Quantitative analysis of cefixime via complexation with palladium(II) in pharmaceutical formulations by spectrophotometry
Syed Njmul Hejz Azmi, Bshir Iql, Nd Sid Hssn Al-Humimi,Imn Rshid Sif Al-Slmni, Noor Ali Sd Al-Ghfri, Nfisur Rhmn
aDepartment of Applied Sciences, Chemistry Section, Higher College of Technology, PO Box 74, Al-Khuwair-133, Muscat, Oman
bDepartment of Chemistry, Aligarh Muslim University, Aligarh-202002, Uttar Pradesh, India
1. Introduction
Cefixime trihydrate (CAS: 79350-37-1, M.W. 507.5) is chemically known as 7-{[2-(2-amino-1,3-thiazol-4-yl)-2(carboxymethoxyimino)acetyl]amino}-3-ethenyl-8-oxo-5thia-1-azabicyclo oct-2-ene-2-carboxylic acid. The drug is available in tablets (200 and 400 mg) and suspension (100 mg per 5-mL spoonful). It is given by mouth in the treatment of susceptible infections including gonorrhoea,otitis media,pharyngitis,lower respiratory-tract infections especially bronchitis, and urinary-tract infections. It comes under third generation cephalosporin, effective against a wide range of sensitive gram-positive, gram-negative and anaerobic bacterial pathogens including betalactamase producing strains.At high concentrations, gastrointestinal side effects, headaches,dizziness and rashes can appear.Therefore,the analysis of cefixime is important for obtaining optimum therapeutic concentration and for quality assurance in pharmaceutical formulations.The drug is officially listed in British Pharmacopoeia [1] which describes a liquid chromatographic method for its assay in bulk form. In order to assure the quantity of cefixime in dosage forms, several methods have been reported which include liquid chromatography-mass spectrometry [2], high performance liquid chromatography[3-6],high performance thin layer chromatography[7,8],derivative spectrophotometry [9], voltammetry [10], and capillary electrophoresis [11].
The literature survey revealed that a number of spectrophotometric methods have been reported for the quantitative analysis of cefixime in pharmaceutical formulations. The estimation of cefixime was performed based on the reaction of drug with ferrihydroxamate [12] and Folin-Ciocalteu reagent at 720 nm [13]. The reaction of cefixime with a mixture of 3-methyl-2-benzothiazolinon hydrazone HCl and ferric chloride [14]; ferric chloride and 2,2-bipyridyl [15] have been utilized for its determination in bulk and dosage forms. Another method is based on the hydrolysis of β-lactum ring of cefixime with sodium hydroxide which subsequently reacts with iodate to liberate iodine in acidic medium.The liberated iodine oxidizes methylene blue to violet colored species of maximum absorption at 640 nm and thus is utilized for its assay in commercial dosage forms [16]. Potassium permanganate oxidizes cefixime in alkaline medium and itself reduces to manganate ion,which was measured at 598 nm [17] for the determination of cefixime in dosage forms.The determination of cefixime has been done by spectrofluorimetry utilizing the reaction of the drug with 2-cyanoacetamide,which exhibits maximum fluorescence intensity at wavelength 378 nm after excitation at 330 nm [18]. The main problem associated with these determinations is the more analysis time with a number of reagents and laborious cleanup procedure prior to analysis. The sample preparation of the drug included enrichment, separation techniques such as liquid-liquid or solidliquid extraction, coprecipitation, electrodeposition to isolate and preconcentrate the drug. Therefore, there is a need for a rapid,simple, accurate and selective spectrophotometric method for the determination of cefixime in pharmaceutical formulations. Spectrophotometry is the best tool for determining drug in the laboratories of research, hospitals and pharmaceutical industries due to its low cost,inherent simplicity,versatility,adaptablity and affordability[19].The proposed method is based on the formation of the yellow colored complex between cefixime and Pd(II) in ethanol-distilled water medium in the presence of buffer solution of pH 3 at room temperature (25±1°C). The complex absorbed maximally at 352 nm and the reaction is utilized for the estimation of drug in pharmaceutical formulations. The reaction conditions are optimized and validated as per the International Conference on Harmonisation guidelines [20].
2. Experimental
2.1. Apparatus
All spectral and absorbance measurements were made on a Helios Alpha UV-vis spectrophotometer (Thermo Electron Corporation, England, UK) with 1 cm matched quartz cells.IR spectra were recorded on an IR Affinity-1 spectrophotometer (Shimadzu, Kyoto, Japan) in wave number region 4000-400 cm-1using KBr pellet technique. pH meter (Hanna,USA) was used to measure the pH of analyte solution.
2.2. Reagents and standards
All reagents used were of analytical reagent grade.9.852×10-4M(0.05%) cefixime trihydrate (CAS: 79350-37-1, M.W.: 507.5)solution was freshly prepared in methanol. The pure cefixime trihydrate(Batch No XMEO 110023)is provided by National Pharmaceutical Industries Company,Oman.The solution was stable up to 12 h.
1.41×10-3M palladium chloride (CAS: 7647-10-1, M.W.:177.32, Iqba Chemie Pvt. Ltd., Mumbai, India) solution was prepared by dissolving 0.025 g palladium chloride in 5 mL of 0.5 M HCl and the mixture was heated until dissolved completely and cooled at room temperature before diluted up to the mark with distilled water in a 100 mL volumetric flask. Buffer solutions of pH ranging from 2.2 to 3.8 were prepared by mixing appropriate volumes of 0.2 M Na2HPO4and 0.1 M citric acid in a total volume of 20 mL[21].Cefixime formulations such as Cefrax 200 mg capsule (National Pharmaceutical Industries Company, Oman) and Suprax 200 mg tablet (Sanofi-aventis, UK) were purchased locally from Scientific Pharmacy (Muscat, Oman).
2.3. Recommended procedure for the determination of cefixime
Aliquots of 0.05-0.7 mL of 0.05% cefixime solution corresponding to 2.5-35 μg/mL were pipetted followed by the addition of 2.5 mL Na2HPO4-citric acid buffer solution of pH 3 into a series of 10 mL standard volumetric flasks. To each flask, 1.4 mL of 1.41×10-3M palladium chloride solution was added and diluted up to the mark with ethanol. The contents of the flask were mixed well and the absorbance was measured at 352 nm against the reagent blank prepared similarly except cefixime within the stability time period of 6 h. The amount of cefixime was obtained either from the calibration graph or the regression equation.
2.4. Determination of cefixime in pharmaceutical formulations
The powder contents of commercially available Cefrax capsule and Suprax tablet(20 in number)of 200 mg strength of cefixime were weighed and grounded. The powder equivalent to 50 mg cefixime were taken in 60 mL methanol and kept for 10 min for complete dissolution of the drug.The mixture was filtered through Whatmann No. 42 filter paper (Whatmann International Limited, Kent, UK) in 100 mL standard volumetric flask. The residue was washed well with 3×10 mL portions of methanol for complete recovery of the drug and diluted up to the mark with methanol. The amount of cefixime was determined following the recommended procedure.
