Three Therapeutic Regimens in Treatment of Community-acquired Pneumonia: A Cost-effectiveness Analysis
DONG Hong-yan, SUN Li-hua
(School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China)
Three Therapeutic Regimens in Treatment of Community-acquired Pneumonia: A Cost-effectiveness Analysis
DONG Hong-yan, SUN Li-hua
(School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China)
Objective To evaluate economic effectiveness of 3 therapeutic regimens in treatment of community-acquired pneumonia, and to provide references for rational drug use and optimal regimen. Methods A retrospective study was conducted with 117 clinical cases of community-acquired pneumonia. Cost-effectiveness analysis of cefmetazole sodium (therapy A n=60), erythromycin (therapy B=28), cefmetazole sodium and erythromycin (therapy C=29) for community-acquired pneumonia were carried out. Results and Conclusion Effective rates of 3 therapeutic regimens were 51.67%, 60.71%, 65.52%, total cost were RMB 3195.01, 2964.80, 3661.56, respectively; cost-effectiveness ratio were 61.83, 48.84, 55.88; incremental cost effectiveness ratio of A and C, B and C is 33.69, 144.86, respectively. Therapy B and C are superior to therapy A for community-acquired pneumonia, while the economic effectiveness of B and C cannot be determined.
cefmetazole; erythromycin; community-acquired pneumonia; cost-effectiveness analysis
Community-acquired pneumonia (CAP), accounting for over 90% of all pneumonias[1], is an infection of the lung parenchyma outside of hospitals, including the pathogen infection with clear incubation period and onset in the latent period after admission[2]. Statistics from WHO and US at the end of the 20th century suggest that CAP is the sixth leading cause of death. Due to insufficient domestic statistics, the often cited but obviously underestimated number—2.5 million pneumonia patients, 125 thousand cases of death annually, shows that CAP has become a major threat for people’s health[3].
At present, the initial treatment of CAP is generally empiric treatment, mainly including the drug use of cephalosporins, quinolones or macrolides alone or combination therapy, and cephalosporins alone or combined macrolides is recommended for the optimization scheme of CAP. Cefmetazole, a kind of semisynthetic cephamycin derivatives, is often referred to as the second generation of cephalosporins, and erythromycin, the presently used macrolide, have become the commonly used antimicrobial agents because of its advantages of efficiency and safety.
Consequently, this study aimed to evaluate the costeffectiveness of cefmetazole and erythromycin in single and combination therapy and to provide reference for rational use of clinical drugs and optimimal regimen.
1 Patients and methods
1.1 Study population
CAP inpatients from a 1st Class, Grade A hospital in Shenyang, Liaoning from 2011.1 to 2012.6 were selected as objects. All objects meet the diagnostic criteria of CAP in “Guidelines for the Diagnosis and Management of Community-Acquired Pneumonia” formulated by Respiratory Branch of Chinese Medical Association in 2006[4]. In addition, such patients were excluded as diagnosed with combined tuberculosis, lung tumor, noninfective interstitial lung disease, pulmonary edema, atelectasis, pulmonary embolism, pulmonary infiltration with eosinophilia, pulmonary vasculitis and other lung diseases, or severe liver and kidney dysfunction, malignant tumor, serious complications.
According to the inclusion/exclusion criteria, a total of 117 patients were included and divided into 3 groups, with 60 (male 39 and female 21) assigned to receive cefmetazole sodium for injection (therapy A), 28 (male 16 and female12) to receive erythromycin for injection (therapy B), 29 (male 11 and female 18) to receive cefmetazole sodium and erythromycin for injection (therapy C). No statistic significance (P>0.05) is found in differences of patients’illness degree and gender and comparability are available.
1.2 Evaluation of efficacy and adverse reaction
The primary efficacy end point was treatment success, defined as clinical recovery, which means the symptoms, signs, laboratory examination and etiology examination all return to normal. Progress was defined as partial response to treatment (i.e., improvement of symptoms, signs, laboratory examination or etiology examination). Treatment failure was defined as either progression of disease or no detectable improvement in the patient’s condition after 72h[5]. Effective rate=recovery case/total case×100%.
At the end of antimicrobial therapy, treatment was considered successful for 51.67% in therapy A, 60.71% in therapy B, 65.52% in therapy C (Table 1), and without any adverse reaction found. There were no significant differences in the rate of treatment efficacy and incidence of adverse reaction (P>0.05).

