Platelet count reduction and outcomes in living liver donors
Jian-Yong Lei and Wen-Tao Wang
Chengdu, China
Platelet count reduction and outcomes in living liver donors
Jian-Yong Lei and Wen-Tao Wang
Chengdu, China
BACKGROUND:Platelet count reduction in living donors after graft harvesting is very common. The mechanisms and the subsequent adverse consequences are not clear. The present study was to explore the mechanisms and the consequences of platelet count reduction in living donors.
METHODS:We collected data from 231 living liver donor patients who donated at our transplant center between July 2002 and August 2009. Baseline and post-operative platelet counts were collected and analyzed. Multivariate logistic regression analysis was used to compare the risk factors for the persistent decrease in platelet counts. Complications and other postoperative recovery were compared between the donors.
RESULTS:Platelet count decreased differently at each of the follow-up intervals, and the average reduction from baseline evaluation to year 3 was 18.2%. A concomitant decrease in white blood cells was observed with platelet count reduction. All of the splenic volumes at the post-operative follow-up time points were significantly higher than those at baseline (P<0.01). Multivariate logistic regression analysis indicated that the graft-to-donor weight ratio was a risk factor for low postoperative platelet counts in living donors at the three followup time points: one week (P=0.047), one month (P=0.034), and three months (P=0.047). At the one week follow-up time, 77 donor platelet counts were higher (group 1) and 151 donor platelet counts were lower (group 2) than baseline levels. Two hemorrhage events (1.3%) were observed in group 2, while three hemorrhage events (3.9%) were observed in group 1 (P=0.211). The overall complication rate was comparable between the two groups (P=0.972).
CONCLUSION:An increase in harvesting graft may decrease platelet counts, but this reduction does not produce short- or long-term damage in living liver donors.
(Hepatobiliary Pancreat Dis Int 2014;13:25-31)
living donor liver transplantation; platelet count reduction;
outcomes
Introduction
Transplantation is the exclusive treatment for patients with end-stage liver disease. However, the increasing death rate of patients on the transplant waiting list has led to the increased use of the riskier approach of living donor liver transplantation (LDLT). The use of LDLT has increased in China because this country harbors a large number of hepatitis B virus (HBV)- infected patients,[1, 2]and social customs prohibit public acceptance of the brain death law. LDLT is criticized for its risks, which include high morbidity[3-6]and donor death.[7-9]Therefore, donor safety is the primary concern in LDLT, and studies have focused on the complications and the quality of life in living donors after donation.[10, 11]Short- and longterm alterations in laboratory test results have been investigated.[12]Trotter et al[12]found that approximately 10% of donors exhibited a platelet count <150×109/L in 2 to 3 years post-donation. Donors with a platelet count ≤150×109/L at 1 year post-donation exhibited significantly lower mean platelet counts (189±32×109/L) compared with the remainder of the cohort (267± 32 × 109/L, P<0.0001). Although Trotter and colleagues revealed this phenomenon, they did not delineate the causes and consequences of platelet count reduction in these donors. However, several hypotheses were introduced in his report, such as elevated portal pressure in donors, reduced thrombopoietin in the remaining liver, and portal vein thrombosis. Although these hypotheses are rational, no evidence has been provided. Therefore, the etiology of reduced platelets is not clear. Another shortage of Trotter's study is that no longterm consequences of platelet count reduction in liver transplant donors were recorded. We also observed this phenomenon and described it in a previous report.[13]The present study was to investigate the possible mechanisms and consequences of persistent platelet count reductions in living liver donors.
Methods
Protocol and data collection
A total of 237 donors underwent partial liver graft harvesting in our transplantation center from July 2002 to August 2009. We retrospectively collected data from these donors from a database: The Chinese Liver Transplant Registry (http://www.cltr.org). The database included pre-operative demographic characteristics, intra-operative data, short- and long-term outcomes, and follow-up data in the outpatient setting. The preoperative data were collected one week prior to donation. A professional secretary performed all donor follow-ups. Only 6 cases were lost to follow-up in the three years after donation, and these six cases were excluded from our analysis. Our routine follow-up time points in the outpatient setting were 1- and 3-month, 1-, 2- and 3-year after discharge.