2.5. Procedure for reference method [15].
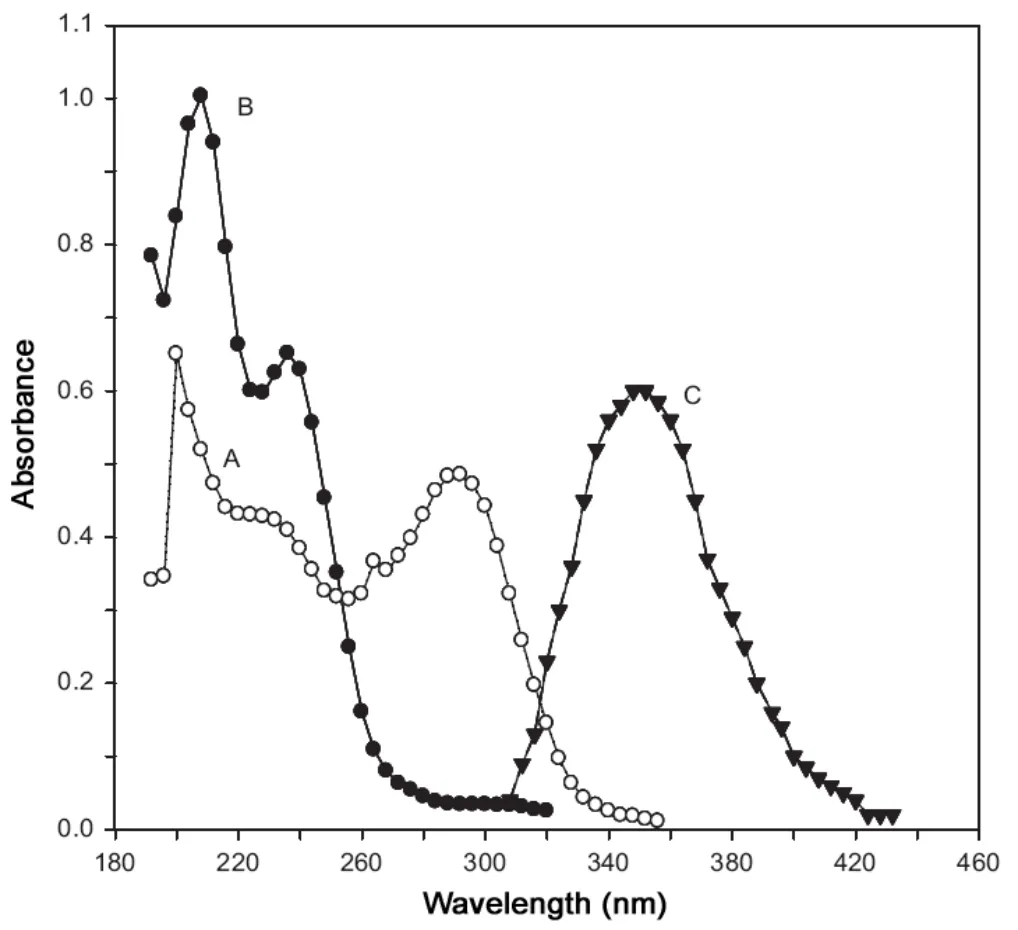
Fig.1 Absorption spectra of (A) 0.1 mL of 9.852×10-4 M cefixime, (B) 0.4 mL of 1.41×10-3 M palladium chloride and(C) 0.6 mL of 9.852×10-4 M cefixime+1.4 mL of 1.41×10-3 M Pd(II)+2.5 mL of Na2HPO4-citric acid buffer solution of pH 2.8.The solution of A is diluted with methanol while the solutions of B and C are diluted with distilled water and ethanol, respectively, in 10 mL standard volumetric flask.
Aliquots of 0.1-1.0 mL of 0.01%cefixime were pipetted into a series of 10 mL standard volumetric flasks. To each flask,0.3 mL of 0.2% ferric chloride solution and 2 mL of 2% 2,2-bipyridyl were added. The contents of the flask were heated at 70°C for 15 min and then cooled.After cooling,the content of the flask was diluted up to the mark with distilled water.The absorbance of the pink colored complex was measured at 520 nm against the reagent blank prepared similarly except cefixime.The amount of cefixime was obtained either from the calibration graph or the regression equation.
2.6. Stoichiometry
The stoichiometric reaction between cefixime and palladium(II) was studied by Job's method of continuous variations[22]. For this purpose, different volumes of 9.852×10-4M cefixime were added with different volumes of 9.852×10-4M palladium(II) chloride, respectively, followed by the addition of 2.5 mL buffer solution of pH 3 in 10 mL standard volumetric flask. The contents of the flask were diluted up to the mark with ethanol. The absorbance was recorded at 352 nm and plotted against the mole fraction of cefixime.
2.7. Validation
The proposed method has been validated for linearity,sensitivity,precision,accuracy,robustness,specificity and evaluation of bias.
2.7.1. Linearity, limit of detection and limit of quantitation The linearity of the proposed method was assessed at nine different concentrations: 2.5, 5, 10, 15, 20, 25, 30, 32.5 and 35 μg/mL. Each concentration level was independently analyzed repeatedly for five times. The absorbance obtained at each concentration was plotted against the concentration of cefixime in μg/mL. The linear regression equation was evaluated by least square treatment of the calibration data. The other statistical parameters of the proposed method were calculated using OriginPro 6.1 Software.
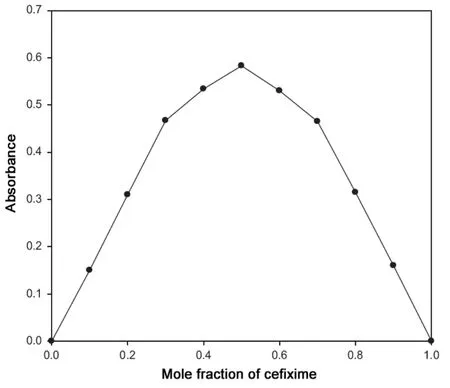
Fig.2 Job's plot for Pd(II)-cefixime complex.
The limit of detection (LOD) and the limit of quantitation(LOQ) for the proposed method were calculated using the following equations [23]:
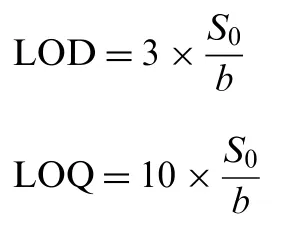
where S0is standard deviation of calibration line and b is the slope.
