCl2·8H2O for esterification of other FFAs and crude non-edible oils with high-acid-value
The effect of different FFAs and crude non-edible oils with high acid value on the initial conversion of methyl esters was analyzed. In the present work four different FFAs (lauric acid, myristic acid, palmitic acid and stearic acid) and two crude non-edible oils (crude jatropha curcas oil and crude euphorbia lathyris oil) were used. The FFAs conversion and conversion of pretreated crude non-edible oils are shown in Table 2. It was discovered that excellent conversion rates were obtained in all cases. Moreover, there were no significant differences for the conversion of different FFAs. The catalyst was also used to catalyze the esterification reaction of some crude non-edible oils with methanol, and the results showed that the conversion of pretreated crude jatropha curcas oil and crude euphorbia lathyris oil reached 83.4% and 77.9%, respectively. Our investigation showed that the ZrOCl2·8H2O catalyst could be a good candidate for manufacture of biodiesel from lowgrade non-edible oils containing high amount of FFAs.

Table 2 Results of esterification of different free fatty acids and high acid value non-edible oils with methanol catalyzed by ZrOCl2?8H2O
3.5 Study on different inorganic salt catalysts for esterification
Esterification reaction was examined over various reported inorganic salt catalysts to compare the applicability and the efficiency of our catalysts, with the results of the esterification reaction presented in Table 3. It can be seen from Table 3 that zirconium oxychloride octahydrate showed higher activity than other inorganic salt catalysts (entries 1—6). Moreover, the zirconium chloride catalyst also had excellent catalytic activity, but zirconium chloride is more expensive than zirconium oxychloride octahydrate. Based onthe above discussion on account of the catalytic activity and the cost of the catalyst, zirconium oxychloride octahydrate is a promising catalyst for biodiesel production process.

Table 3 Comparison of different inorganic salts catalysts for the esterification
4 Conclusions
In this study, the low-cost and reusable zirconium oxychloride octahydrate catalyst has been determined as an efficient catalyst for biodiesel production from FFAs. The catalyst was effective in the esterification reaction with a FFA conversion of 97.0% under the optimum reaction conditions. The catalytic activity of ZrOCl2·8H2O only decreased slightly after three times of recycling. Furthermore, the catalyst has a good catalytic activity for converting various FFAs and non-edible oils upon reacting on various short-chain alcohols. Compared to various reported inorganic salt catalysts, ZrOCl2·8H2O showed higher activity than other catalysts, indicating that the catalyst can be regarded as a promising catalyst candidate for biodiesel production in the industry.
Acknowledgements:This work was financially supported by the Chunhui Project of the Ministry of Education of China (Z122007).
[1] Girish N, Niju S, Meera Sheriffa Begum K M, et al. Utilization of a cost effective solid catalyst derived from natural white bivalve clam shell for transesterification of waste frying oil [J]. Fuel, 2013, 111: 653-658
[2] Zhang Q Y, Xu C, Zhou K Z, et al. Synthesis of biodiesel with silica gel column chromatography catalyzed with immobilized K2CO3[J]. Journal of Fuel Chemistry and Technology, 2011, 39(10): 754-758 (in Chinese)
[3] Badday A S, Abdullah A Z , Lee K T. Transesterification of crude Jatropha oil by activated carbon-supported heteropolyacid catalyst in an ultrasound-assisted reactor system [J]. Renewable Energy, 2014, 62: 10-17
[4] Karabas H. Biodiesel production from crude acorn (Quercus frainetto L.) kernel oil: an optimisation process using the Taguchi method [J]. Renewable Energy, 2013, 53: 384-388
[5] Jiménez-Morales I, Santamaría-González J, Maireles-Torres P, et al. Zirconium doped MCM-41 supported WO3solid acid catalysts for the esterification of oleic acid with methanol [J]. Applied Catalysis A: General, 2010, 379: 61-68
[6] Zhang Q Y, Li H, Qin W T, et al. Solid acid used as highly efficient catalyst for esterification of free fatty acids with alcohols [J]. China Petroleum Processing and Petrochemical Technology, 2013, 15(1): 19-24
[7] Zi J F. Synthesis of butyl oleate catalyzed by compound inorganic salts [J]. Industrial Catalysis, 2013, 21(1): 50-52 (in Chinese)
[8] Gao D Z, Wang L, Liu L H. Comparison of catalytic activity of inorganic salts in the esterification of high acid value oil [J]. Shandong Chemical Industry, 2009, 38(8): 7-9 (in Chinese)
[9] Cheng D Y, Li D S. Preparation of isoamyl butyrate using heterogeneous inorganic ferric salt catalyst [J]. Natural Gas Chemical Industry, 2004, 29(5): 65-67 (in Chinese)
[10] Park Y M, Lee D W, Kim D K, et al. The heterogeneous catalyst system for the continuous conversion of free fatty acids in used vegetable oils for the production of biodiesel [J]. Catalysis Today, 2008, 131: 238-243
[11] Corro G, Ba?uelos F, Vidal E, et al. Measurements of surface acidity of solid catalysts for free fatty acids esterification in Jatropha curcas crude oil for biodiesel production [J]. Fuel, 2014, 115: 625-628
[12] Zhang Q Y, Li H, Liu X F, et al. Modified porous Zr-Mo mixed oxides as strong acid catalysts for biodiesel production [J]. Energy Technology, 2013, 1(12): 735-742.
[13] He L Q, Qin S J, Chang T, et al. Biodiesel synthesis from the esterification of free fatty acids and alcohol catalyzed by long-chain Br?nsted acid ionic liquid [J]. Catalysis Science & Technology, 2013, 3(4): 1102-1107
[14] Su C H. Kinetic study of free fatty acid esterification reaction catalyzed by recoverable and reusable hydrochloric acid [J]. Bioresource Technology, 2013, 130: 522-528
[15] Salis A, Pinna M, Monduzzi M, et al. Biodiesel production from triolein and short chain alcohols through biocatalysis [J]. Journal of Biotechnology, 2005,119: 291-299
[16] Mao L X. Comparison of catalytic activity of some inorganic salts in esterification of propanoic acid with butanol [J]. Fine Chemical Intermediates, 2001, 31(6): 34-35 (in Chinese)
[17] Santos J S, Dias J A, Dias S C L, et al. Acidic characterization and activity of (NH4)xCs2.5-xH0.5PW12O40catalysts in the esterification reaction of oleic acid with ethanol [J]. Applied Catalysis A: General, 2012, 443-444: 33-39
[18] Tang B H. Synthesis of ethyl acetate catalyzed by inorganic salt [J]. Advanced Materials Research, 2012, 455-456: 1060-1063
Recieved date: 2013-11-25; Accepted date: 2013-12-12.
Prof. Ma Peihua, E-mail: sci.phma@ gzu.edu.cn.
- 中國煉油與石油化工的其它文章
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 1. Single-Event Microkinetic (SEMK) Modeling
- Synthesis of Environmentally Friendly Magnesium Linoleate Detergent
- Alkylation of o-Xylene with Styrene over Modified Mordenite for Environmentally Friendly Synthesis of PXE
- Influence of Gas Density on Hydrodynamics in a Bubble Column
- Dispersion Performance of Methanol-Diesel Emulsified Fuel Prepared by High Gravity Technology
- Preparation and Catalytic Performance of Silica-Supported Cr(acac)3/PNP for Ethylene Tetramerization