Protection of human subjects
All procedures were performed with approval from the Ethics Committee of Sichuan University, and local permission was obtained. All donations were voluntary and altruistic. All donors must have exhibited at least a one-third degree of consanguinity with the recipients as verified by the Health Administrative Departments and the Public Security Organs or a DNA test. We informed the donors and their families of the possible risks of donor hepatectomy. Written consent was provided by the donors for the storage of their information in the hospital database and its use in research. The inclusion and exclusion criteria and surgical techniques that were used in this study have been described previously.[14, 15]
Statistical analysis
Descriptive data were expressed as means. Continuous variables were compared with independent samples using the non-parametric Wilcoxon's rank-sum test because some of the measurements did not follow a normal distribution. Categorical data were compared using the Chi-square test or Fisher's exact test when necessary. Inclusion of variables in the final model was based on biological and statistical considerations. Multivariate logistic regression analysis was used to identify factors decreased. Statistical analyses were performed using the SAS statistical software package (version 9.1.3; SAS Institute, Inc., Cary, NC, USA), and a two-sided P value <0.05 was considered to be statistically significant.
CT for splenic volume
Multi-row-detector CT scans for volumetric measurements were performed to evaluate graft size, hepatic vascular anatomy (including hepatic artery, portal vein, and hepatic vein), and the remaining donor liver size, and magnetic resonance imaging (MRI) was used to evaluate the biliary tract. After the donation, the donor participated in routine follow-up examinations, which included CT scans. We retrieved all of the imaging examination data, which were transferred to a SYNAPSE computer workstation (Fujifilm Co., Tokyo, Japan). Splenic volumes were measured using new software developed by the Fujifilm Company.
Results
Baseline demographic characteristics and intraoperative data
The mean donor age of the 231 living donors was 35.2 years (range 19 to 61). Twenty-one donor serum tests were positive for anti-hepatitis B virus core antibody (HBcAb), but HBV DNA tests were negative. Enhanced CT or MRI revealed no cirrhosis in these 21 donors. The demographic characteristics of the 231 living liver donors at the time of evaluation are detailed in Table 1. The operative details of all 231 donors were collected retrospectively. The majority of the grafts (190 cases, 82.3%) were taken from the right lobe for adult recipients. All left lateral lobe recipients were children, with the exception of two patients. The mean graft-todonor weight ratio was 0.88% in the 231 donors.
Alterations in platelet and white blood cell counts (WBCs)
Platelet counts at baseline were 247.6×109/L. Different degrees of platelet count reductions were observed at each of the follow-up intervals. The average reduction in platelet count from baseline to week one was 5.3%. A slight rebound was observed at one month compared with one week after donation, but persistent platelet count reductions were observed thereafter. The average reduction from baseline to year 3 was 18.2% (Fig. 1). Reductions in platelet counts diminished over time. Scatter plots of patient platelet counts at post-donation time points versus baseline values are illustrated in Fig. 2. The points below the equivalence line indicate that patients whose post-donation time points were lower than baseline levels, and the points above the line indicate the levels that were higherthan the baseline. Most of the one-month time points clustered together on the two sides of the equivalence line, which is different from the other follow-up time points (Fig. 2). The repeated measures ANOVA showed significant differences in platelet counts at different time points during follow-up (F=59.172, P<0.001; Fig. 1).
We also observed changes in the WBCs. The average WBC of the 231 donors at baseline was 8.6×109/L, and this level increased to 10.2×109/L one week after donation (normal 4-10×109/L). A decrease in WBC was observed at several post-donation time points, but the decrease was smaller and not persistent (Fig. 3). We also examined the correlation between platelet counts and WBCs to detect the relationship between the decrease of platelet count and portal hypertension. Significant correlations between these factors were observed at the six follow-up time points (P<0.05): r=0.26 at baseline, r=0.22 at 1 week, r=0.22 at 1 month, r=0.29 at 3 months, r=0.33 at 1 year, r=0.37 at 2 years, and r=0.38 at 3 years. The degree of the correlations between platelet counts and WBCs was stronger over time.
The mean splenic volume for the 231 donors was 142.0 cm3(range 65 to 389) at the evaluation point. One week after donation, 46 patients underwent CT scans, which identified a mean splenic volume of 160.6 cm3(range 86 to 321). The splenic volume of the 183 donors increased to 168.1 cm3one month after donation (range 85 to 312). The splenic volume reached its peak of 173.0 cm3(154 cases; range 93 to 300) three months after the operation. However, one year later, the mean volume of the spleens was slightly reduced but still significantly higher than that at the baseline level (P<0.05; Fig. 1). All of the splenic volumes at the post-operative followup time points were significantly higher than those at baseline (P<0.01). Significant correlations between splenic volume and platelet counts were observed at all follow-up time points (P<0.05): r=-0.19 at baseline, r=-0.32 at 1 week, r=-0.23 at 1 month, r=-0.35 at 3 months, r=-0.33 at 1 year, r=-0.29 at 2 years, and r=-0.36 at 3 years.