2.7.2. Precision
The precision of the proposed method was evaluated by intraday and inter-day precisions at three concentration levels(5.0, 15.0 and 30.0 μg/mL) of cefixime. Each concentration level was independently analyzed repeatedly for five times within a day (intra-day precision) and over five consecutive days(inter-day precision). The standard deviation (SD) and %relative standard deviation (RSD) were calculated.
2.7.3. Accuracy
The accuracy of the proposed method was tested by analyzing freshly prepared capsule and tablet formulated drug solution in five replicates. The same drug solution was also tested by reference method. The percent recovery and standard deviations of the two methods were compared and tested for accuracy of the proposed method.
The accuracy of the proposed method was also checked by standard addition technique. In this technique, 0.4 mL of the 0.5 mg/mL of the formulated capsule (or tablet) sample solution was spiked separately with 0, 0.05, 0.1, 0.15 and 0.2 mL of the reference drug sample solution in 10 mL standard volumetric flask and diluted up to the mark with ethanol. Each level was independently analyzed repeatedly for five times. The nominal value of the cefixime concentration in capsule and tablet was determined by dividing the obtained intercept by slope.
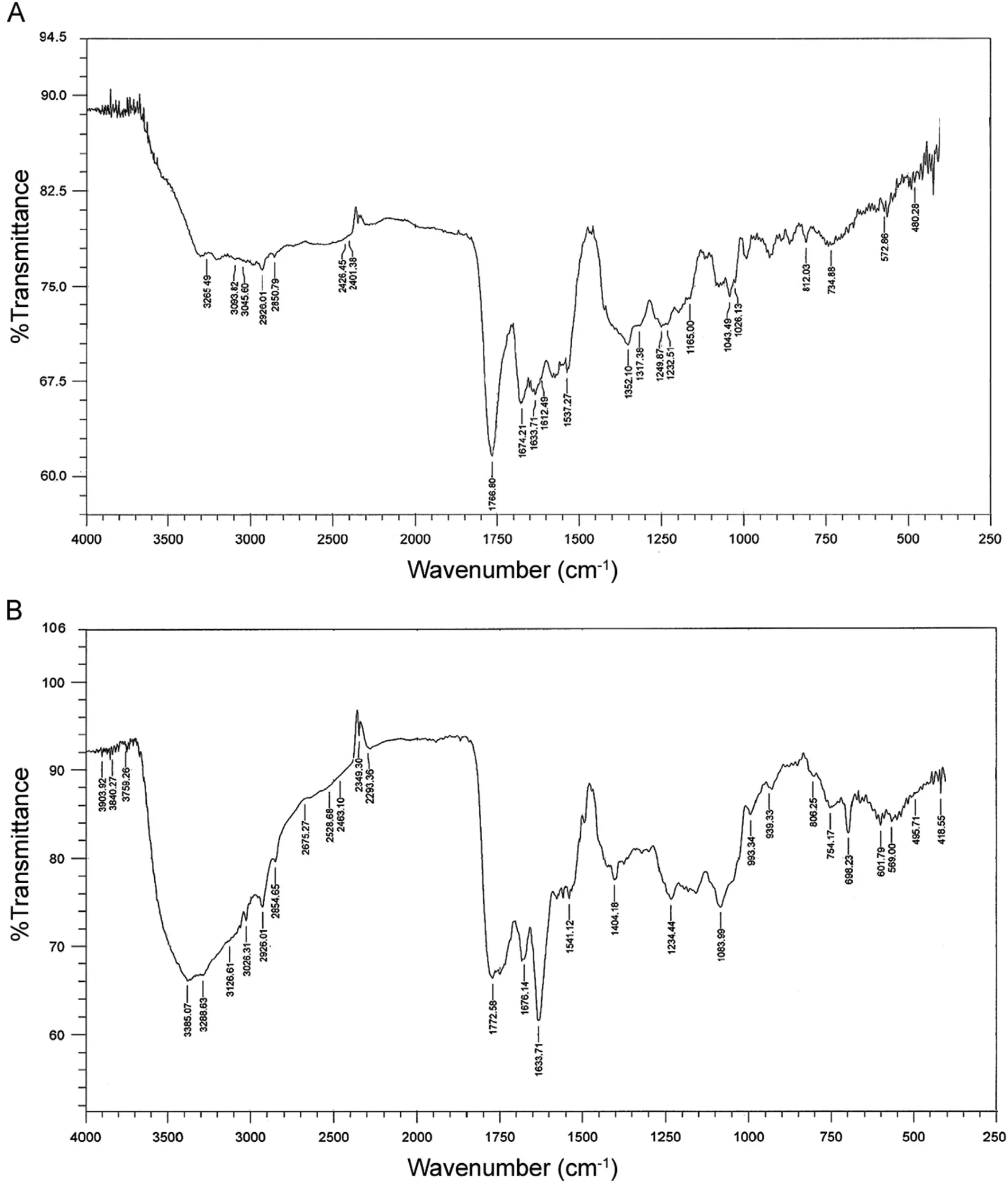
Fig.3 Infra-red spectra of (A) pure cefixime and (B) Pd-cefixime complex.
2.7.4. Specificity
The specificity of the proposed method was investigated by observing any interference encountered from common excipients of the pharmaceutical formulations such as starch,fructose,glucose,lactose,sodium benzoate and phenyl alanine at 30 μg/mL cefixime.
2.7.5. Robustness
The robustness of the proposed method was evaluated by challenging each operational parameter of the proposed method such as: 1.4±0.2 mL of 1.41×10-3M palladium chloride; buffer solution of pH 3±0.2; working temperature,25C±1°C, color development time, immediately.