Table 1 Clinical efficacy evaluation of 3 therapies
1.3 Cost data analyzed
The pharmacoeconomic analysis was performed from the perspective of society as a whole. The treatment costs considered included direct, indirect, and intangible costs. Although direct costs include both direct medical costs and direct non-medical costs, as the latter vary widely in different situations and are difficult to calculate accurately, we did not consider them in this analysis. Likewise, we did not consider indirect costs, as the costs arising from lost wages are difficult to determine, or intangible costs (e.g. those due to the pain or discomfort caused by the disease or the implementation of preventive, diagnostic and other medical procedures) as they are difficult to measure accurately in monetary terms[6,7]. Therefore, costs in this study refer to direct medical cost, including antimicrobial drug costs and the costs of hospitalizations and examinations. All costs were converted to current value of 2012 by the discount rate of 8%.
For the 3 therapies, the antimicrobial drug costs were obtained by multiplying the price per gram of drug (P) by the daily dose per patient (Q) and the average duration of treatment (T), the costs of hospitalizations and examinations were derived by multiplying the average daily costs of hospitalization and examination per patient (Cave) by the average length of stay (T’). The cost equation was as follows:
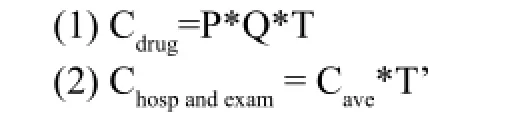
Cefmetazole sodium was provided by Sichuan public credibility pharmaceutical Co. and erythromycin by Dalian merro companies. Drug prices were derived from the hospital drug database, the price of cefmetazole sodium (1g*10 branch/box) and erythromycin (0.3g:300000U*50 branch/box) was 472.9yuan/box (47.29yuan/branch) and 74.5yuan/box (1.49yuan/branch), respectively.
Based on the cost equation, the antimicrobial drug costs and the costs of hospitalizations and examinations of therapy A, B, C were 487.42, 15.46, 524.62 yuan (Table 2) and 2707.59, 2949.34, 3136.94 yuan (Table 3). Therefore, the total direct medical cost of 3 therapies were determined to be 3195.01, 2964.80, 3661.56 yuan, respectively.
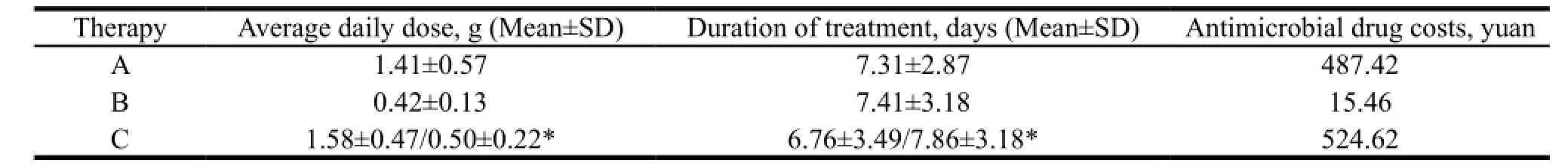
Table 2 The antimicrobial drug costs of 3 therapies

Table 3 The costs of hospitalizations and examinations of 3 therapies
1.4 Sensitivity analyses
Due to different degrees of uncertainty in successful treatment and cost data, 2 one-way sensitivity analyses were performed in order to test the strength of the study’s conclusions over a range of assumptions.
2 Results
2.1 Cost-effectiveness analyses
Based on the above treatment efficacy and cost data, we determined cost-effectiveness ratios for therapy A, B, C (Table 4). The results showed that therapy B was superior to therapy A; compared with A and B, therapy C showed better clinical effect with higher total costs, thus unable to directly compare the economy only when incremental analysis is adopted. According to the results of incremental analysis, the incremental cost effectiveness ratio (ICER) of therapy A and C was 33.69 (<61.83), which means therapy C was superior to therapy A; the ICER of therapy B and C was 144.86 (>48.84), thus unable to determine their economy because of not given cost-effectiveness threshold. Accordingly, therapy B and C is superior to therapy A, yet the comparative economy is unable to be determined.