Table 1.Characteristics of 231 living liver donors at the time of evaluation
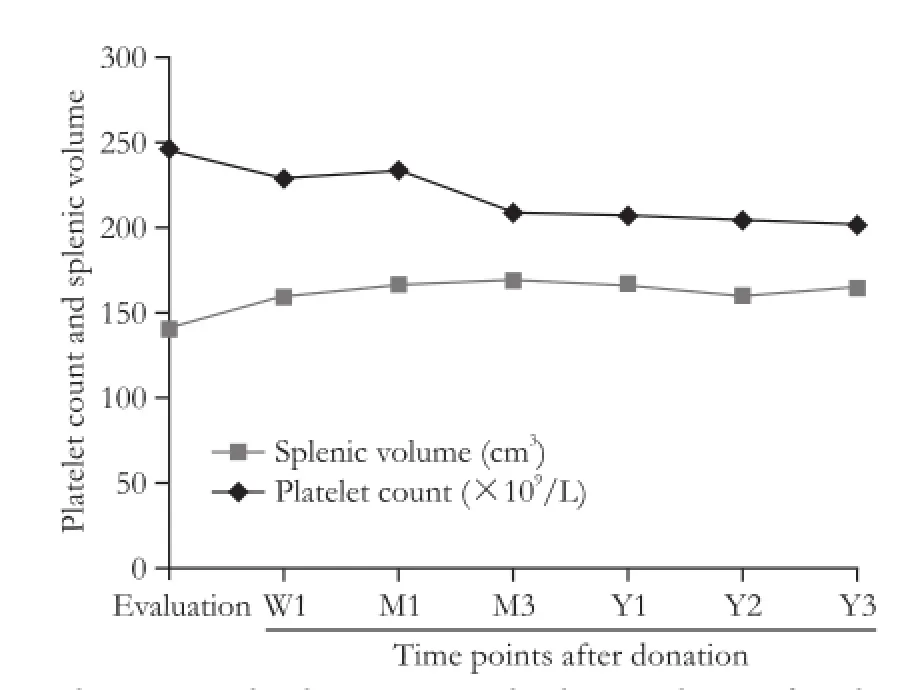
Fig. 1.Changes in platelet counts and splenic volume after donation.
Risk factors for platelet count reductions
Multivariate data (donor age, gender, history of alcohol use, BMI, blood group, graft type, graft size, operative time, graft-to-donor weight ratio, blood loss, and HBcAb) were analyzed using stepwise multivariate logistic regression analysis (step-down) to identify the risks of lower platelet counts. Graft-to-donor weight ratios were significantly correlated with decreased platelet counts at one week (P=0.002, OR=1.045, 95% CI: 1.011-1.119), one month (P=0.034, OR=1.243, 95% CI: 1.119-1.321) and three months (P=0.047, OR=1.131, 95% CI: 1.102-1.211). The P values were greater than 0.05 at one year, two years and three years after donation. However, graft size and graft type were not risk factors for decreased platelet counts (P>0.1). We investigated the correlation between graft-to-donor weight ratios and platelet counts at each follow-up time point. The correlations at one week (P=0.032), one month (P=0.002) and 3 months (P=0.012) were significant, butno correlations were observed in the long-term followup (more than one year). Therefore, the graft-to-donor weight ratio can predict platelet count reductions during the first year after donation. Furthermore, we observed significant correlations between splenic volume and graft-to-donor weight ratio at three follow-up time points (P<0.05): r=0.13 at 1 month, r=0.17 at 3 months, and r=0.14 at 1 year.