2.7.6. Applicability and evaluation of bias
The point and interval hypothesis tests have been performed to compare the results of the proposed method with those of the reference method at 95% confidence level. The bias was
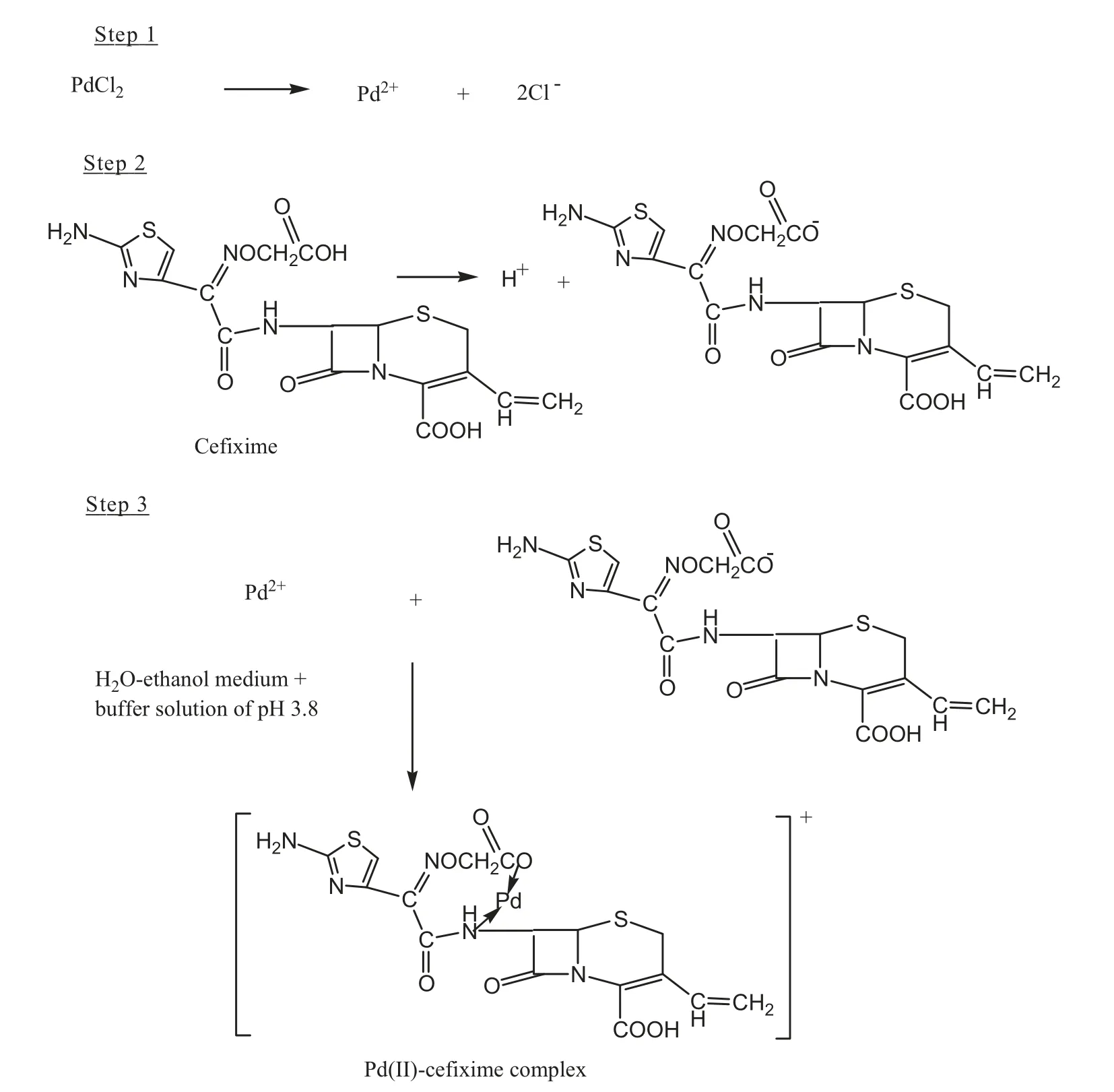
Scheme 1 Reaction sequence of the proposed method.
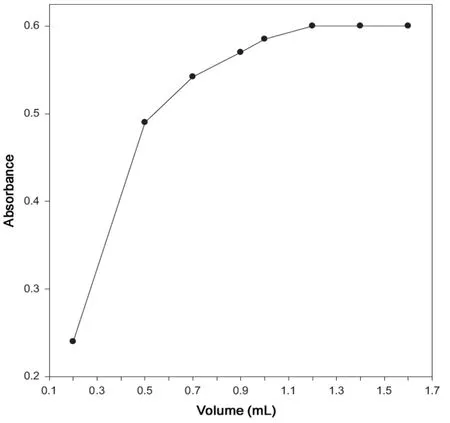
Fig.4 Effect of the volume of 1.41×10-3 M palladium chloride.
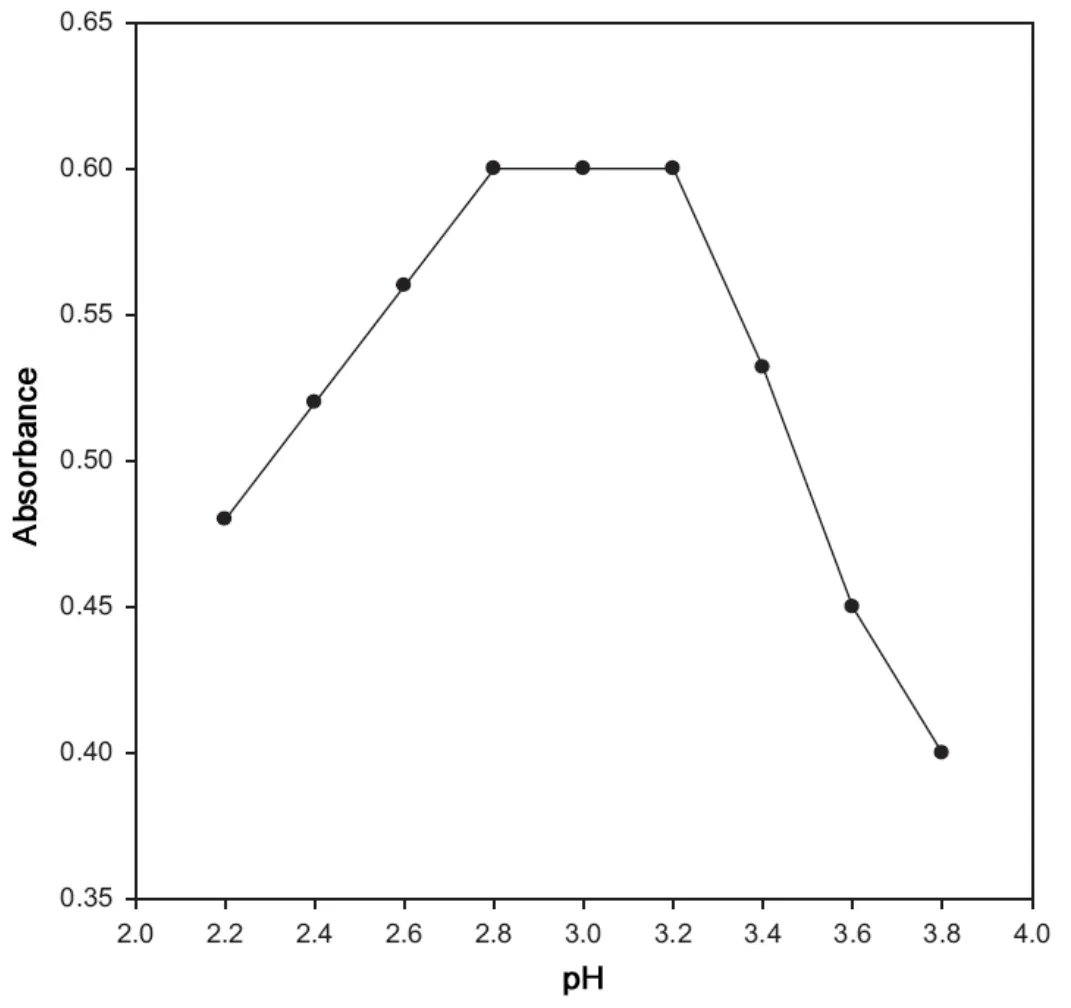
Fig.5 Effect of pH on the absorbance of Pd(II)-cefixime complex.