Table 4 Cost-effectiveness of 3 therapies for CAP
2.2 Sensitivity analysis of drug price
The first one-way sensitivity analysis evaluated the impact of drug price on the results. The highest retail price and the bidding price in various provinces and cities show that the price of cefmetazole sodium (1g/branch) ranged from 41.13 to 47.3 yuan while that of erythromycin (0.3g:300000U/branch) ranged from 1.29 to 1.5 yuan. With other conditions remaining unchanged, we increased the price of the two drugs by 5%, 10%, 15% to calculate the influences on the analysis result. The result showed that variations in the drug price wouldn’t influence the conclusions, that is, the economy of therapy B and C is better than C, while the comparative economy between B and C couldn’t be identified.
2.3 Sensitivity analysis of treatment success rate
The second one-way sensitivity analysis evaluated the impact of treatment success rate on the results. Based on domestic literatures, the treatment success rate of therapy A varied from 80% to 96%, while only one paper provided that of therapy B, 96%. No treatment success rate of therapy C is found. With other conditions remaining unchanged, we increased the treatment success rate by 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90% for therapy A, and 10%, 20%, 30%, 40%, 50%, 60% for therapy B. The result showed that in general, increase of the treatment success rate of A and B in no more than 10% will not affect the conclusions.
The absolute determination analysis was adopted as it was unable to obtain the range of changes for treatment success rate of therapy C. Assume that therapy B and C have the same cost-effectiveness ratio with other conditions remaining unchanged, the minimum treatment success rate for therapy C was 74.98% when superior to therapy B, means the treatment success rate of therapy C changes in no more than 74.98% will not affect the conclusions.
3 Discussions
This pharmacoeconomic evaluation has shown that therapy B and C were better cost-effective options compared with A, while it was unable to compare B and C. Means of expanding samples or other methods were needed for further comparative analysis of the economy of B and C. In addition, far more clinical medications are available other than the three ones here. Therefore, the conclusion is only able to prove that B and C are better therapies in terms of economy. Whether they are better choices among all the available medications for such illness remains to be examined through the comprehensive evaluation of all alternatives.
[1] LI Kai, XU Hong-bing, MA Ai-xia. Analysis of Pharmacoeconomics Evaluation Programs of Community-acquired Pneumonia in Some Community [J]. China Pharmacy, 2009, 20 (2): 87-90.
[2] The Institute of Chinese Medicine Medical Professional Committee, Branch Department of Lung Disease. Community-acquired Pneumonia in TCM Diagnosis and Treatment Guidelines (2011) [J]. Journal of Traditional Chinese Medicine, 2011, 52 (21): 1883-1888.
[3] HE Li-xian, CHEN Xue-hua. Controversy of the Pathogen Spectrum Structure and Initial Empiric Treatment of Community-acquired Pneumonia [J]. Chinese Journal Practical Internal Medicine, 2007, 27 (2): 110-113.
[4] The Chinese Medical Association Epidemiology Branch of Breathing. Guidelines for the Diagnosis and Management of Community-Acquired Pneumonia [J]. The Tuberculosis and Respiratory Journal, 2006, 29 (10): 651-655.
[5] XIE Song-mei, ZHAO Ming, YANG Jin-bo, et al. Considerations and Determination on the Evaluation Criterion for Clinical Efficacy of Antibacterial Agents in China [J]. Chinese Journal of Clinical Pharmacology, 2008, 24 (5): 466-468.
[6] Research Group of “The China Pharmacoeconomics Evaluation Guidelines”. China Guidelines for Pharmacoeconomic Evaluations (2011) [J]. China Journal of Pharmaceutical Economics, 2011, (3): 6-48.
[7] SUN Li-hua. Pharmacoeconomic (Second Edition) [M]. Beijing: China Medical Science Press, 2010: 14-16.
Author’s information: SUN Li-hua, Professor. Major research area: Pharmacoeconomics, medical investment performance and management, etc. Tel: 024-23986553, E-mail: slh-3632@163.com
- 亞洲社會藥學雜志的其它文章
- Marine Pharmaceutical Industry Development in Developed Countries and Its Enlightenment
- Study on the Establishment of Efficiency Evaluation System of Drug Supervision and Sampling Test
- New Version of GDP in EU and Its Enlightenment
- FDA Practices: GMP Quality System Inspection and Evaluation
- Family Economic Risk Caused by Five Major Chronic Diseases among Urban Residents: A Comparative Study Based on the Data from 9 Cities in China
- Pharmacovigilance System for Pharmaceutical Enterprises in EU: Regulation and Its Enlightenment