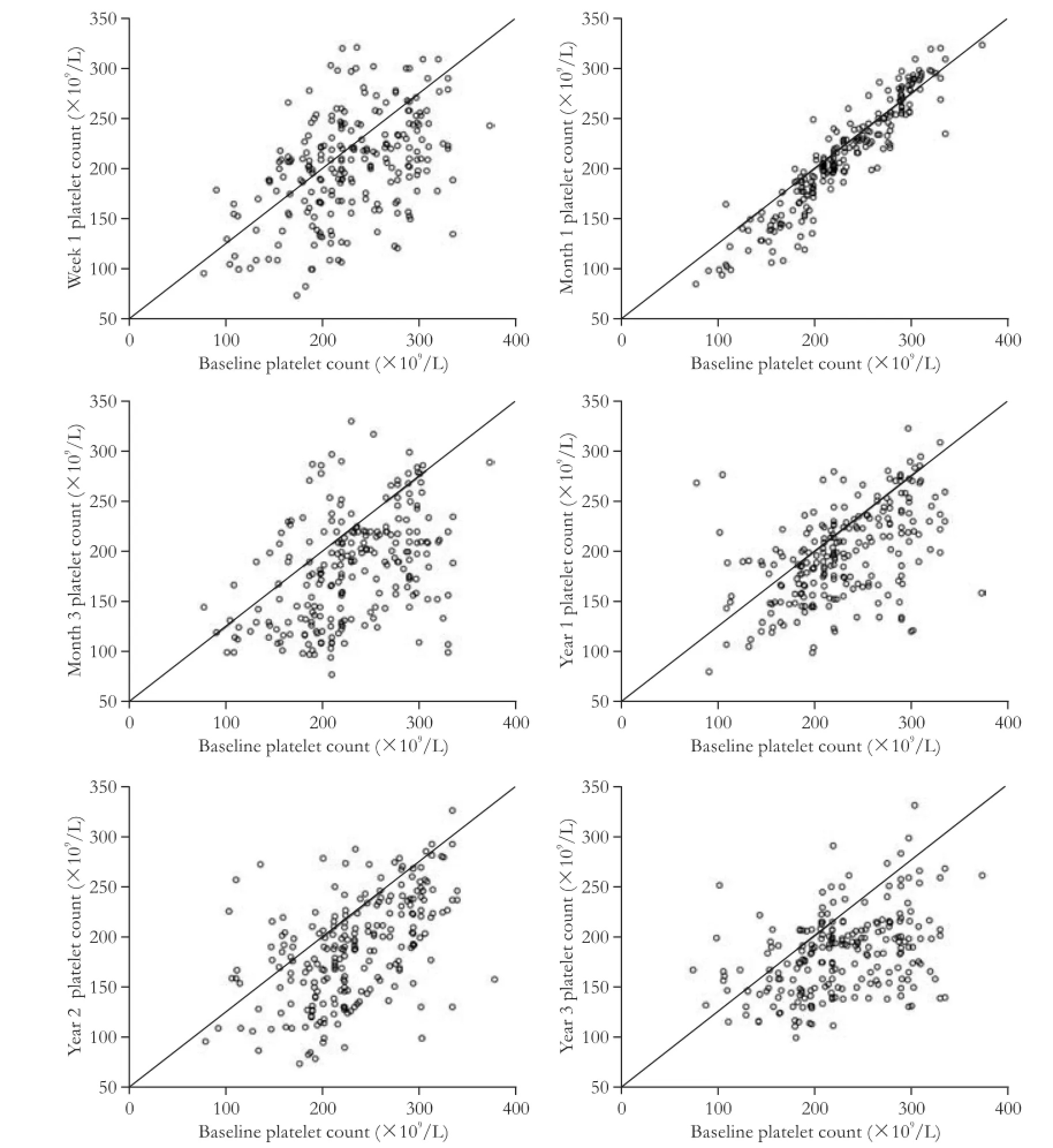
Fig. 2.Platelet count changes compared with baseline at each follow-up time point.