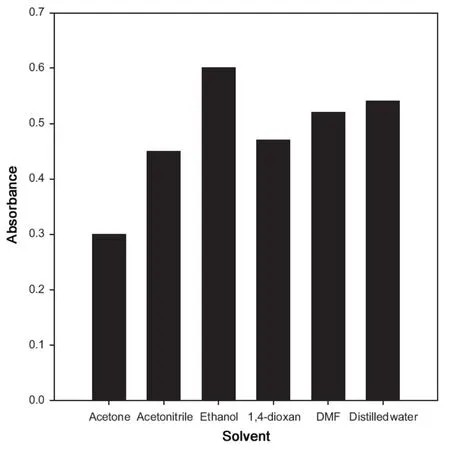
Fig.6 Effect of solvent on the absorbance of Pd(II)-cefixime complex.
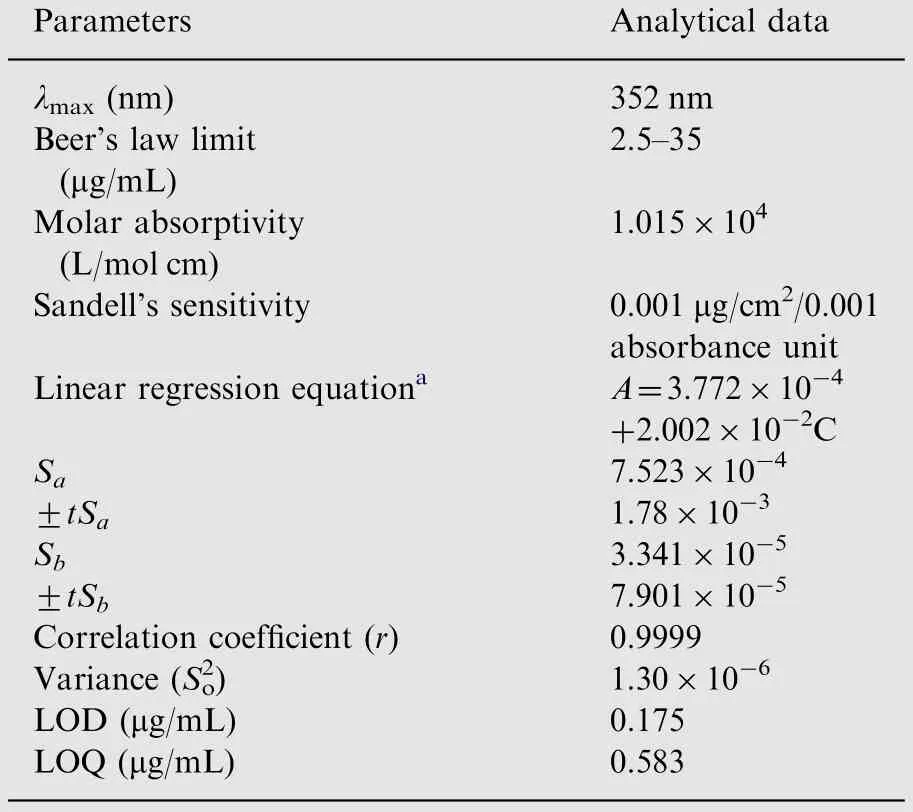
Table 1 Optical and regression characteristics of the proposed method.
aWith respect to A=a+bC, where C is the concentration in μg/mL and A is absorbance.±tSaand±tSbare the confidence evaluated by an interval hypothesis test based on the mean values of the proposed method and the reference method.The proposed method is considered acceptable when its true mean is within ±2.0% of that of the reference method. The lower(θL)and the upper(θU)acceptance limits can be calculated by the following quadratic equation [24]:
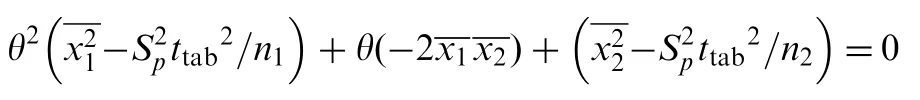
3. Results and discussion
3.1. UV-visible spectrophotometric spectra analysis
The absorption spectrum of methanolic solution of cefixime absorbed maximally at 210 and 290 nm whereas the absorption spectrum of aqueous solution of palladium chloride (pH 2.1)showed two bands at 208 and 236 nm. When the two solutions were mixed together,a red shift in the wavelength is observed due to the complexation reaction between cefixime and Pd(II) in the presence of acidic buffer solution. The Pd(II)-cefixime complex exhibited only one band with λmaxof 352 nm. The absorption spectra of cefixime, palladium chloride and Pd(II)-cefixime complex are shown in Fig.1. The absorbance measurement at 352 nm as a function of cefixime concentration is exploited to develop a new, accurate and rapid spectrophotometric method for the determination of cefixime in pharmaceutical formulations.The reaction was carried out at room temperature(25°C)and the coloured complex was stable up to 6 h.
3.2. Stoichiometry
The stoichiometric ratio between cefixime and Pd(II) was evaluated by Job's method of continuous variations. The Job's plot (Fig.2) has confirmed that 1 mol of cefixime was reacted with 1 mol of Pd(II). Thus, the stoichiometry of the complex is established and found to be 1:1. The apparent formation constant (Kf) for the complex between Pd(II) and cefixime is calculated using the following expression:
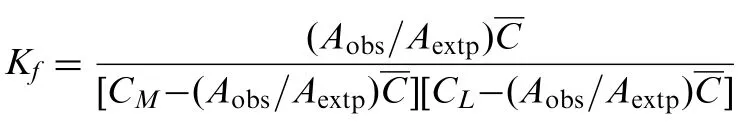
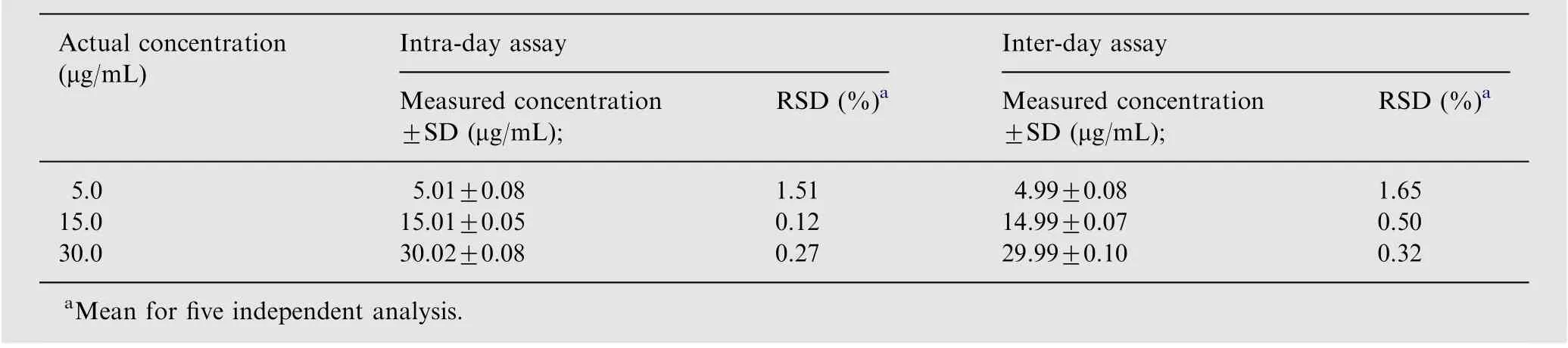
Table 2 Precision of the proposed method.