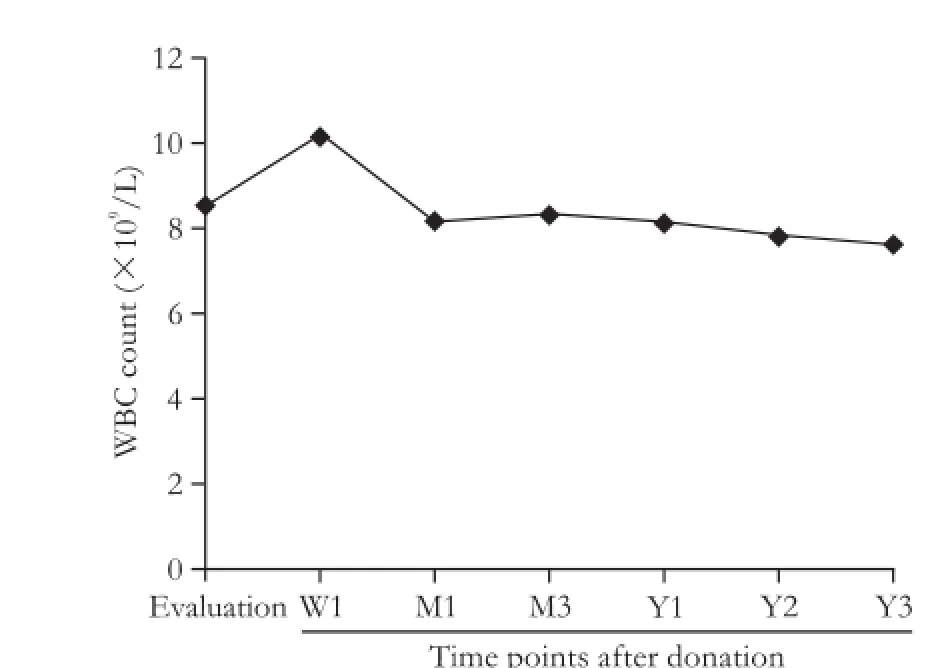
Fig. 3.Change in white blood cell counts after donation.
Impact of platelet count reduction
One week after donation, 77 donor platelet counts were higher (group 1), and 151 donor platelet countswere lower (group 2) than baseline levels. The platelet counts of 3 donors were comparable with baseline values. No differences in pre-operative and intra-operative data were observed between the two groups. We compared post-operative factors between the two groups while the donors were in the hospital. Complications in the two donor groups were graded using the Clavien system, and the results are compared in Table 2. In the followup CT or MRI scan, no signs of portal hypertension such as gastric esophagus varicosity were observed, and no portal hypertensive complications such as gastroesophageal varices bleeding were observed in any of the donors. The overall complication rate in group 1 was 18.2%, which was comparable with that in group 2 (18.5%; P=0.972). Meanwhile, the overall rates of severe complications (grade III) were 5.2% in group 1 and 4.0% in group 2 (P=0.671). Three cases (3.9%) in group 1 and two cases (1.3%) in group 2 (P=0.211) exhibited post-operative bleeding events. One 27-year-old male donor complained of intense incisional pain 81 hours after donation, and ultrasound examination revealed a wound hematoma. The incision was partially divided, and the hematoma was evacuated. The patient was discharged 21 days after donation because of the delayed healing of the incision. Four donors were diagnosed with intraabdominal hemorrhage (persistent hemorrhagic liquid extraction and hemoglobin reduction) after the operation. These donors received conservative treatment, but the hemostatic treatment failed in two donors who then underwent reoperation. The source of bleeding was identified in one donor, and no bleeding was observed after the reoperation. One case exhibited bleeding from the liver wound, and a small artery hemorrhage was identified on the diaphragm.
We also compared other post-operative data between the two groups. The post-operative blood transfusion rates were 3.9% (3 cases) in group 1 and 3.3% (5 cases) in group 2 (P=0.821). The mean blood transfusion volumes after operation of the 3 donors in group 1 were 344.4 mL and 280.0 mL of the 5 donors in group 2 (P=0.420). Post-operative blood transfusions were primarily required because of hemorrhage after donation. Differences in intensive care unit stays, hospital stay, overall cost (USD) and other postoperative data were not statistically significant (P>0.05).
Discussion
LDLT is performed worldwide, and various aspects of donor safety have been evaluated, including complications, quality of life, and long-term outcomes. However, few reports have focused on the changes in laboratory tests after donation.[12, 16]Trotter et al[12]fi rst reported reductions in platelet counts after liver donations and investigated the possible underlying mechanisms. In our previous study, we also found a persistent decrease of platelet counts after donation.[13]However, no studies investigated the mechanisms and consequences of platelet count reduction in living donors. The present study explored the possible risk factors and adverse outcomes of platelet count reduction in living donors.
The etiology of platelet count reduction has not been delineated previously. Our study demonstrated that the graft-to-donor weight ratio was a risk factor and a potential predictor of platelet count reduction after donation. The volume of the liver is reduced after part of the liver is harvested, but portal vein flow is not changed. These modifications increase the portal blood flow per unit volume of the liver (i.e., sinusoidal hyperperfusion) and elevate portal pressure. Higher portal pressures produce splenomegaly and decrease platelet and WBCs.[17, 18]Our study revealed significant correlations between platelet and WBCs at every post-donation time point, and the degree of correlation strengthened over time, with the exception of the one week post-operation time point. We also found a negative correlation between platelet count and splenic volume at followup time. These findings strongly suggested that the reduction in platelet count after donation was mainly caused by splenomegaly. The remaining liver begins toregenerate in the first week after resection,[19, 20]and this process continues until the mean liver volume of right lobe donors is approximately 90% of the original liver volume six months after donation.[21]The liver volumes of left lobe and left lateral lobe donors reach 90% of the pre-operative liver volume one year after donation.[22]Liver regeneration terminates when the liver regains its original size in approximately 1 year,[23]and the high portal pressures are back to normal between 6 months and one year. The significant correlation between graftto-donor weight ratios and low platelet counts was observed at one week, one month and three months after donation, but this correlation terminated at one year. These results suggest that high portal pressure is an underlying factor for lower platelet counts after donation in the first year. Portal vein thrombosis has been reported in less than 1% of living liver donors.[24, 25]This condition can aggravate portal pressure, produce splenomegaly and, subsequently, lower platelet count. However this condition is rare and cannot explain platelet count reductions in all donors. Our study did not detect this condition. These two mechanisms were valid in the first year. However, the mechanisms of persistent splenomegaly (>1 year) and chronic persistent platelet count reduction (>1 year) are not known, and more investigations on this mechanism are required.
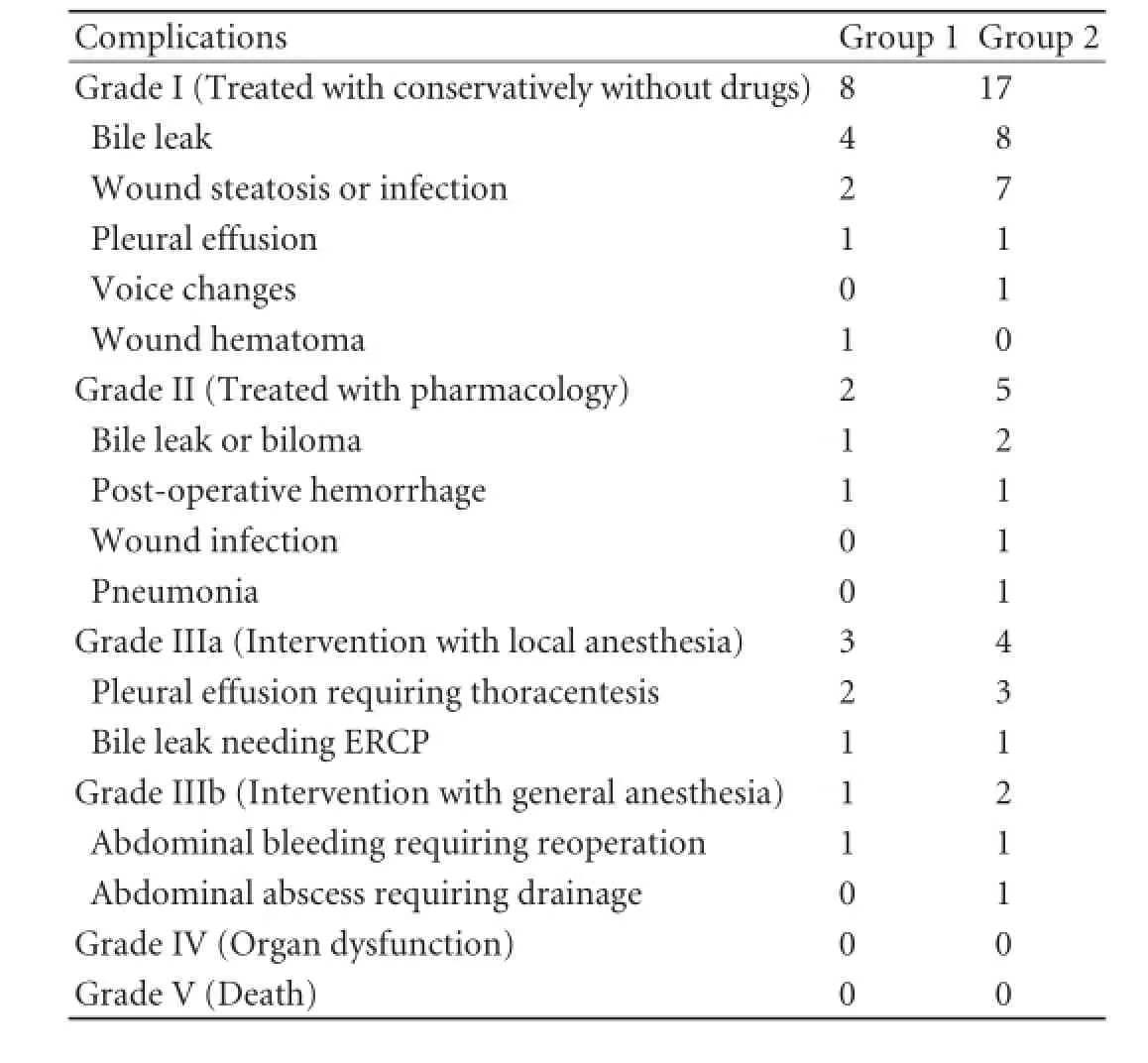
Table 2.Complications in the two groups classified using the Clavien system
Blood platelets are nuclear disc-shaped cells that are generated from megakaryocytes in the bone marrow. Normal platelet function is critical for primary hemostasis, which is the initial phase of hemostasis after vascular injury. Disorders of platelet number or function may result in defective hemostasis. The disorders may be primary hematologic disorders or secondary to other underlying medical conditions.[26, 27]Reductions in platelet count significantly correlate with post-operative blood loss in cardiopulmonary bypass patients.[28]In our study, however, the rates of hemorrhagic events were similar between the two groups one week after donation. These reductions produced no further grade II and III complications and no further blood transfusions in the platelet-reduced group. The latest hemorrhagic event occurred 81 hours after donation with a diagnosis of wound hematoma. Other differences between the two groups were not statistically significant. Therefore, there is no potential harm due to reduced platelet counts in donors.