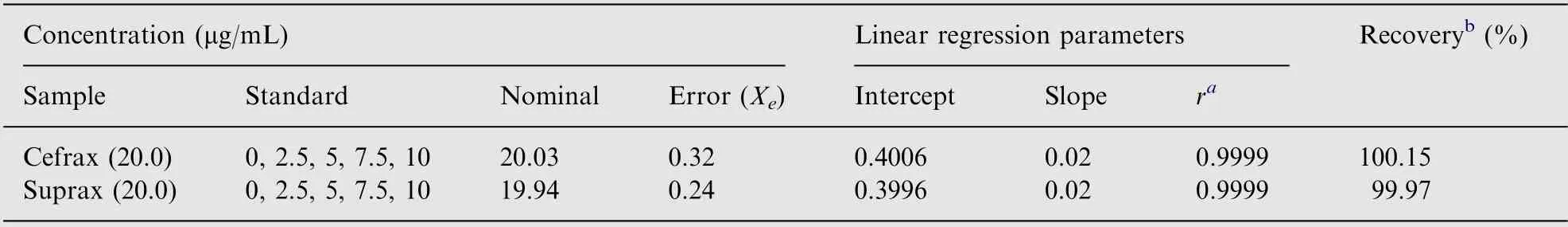
Table 3 Test of accuracy in Cefrax capsule and Suprax tablet by standard addition technique.
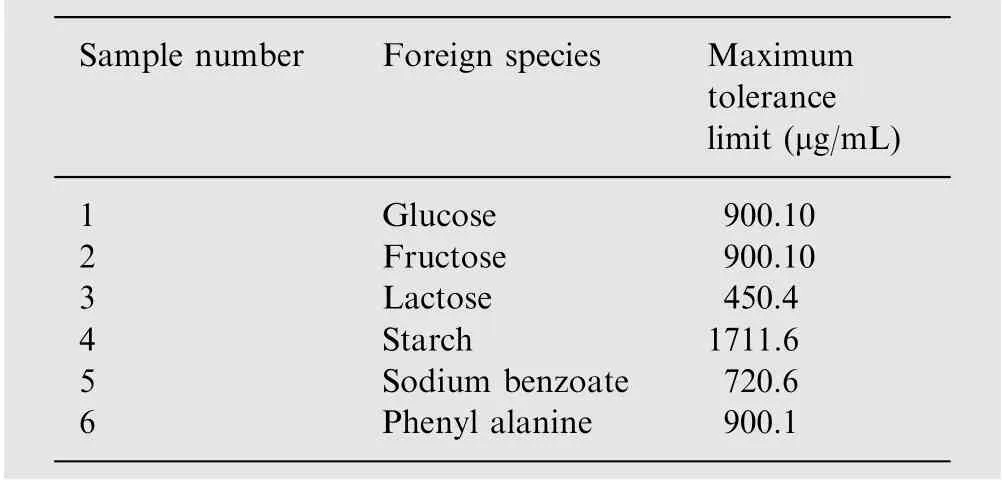
Table 4 Effect of foreign species on the determination of 30 μg/mL cefixime.
3.3. Infra red spectra analysis
The infra-red spectra of pure cefixime and Pd(II)-cefixime complex are shown in Fig.3A and B, respectively. Cefixime has -NH2,-COOH, -CONH and C=O lactam groups which are the potential sites for coordination with metal ions.Comparison of an IR spectrum of the complex with those of pure cefixime indicates that the lactam (C=O) band appears at 1766 cm-1in the pure cefixime while the complex shows this band at 1772 cm-1suggesting that no coordination occurs with palladium ion. The amide carbonyl band, ν(C=O)-NH in the pure cefixime appears at 1674 cm-1with a weak shoulder at 1633 cm-1while the Pd(II)-cefixime complex shows this band at 1676 cm-1with a prominent peak at 1633 cm-1suggesting that cefixime coordinated with Pd(II)through the nitrogen atom.The asymmetrical and symmetrical stretching bands of carboxylate groups change from 1537 to 1541 cm-1and 1352 to 1404 cm-1, respectively, due to the coordination. The Pd-N stretching vibration occurs at 495 cm-1[25]. A tentative mechanism for the complexation of Pd(II)-cefixime complex is given in Scheme 1.
3.4. Optimization of variables
The optimization of variables was investigated by testing reaction time, concentration of cefixime, solvents and buffer solution of different pH.
The effect of reaction time on the absorbance of Pd(II)-cefixime complex and its stability was investigated. The Pd(II)-cefixime complex got stabilized immediately at 25±1°C and remained stable for 6 h.
The effect of the volume of 1.41×10-3M palladium on the absorbance of complex was investigated in the range 0.2-1.6 mL. It is evident from Fig.4 that the maximum absorbance was obtained with 1.2 mL of 1.41×10-3M palladium.Above this volume up to 1.6 mL of 1.41×10-3M palladium,the absorbance remained unchanged. Therefore, 1.4 mL of 1.41×10-3M palladium chloride was used in the determination process of cefixime.
The influence of pH on the absorbance of the Pd(II)-cefixime complex with 30.0 μg/mL cefixime was investigated in the pH range of 2.2 to 3.8 using disodium hydrogen phosphate-citric acid buffer. The maximum absorbance was obtained in the pH range of 2.8-3.2 and above this, the absorbance value decreased (Fig.5 ). Therefore, the buffer solution of pH 3.0 was selected for all absorbance measurements in the determination of cefixime in pharmaceutical formulations.
The effects of solvents such as methanol, acetone, acetonitrile, ethanol, 1,4-dioxan, dimethylformide (DMF) and distilled water were investigated on the absorbance of the Pd(II)-cefixime complex. The absorbance of Pd(II)-cefixime complex using 30.0 μg/mL cefixime diluted with various solvents is recorded at 352 nm and shown in Fig.6. It is clear from the figure that the highest absorbance was obtained in ethanol.Therefore, ethanol was selected as the best solvent for the determination of cefixime in drug formulations.