Our analysis has several shortcomings because of the data obtained from a single center. Multicenter data should be more representative to the donor population. The longest follow-up period in our study was 3 years; follow-up periods should be extended to observe the changes in the platelet counts. In addition, not every donor had data for each of the follow-up intervals. We believe that a larger number of patients, a multicenter study design, and a longer follow-up would minimize these limitations.
In conclusion, graft-to-donor weight ratio is the only risk factor for platelet count reduction, and this factor may predict low platelet counts one year after donation in living donors. Splenomegaly is the main cause of the lower platelet counts. Fortunately, this persistent postdonation decrease of platelets does not cause any serious consequences.
Contributors:WWT proposed the study. LJY and WWT performed research and wrote the first draft. LJY analyzed the data. Both authors contributed to the design and interpretation of the study and to further drafts. WWT is the guarantor.
Funding:None.
Ethical approval:All procedures were performed with approval by the Ethics Committee of Sichuan University.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Hwang S, Moon DB, Lee SG, Park KM, Kim KH, Ahn CS, et al. Safety of anti-hepatitis B core antibody-positive donors for living-donor liver transplantation. Transplantation 2003;75: S45-48.
2 Lee KH, Wai CT, Lim SG, Manjit K, Lee HL, Da Costa M, et al. Risk for de novo hepatitis B from antibody to hepatitis B core antigen-positive donors in liver transplantation in Singapore. Liver Transpl 2001;7:469-470.
3 Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology 2008;135:468-476.
4 Gruttadauria S, Marsh JW, Vizzini GB, di Francesco F, Luca A, Volpes R, et al. Analysis of surgical and perioperative complications in seventy-five right hepatectomies for living donor liver transplantation. World J Gastroenterol 2008;14: 3159-3164.
5 Marsh JW, Gray E, Ness R, Starzl TE. Complications of right lobe living donor liver transplantation. J Hepatol 2009;51: 715-724.
6 Adcock L, Macleod C, Dubay D, Greig PD, Cattral MS, McGilvray I, et al. Adult living liver donors have excellent long-term medical outcomes: the University of Toronto liver transplant experience. Am J Transplant 2010;10:364-371.
7 Ringe B, Xiao G, Sass DA, Karam J, Shang S, Maroney TP, et al. Rescue of a living donor with liver transplantation. Am J Transplant 2008;8:1557-1561.
8 Trotter JF, Adam R, Lo CM, Kenison J. Documented deaths of hepatic lobe donors for living donor liver transplantation. Liver Transpl 2006;12:1485-1488.
9 Melloul E, Dondero F, Paugam-Burtz C, Bouadma L, Arnulf B, Belghiti J. Living liver donor death related to complications of myeloma. Liver Transpl 2009;15:326-329.
10 Jin SG, Xiang B, Yan LN, Chen ZY, Yang JY, Xu MQ, et al. Quality of life and psychological outcome of donors after living donor liver transplantation. World J Gastroenterol 2012;18:182-187.
11 Togashi J, Sugawara Y, Tamura S, Yamashiki N, Kaneko J, Aoki T, et al. Donor quality of life after living donor liver transplantation: a prospective study. J Hepatobiliary Pancreat Sci 2011;18:263-267.
12 Trotter JF, Gillespie BW, Terrault NA, Abecassis MM, Merion RM, Brown RS Jr, et al. Laboratory test results after living liver donation in the adult-to-adult living donor liver transplantation cohort study. Liver Transpl 2011;17:409-417.
13 Lei J, Yan L, Wang W. Donor safety in living donor liver transplantation: a single-center analysis of 300 cases. PLoS One 2013;8:e61769.
14 Shi ZR, Yan LN, Li B, Wen TF. Evaluation of standard liver volume formulae for Chinese adults. World J Gastroenterol 2009;15:4062-4066.
15 Taketomi A, Kayashima H, Soejima Y, Yoshizumi T, Uchiyama H, Ikegami T, et al. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation 2009;87:445-450.
16 Li C, Mi K, Wen TF, Yan LN, Li B. Outcome comparison of right hepatectomy for living liver donation versus for hepatic patients without cirrhosis. J Gastrointest Surg 2011;15:982- 987.
17 Eyraud D, Granger B, Ionescu C, Fratéa S, Darnat S, Vaillant JC, et al. Thrombocytopenia, splenomegaly, and portal blood fl ow in patients who have undergone liver transplantation for cirrhosis. Liver Transpl 2012;18:340-346.
18 Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology 2008;47:153-159.
19 Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation 2000;69:1375-1379.
20 Kawasaki S, Makuuchi M, Ishizone S, Matsunami H, Terada M, Kawarazaki H. Liver regeneration in recipients and donors after transplantation. Lancet 1992;339:580-581.
21 Ibrahim S, Chen CL, Wang CC, Wang SH, Lin CC, Liu YW, et al. Liver regeneration and splenic enlargement in donors after living-donor liver transplantation. World J Surg 2005;29: 1658-1666.
22 Haga J, Shimazu M, Wakabayashi G, Tanabe M, Kawachi S, Fuchimoto Y, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl 2008;14:1718-1724.
23 Miwa S, Miyagawa S. Liver regeneration after hepatectomy. Nihon Geka Gakkai Zasshi 2004;105:654-657.
24 Lo CM. Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation 2003;75:S12-15.
25 Coelho JC, de Freitas AC, Matias JE, de Godoy JL, Zeni Neto C, Parolin MB, et al. Donor complications including the report of one death in right-lobe living-donor liver transplantation. Dig Surg 2007;24:191-196.
26 Weiss HJ. Platelet physiology and abnormalities of platelet function (first of two parts). N Engl J Med 1975;293:531-541.
27 Rodgers GM. Overview of platelet physiology and laboratory evaluation of platelet function. Clin Obstet Gynecol 1999;42: 349-359.
28 Holloway DS, Summaria L, Sandesara J, Vagher JP, Alexander JC, Caprini JA. Decreased platelet number and function and increased fibrinolysis contribute to postoperative bleeding in cardiopulmonary bypass patients. Thromb Haemost 1988;59: 62-67.
Received January 22, 2013
Accepted after revision June 26, 2013
AuthorAffiliations:Liver Transplantation Center, West China Hospital of Sichuan University, Chengdu 610041, China (Lei JY and Wang WT)
Wen-Tao Wang, MD, PhD, Liver Transplantation Center, West China Hospital of Sichuan University, Chengdu 610041, China (Tel/Fax: 86-28-85422867; Email: ljydoctor@163.com)
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60003-5
 Hepatobiliary & Pancreatic Diseases International2014年1期
Hepatobiliary & Pancreatic Diseases International2014年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Samaritan donor interchange in living donor liver transplantation
- lntrahepatic Glissonian approach and outflow vascular occlusion during partial hepatectomy
- Complex hepatic outflow reconstruction in domino liver transplantation
- Novel en-bloc resection of locally advanced hilar cholangiocarcinoma: the Rex recess approach
- KAI1 inhibits lymphangiogenesis and lymphatic metastasis of pancreatic cancer in vivo
- Effect of CD74 on the prognosis of patients with resectable pancreatic cancer