3.5. Validation
3.5.1. Linearity, LOD and LOQ
Under the optimized experimental conditions, the calibration graph was constructed by plotting the absorbance against initial concentration of cefixime at nine independent concentration levels.Beer's law is obeyed in the concentration ranges of 2.5-35 μg/mL with apparent molar absorptivity of 1.015×104L/mol cm and Sandell's sensitivity of 0.001 μg/cm2/0.001 absorbance unit. The linear regression equation is obtained by statistical treatment of the calibration data which is fitted with the straight line equation in the form of A=a+bC, where A is absorbance at 352 nm, C is concentration in μg/mL, b is slope and a is intercept of calibration line.The high value of correlation coefficient (0.9999) indicated excellent linearity (Table 1). The experimental intercept of the calibration line was tested for significance of deviation from the theoretical intercept, i.e., zero. For this justification,t-value calculated from relation, t=a/Sa[26] is found to be 0.501,which did not exceed the tabulated t-value(2.365,ν=7)at 95% confidence level. This indicated that the intercept inthe calibration equation of the proposed method is not significantly different from zero. Thus, the proposed method is free from procedural error.
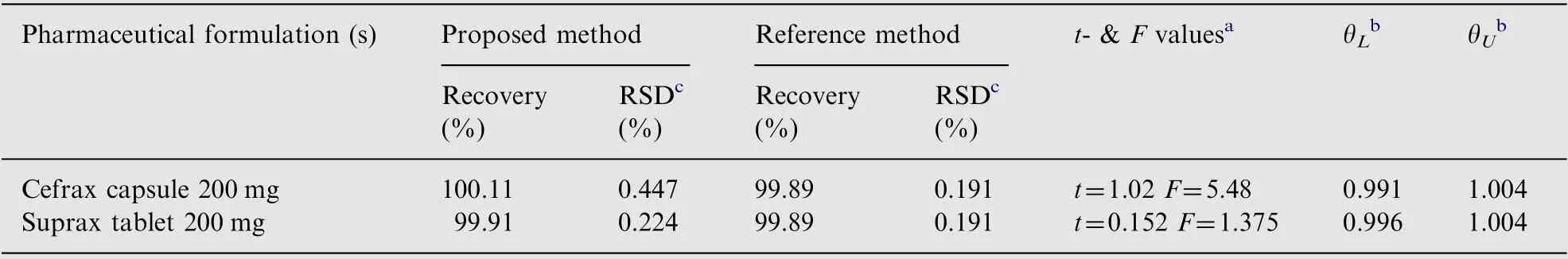
Table 5 Significance of testing: Point and interval hypothesis tests for the determination of cefixime in pharmaceutical formulations at 95% confidence level.
3.5.2. Precision
The intra-day and inter-day precisions were evaluated by determining the concentration of cefixime at lower, middle and upper concentration levels for five repeated times within the same day and on five consecutive days, respectively. The results and their statistical analysis are summarized in Table 2.It is clear from the table that the values of RSD(intra-day and inter-day precisions) were in the ranges of 0.12-1.65%,respectively. It can be seen from the table that RSD values were precise and can be used to determine cefixime in the pharmaceutical formulations.
3.5.3. Accuracy
The accuracy of the proposed method was investigated by performing recovery experiments through standard addition technique. The absorbance is recorded for all standard addition solutions and the results of analyses are summarized in Table 3. It is clear from the table and the graph that the linearity of the regression line for capsule and tablet formulation samples were good. It is evident from the figure that the concentration of cefixime in pharmaceutical formulations is given by intercept/slope. The ratio of the intercept and the slope of the regression line is subjected to error (SxE). SxEis calculated from the following expression
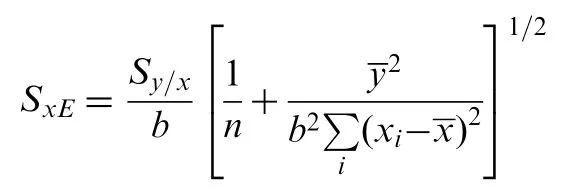
and found to be 0.32 and 0.24 μg/mL,respectively,for capsule and tablet formulation samples. The confidence limit for the concentration of cefixime in pharmaceutical formulations is calculated byxE±tSxEat n-2 degrees of freedom and found to be 20.03±0.32 and 19.94±0.24 μg/mL,respectively.The most attractive feature of the proposed method using standard addition method is its relative freedom from various excipients found in drug formulations.
3.5.4. Specificity
The effect of excipients added as additives on the determination of 30 μg/mL cefixime was studied. For this purpose, the varying concentrations of excipients such as starch, fructose,glucose, lactose, sodium benzoate and phenyl alanine with 30 μg/mL cefixime were taken and the absorbance was recorded to know the concentration of cefixime.Table 4 shows the maximum tolerance value of the studied excipients. The maximum tolerance value was taken, when the absorbance value did not exceed ±2% on addition of excipients.
3.5.5. Robustness
The robustness of the proposed method was established by deliberately changing the volume of 1.41×10-3M Pd(II),1.4 mL (±0.2 mL) for the determination of cefixime. The capsule and tablet formulated sample solutions containing 10 μg/mL cefixime were analyzed five times repeatedly by the proposed method. Percentage recovery and RSD were found to be 100.11% and 0.447% for capsule and 99.91% and 0.224% for tablet, indicating the robustness of the proposed method.
3.5.6. Applicability and evaluation of bias
The applicability of the proposed method for the determination of cefixime in pharmaceutical formulation samples has been studied.Results of the proposed method were statistically compared with those of reference method using point and interval hypothesis tests. The paired t- and the F-values at 95%confidence level were calculated and found to be less than the tabulated t-(2.036 at υ=8) and the F-values (6.39 at υ=4,4) at 95% confidence level [27], thus confirming no significant difference between the performance of the proposed method and the reference method (Table 5). The bias calculated by interval hypothesis test in the form of lower limit(θL)and upper limit (θU) were in the range of 0.98-1.02. Thus, the proposed method is suitable for routine analysis of cefixime in dosage forms. The speed of analysis and less number of reagents utilized in the proposed method are the main advantages of the proposed method as compared to reference method.
4. Conclusions
The proposed method is a simple, rapid and accurate for the determination of cefixime in capsule and tablet formulations.The method has advantage of using a commonly available solvent, i.e., ethanol with the use of palladium as a reagent.The proposed method has avoided the use of heating the reaction mixture and can be used as an alternate method for routine quality control analysis of cefixime in pharmaceutical and biological samples.
Acknowledgements
The authors are grateful to Ministry of ManPower (Higher College of Technology) Muscat, Sultanate of Oman and Aligarh Muslim University, Aligarh, India for facilities. The authors wish to express their gratitude to National Pharmaceutical Industries Company, Oman for the sample of reference standard of cefixime.
[1] British Pharmacopoeia, vol. I, Her Majesty Stationary Office,London, UK, 2009, pp. 1139-1144.
[2] F.Meng,X.Chen,Y.Zeng,et al.,Sensitive liquid chromatographytandem mass spectrometry method for the determination of cefixime in human plasma: application to a pharmacokinetic study, J.Chromatogr. B Analyt. Technol. Biomed. Life Sci. 819 (2005)277-282.
[3] K.A. Raj, D. Yada, D. Yada, et al., Determination of cefixime trihydrate and cefuroxime axetil in bulk drug and pharmaceutical dosage forms by HPLC,Int.J.ChemTech.Res.2(2010)334-336.
[4] D. Zendelovska, T. Stafilov, P. Milo ˇsevski, High-performance liquid chromatographic method for determination of cefixime and cefotaxime in human plasma, Bull. Chem. Technol. Macedonia 22 (2003) 39-45.
[5] E.H.K. Adam, A.E.M. Saeed, I.E. Barakat, Development and validation of a high performance liquid chromatography method for determination of cefixime trihydrate and its degraded products formed under stress condition of UV light, Int. J. Pharm.Sci. Res. 3 (2012) 469-473.
[6] K. Kathiresan, R. Murugan, M.S. Hameed, et al., Analytical method development and validation of cefixime and dicloxacillin tablets by RP-HPLC, Rasayan J. Chem. 2 (2009) 588-592.
[7] M.M. Deshpandea, V.S. Kastureb, S.A. Gosavib, Application of HPLC and HPTLC for the simultaneous determination of cefixime trihydrate and ambroxol hydrochloride in pharmaceutical dosage form, Eurasian J. Anal. Chem. 5 (2010) 227-238.
[8] K.S. Khandagle, S.V. Gandhi, P.B. Deshpande, et al., High performance thin layer chromatographic determination of cefixime and ofloxacin in combined tablet dosage form, J. Chem.Pharm. Res. 2 (2010) 92-96.
[9] V. Shah, H. Raj, Development and validation of derivative spectroscopic method for simultaneous estimation of cefixime trihydrate and azithromycin dihydrate in combined dosage form,Int. J. Pharm. Sci. Res. 3 (2012) 1753-1760.
[10] R. Jain, V.K. Gupta, N. Jadon, Voltammetric determination of cefixime in pharmaceuticals and biological fluids,Anal.Biochem.407 (2010) 79-88.
[11] K.A. Raj, Determination of cefixime trihydrate and cefuroxime axetil in bulk drug and pharmaceutical dosage forms by electrophoretic method, Int. J. ChemTech. Res. 2 (2010) 337-340.
[12] D. Agbaba, S. Eric, K. Karljikovic-Rajic, et al., Spectrophotometric determination of certain cephalosporins using ferrihydroxamate method, Spectrosc. Lett. 30 (1997) 309-319.
[13] D.G. Shankar, K. Sushma, R.V. Lakshmi, et al., UV and visible spectrophotometric methods for the determination of cefixime,Indian Drugs 38 (2001) 617-619.
[14] P.B. Shah, K. Pundarikakshudu, Spectrophotometric, difference spectroscopic, and high-performance liquid chromatographic methods for the determination of cefixime in pharmaceutical formulations, J. AOAC Int. 89 (2006) 987-994.
[15] S.I. Pasha, A.S. Kumar, K. Sravanthi, et al., New visible spectrophotometric method for the determination of cefixime trihydrate in pharmaceutical formulations, Orient. J. Chem. 28(2012) 571-574.
[16] B.S. Virupaxappa1, K.H. Shivaprasad, M.S. Latha, A simple method for the spectrophotometric determination of cefixime in pharmaceuticals, Asian J. Res. Chem. 4 (2011) 1275-1277.
[17] A. Kumar, L. Kishore, A. Nair, et al., Kinetic spectrophotometric method for the estimation of cefixime in pharmaceutical formulations, Der Pharma Chemica 3 (2011) 279-291.
[18] J.Shah,M.R.Jan,S.Shah,et al.,Spectrofluorimetric method for determination and validation of cefixime in pharmaceutical preparations through derivatization with 2-cyanoacetamide,J. Fluoresc. 21 (2011) 579-585.
[19] D.A.C. Czegan, D.K. Hoover, UV-visible spectrometers: versatile instruments across the chemistry curriculum, J. Chem. Educ.89 (2012) 304-309.
[20] International Conference on Harmonisation, Food and Drug Administration, ICH Harmonised Tripartite Guideline—Text on Validation of Analytical Procedures. Rockville, MD, USA. Fed.Regist. 60 (1995), pp. 11260-11262.
[21] H.T.S.Britton,Chapter XVI:Solutions of Known Hydrogen Ion Concentration, Hydrogen Ions, vol. I, Chapman and Hall Ltd.,London, 1942, p. 304.
[22] J.M.Bosque-Sendra,E.Almansa-Lopez,A.M.Garcia-Campana,et al., Data analysis in the determination of stoichiometries and stability contants of complexes, Anal. Sci. 19 (2003) 1431-1439.
[23] J.C. Miller, J.N. Miller, Chapter 5: Errors in Instrumental Analysis;Regression and Correlation,In:Statistics for Analytical Chemistry,third ed., Ellis Horwood PTR Prentice Hall,London,1993, pp. 115-117.
[24] C. Hartmann, J. Smeyers-Verbeke, W. Pinninckx, et al., Reappraisal of hypothesis testing for method validation: detection of systematic error by comparing the means of two methods or of two laboratories, Anal. Chem. 67 (1995) 4491-4499.
[25] K. Nakamoto, Chapter I: Applications in Coordination, Organometallic and Bioinorganic Chemistry, In: Infrared and Raman Spectra of Inorganic and Coordination Compounds,Part B,sixth ed., John Wiley and Sons, Inc., Hoboken, New Jersey, USA,2009, pp. 9.
[26] V.V. Nalimov, The Application of Mathematical Statistics to Chemical Analysis, Pergamon Press, Oxford, 1963, pp. 167-189.
[27] J. Mendham, R.C. Denney, J.D. Barnes, et al., Statistics:Introduction to Chemo Metrics.In: Vogel's Textbook of Quantitative Chemical Analysis, sixth ed., Pearson Education, Singapore,2002, pp. 137.
 Journal of Pharmaceutical Analysis2013年4期
Journal of Pharmaceutical Analysis2013年4期
- Journal of Pharmaceutical Analysis的其它文章
- LC-MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats
- Characterization of phloroglucinol derivatives and diterpenes in Euphorbia ebracteolata Hayata by utilizing ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry
- Application of analytical instruments in pharmaceutical analysis
- Investigation of the interaction between indigotin and two serum albumins by spectroscopic approaches
- In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia
- Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry
