Stem cell transplantation for treating Duchenne muscular dystrophy A Web of Science-based literature analysis*
Xiaofeng Yang
Cell Therapy Center, Chinese PLA 463 Hospital, Shenyang 110042, Liaoning Province, China
Stem cell transplantation for treating Duchenne muscular dystrophyA Web of Science-based literature analysis*
Xiaofeng Yang
Cell Therapy Center, Chinese PLA 463 Hospital, Shenyang 110042, Liaoning Province, China
OBJECTlVE:To identify global research trends in stem cell transplantation for treating Duchenne muscular dystrophy using a bibliometric analysis of Web of Science.
DATA RETRlEVAL:We performed a bibliometric analysis of studies on stem cell transplantation for treating Duchenne muscular dystrophy from 2002 to 2011 retrieved from Web of Science.
SELECTlON CRlTERlA:Inclusion criteria: (a) peer-reviewed published articles on stem cell transplantation for treating Duchenne muscular dystrophy indexed in Web of Science; (b) original research articles, reviews, meeting abstracts, proceedings papers, book chapters, editorial material, and news items; and (c) publication between 2002 and 2011. Exclusion criteria: (a) articles that required manual searching or telephone access; (b) documents that were not published in the public domain; and (c) corrected papers.
MAlN OUTCOME MEASURES:(1) Annual publication output; (2) distribution according to subject areas; (3) distribution according to journals; (4) distribution according to country; (5) distribution according to institution; (6) distribution according to institution in China; (7) distribution according to institution that cooperated with Chinese institutions; (8) top-cited articles from 2002 to 2006; (9) top-cited articles from 2007 to 2011.
RESULTS:A total of 318 publications on stem cell transplantation for treating Duchenne muscular dystrophy were retrieved from Web of Science from 2002 to 2011, of which almost half derived from American authors and institutes. The number of publications has gradually increased over the past 10 years. Most papers appeared in journals with a focus on gene and molecular research, such asMolecular Therapy,Neuromuscular Disorders, andPLoS One. The 10 most-cited papers from 2002 to 2006 were mostly about different kinds of stem cell transplantation for muscle regeneration, while the 10 most-cited papers from 2007 to 2011 were mostly about new techniques of stem cell transplantation for treating Duchenne muscular dystrophy.
CONCLUSlON:The publications on stem cell transplantation for treating Duchenne muscular dystrophy were relatively few. It also needs more research to confirm that stem cell therapy is a reliable treatment for Duchenne muscular dystrophy.
pseudohypertrophic muscular dystrophy; Duchenne muscular dystrophy; Becker muscular dystrophy; stem cell; myoblast; exon skipping; dystrophin gene; motor function; cell transplantation; regenerative myogenesis; neural regeneration
Research Highlights
(1) We performed a bibliometric analysis of published studies on stem cell transplantation for treating Duchenne muscular dystrophy from 2002 to 2011 retrieved from Web of Science.
(2) We analyzed the distributions according to institutions in China and according to institutions that cooperated with Chinese institutions to provide information on the research status in China.
(3) We analyzed the most-cited articles from 2002 to 2006 and from 2007 to 2011 to identify changes in research focus regarding the use of stem cell transplantation for Duchenne muscular dystrophy over the past 10 years.
lNTRODUCTlON
Duchenne muscular dystrophy is an inherited myopathy involving skeletal muscle and myocardium, and is the commonest neurogenetic disorder[1-2]. Duchenne muscular dystrophy is a neuromuscular, X-linked recessive condition, in which mutation of the dystrophin gene leads to complete or partial lack of dystrophin protein on the myolemma[3]. Becker muscular dystrophy differs from Duchenne muscular dystrophy because of different spatial structural changes and functional incapacitation of the dystrophin protein. Duchenne muscular dystrophy is a serious condition with a poor prognosis, while Becker muscular dystrophy is a milder condition and has a more favorable prognosis[4-5].
Duchenne muscular dystrophy is known to result from a lack of dystrophin in the muscle. Dystrophin is a cytoskeletal protein expressed on the surface of the endochylema of the sarcolemma in skeletal muscle, cardiac muscle, smooth muscle and brain[6]. Dystrophin protein is encoded by a gene located on chromosome Xp2l. The dystrophin gene is one of the largest known genes, containing 79 exons. About 60% of dystrophin gene mutations are deletions, while the other 40% involve mutations other than deletions. These mutations alter the structure and function of the encoded dystrophin protein, leading to Duchenne muscular dystrophy[7].
Treatments, including symptomatic, supportive, drug, physical and orthopedic treatments, can improve the patient’s condition but cannot effectively prevent disease progression. However, gene therapy and stem cell transplantation are expected to become effective treatments for patients with Duchenne muscular dystrophy. Many recent studies have used stem cell transplantation to treat Duchenne muscular dystrophy in a rat model, with improvements in pathology, physiology, biochemistry, dystrophin expression and motor function[8].
Stem cell transplantation for Duchenne muscular dystrophy may involve bone marrow stromal cells, hematopoietic stem cells, or muscle stem cells. Myofibers are polynucleated megacells without the capacity to differentiate, while normal stem cells contains the normal dystrophin gene, and can further differentiate into osteoblasts, cartilage cells, adipose cells and even sarcoblasts in the appropriate nutritive media. The aim of stem cell transplantation is to generate functional dystrophin protein.
In this study, we analyzed the research trends in stem cell transplantation for treating Duchenne muscular dystrophy, based on a bibliometric analysis of papers in Web of Science from 2002 to 2011[2].
DATA SOURCES AND METHODOLOGY
Data retrieval
This study used bibliometric analyses to quantitatively and qualitatively investigate research trends in studies of stem cell transplantation for treating Duchenne muscular dystrophy. We searched Web of Science, a research database of publications and citations selected and evaluated by the Institute for Scientific Information in Philadelphia, PA, USA, using the key words “duchenne muscular dystrophy” “Duchenne muscular dystrophy”and “stem cell”. We limited the period of publication from 2002 to 2011 and compiled a bibliography of all articles related to stem cell transplantation for Duchenne muscular dystrophy. We downloaded the data on June 1st, 2012.
lnclusion criteria
The inclusion criteria were as follows: (1) published peer-reviewed articles on the use of stem cell transplantation for treating Duchenne muscular dystrophy, including original research articles, reviews, meeting abstracts, proceedings papers, book chapters, editorial material, and news items, which were indexed in Web of Science; (2) year of publication 2002-2011; and (3) citation database was Science Citation Index Expanded.
Exclusion criteria
We excluded articles that required manual searching or telephone access, documents that were not published in the public domain, and a number of corrected papers from the total number of articles analyzed.
The outcomes of all articles referring to the use of stem cell transplantation for treating Duchenne muscular dystrophy were assessed using the following criteria. (a) Annual publication output on stem cell transplantation for Duchenne muscular dystrophy included in Web of Science from 2002 to 2011. (b) Publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 according to subject areas. (c) Publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 according to journals. (d) Publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 according to country. (e) Publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 by institution. (f) Distribution of publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 by institution in China. (g) Distribution of publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 by institutions that cooperated with Chinese institutions. (h) Most-cited papers on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2006. (i) Most-cited papers on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2007 to 2011.
RESULTS
Annual publication output relating to stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 (Figure 1)
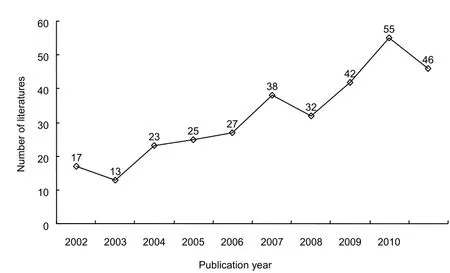
Figure 1 Annual number of publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011.
A total of 318 publications on stem cell transplantation for Duchenne muscular dystrophy were retrieved from Web of Science from 2002 to 2011. The number of relevant publications gradually increased over the 10-year study period. Seventeen papers were published and included in Web of Science in 2002, but the number of published papers had increased to 55 in 2010. However, there were slight decreases in the numbers of papers published in 2003, 2008 and 2011.
Distribution of subject areas in publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 (Table 1)
As shown in Table 1, among the subject categories related to stem cell transplantation for Duchenne muscular dystrophy from 2002 to 2011, most studies (105 papers) were in the field of cell biology, which accounted for 33.019%. The second-highest number of studies (86 papers, 27.044%) was in the field of research/experimental medicine. And in the field of biotechnology/applied microbiology, genetics/heredity and biochemistry/molecular biology, more than 50 papers on stem cell transplantation for Duchenne muscular dystrophy were published.

Table 1 Top 10 subject areas for publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011
Distribution of output according to journal for publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 (Table 2)

Table 2 Top 10 journals selected on the basis of number of publications on stem cell transplantation for Duchenne muscular dystrophy between 2002 and 2011
As shown in Table 2,Molecular Therapypublished 21 papers, followed byNeuromuscular Disorders,PLoS OneandHuman Gene Therapy, which published 12, 10 and 8 papers, respectively. The other six top journals areGene Therapy, Human Molecular Genetics,Journal of Clinical Investigation, Muscle & Nerve, Stem CellsandExpert Opinion on Biological Therapy.
Of the top 10 journals, seven journals were from the USA and another three journals were from the UK.
Molecular Therapyis the monthly publication of the American Society of Gene Therapy. The publisher is Nature Publishing Group in USA. It publishes 230 papers in 12 issues per year.
Neuromuscular Disordersis an international, multidisciplinary journal, which covers all aspects of neuromuscular disorders. The publisher is Pergamon-Elsevier Science ltd in USA. It publishes 78 papers in 12 issues per year.
PLoS Oneis published by the Public Library of Science (PLoS), a nonprofit organization in USA. It publishes 13 781 papers per year.
Human Gene Therapyis a journal covering all aspects of human gene therapy. The publisher is Mary Ann Liebert inc in USA. It publishes 145 papers in 18 issues per year.
Distribution of output according to country and institution for publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 (Figures 2, 3)
The analysis of the contributions of different countries/states to publications was based on journal articles in which the address and affiliation of at least one author were provided. A total of 318 articles were analyzed according to country and institution.
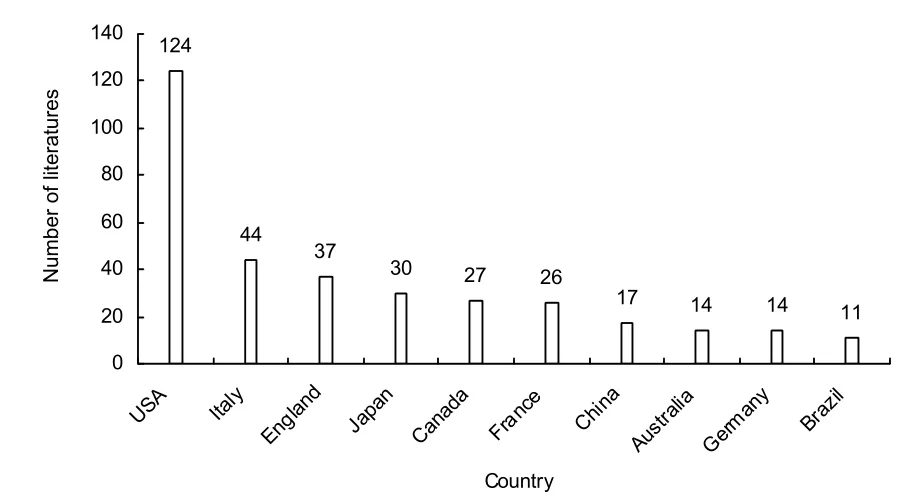
Figure 2 The top 10 countries publishing papers on stem cell transplantation for Duchenne muscular dystrophy from 2002 to 2011.
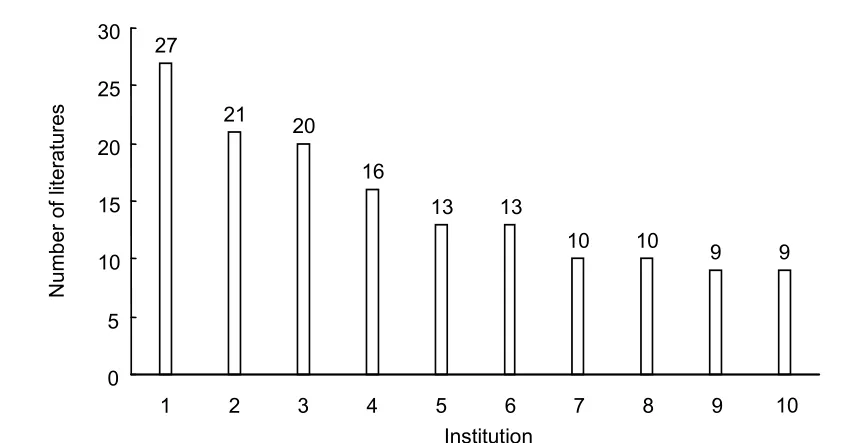
1: University of Pittsburgh, USA; 2: University of Milan, Italy; 3:University of Washington, USA; 4: Children's Hospital of Pittsburgh, USA; 5: Children's Hospital, Boston, USA; 6: Harvard University, USA; 7: University of Pavia, Italy; 8: University of Western Australia, Australia; 9: French National Center for Scientific Research, France; 10: Laval University, Canada.
From Figure 2, it is clear that most papers on stem cell transplantation for Duchenne muscular dystrophy were published in the USA (124 papers), followed by Italy (44 papers) and the UK (37 papers). China published 17 papers, ranking 7th. The other prolific countries are Japan, Canada, France, Australia, Germany and Brazil.
From Figure 3, the University of Pittsburgh, University of Milan and University of Washington were the most prolific research institutes. Five of the top 10 research institutes publishing in this field were in the USA, two were in Italy, and one institute each was in Australia, Canada and France.
Distribution according to institutions in China for publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 (Table 3)
As shown in Table 3, Sun Yat-Sen University was the most prolific research institute in China regarding the publication of papers on stem cells transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011. Sun Yat-Sen University published seven papers.
But we found that the number of papers published by Chinese institutions was still very few.
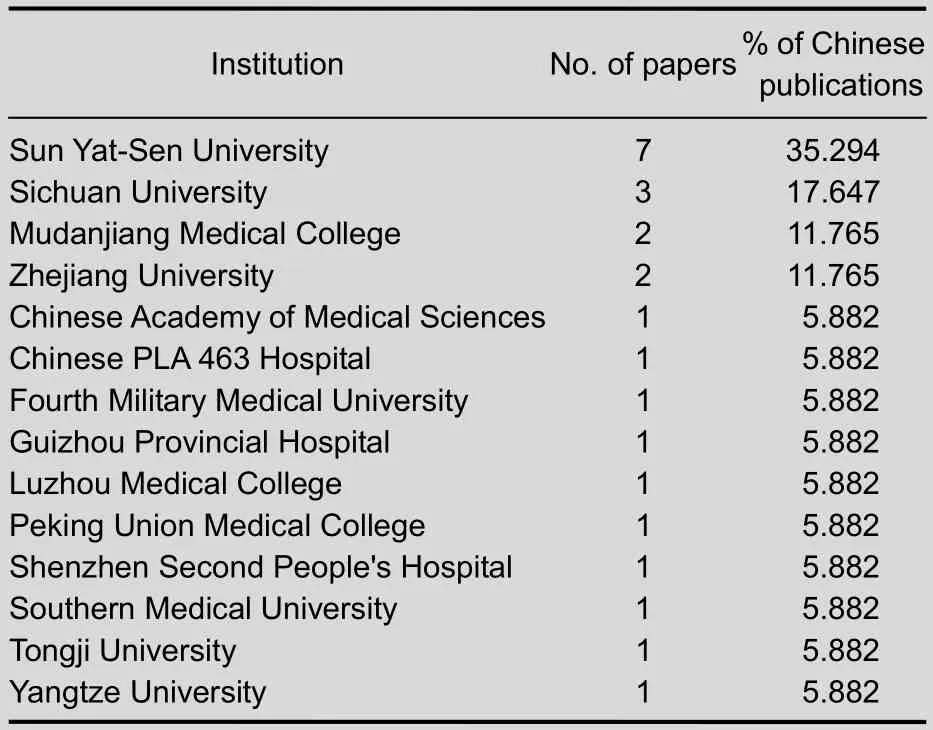
Table 3 The top 10 Chinese institutes publishing papers on stem cell transplantation for Duchenne muscular dystrophy from 2002 to 2011
Distribution according to overseas institutions that cooperated with Chinese institutions for publications on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011
As shown in Table 4, most institutions that cooperated with Chinese institutions on stem cells transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2011 were from Japan and Germany. The National Institute of Child Health and Human Development in Japan published two papers after cooperated with Chinese institutions. However, the international cooperation with China in the field of stem cell transplantation for Duchenne muscular dystrophy has been rare to date.
Highly-cited papers on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2002 to 2006 (Table 5)

Table 4 The top 10 overseas institutions that cooperated with Chinese institutions regarding publications on stem cell transplantation for Duchenne muscular dystrophy from 2002 to 2011
A total of 105 papers on stem cell transplantation for Duchenne muscular dystrophy were cited in Web of Science from 2002 to 2006. Most of the 15 most-cited papers were about different kinds of stem cell transplantation for muscle regeneration. The stem cells used were muscle stem cells, mesoangioblast stem cells, mesenchymal stem cells, adipose-derived stem cells, hematopoietic stem cell, and embryonic stem cells. Most of the studies were based on animal experiments. The paper “Cellular and molecular regulation of muscle regeneration”[9]published in 2004 was cited 696 times, which was more times than any other paper. It was published by the journalPhysiological Reviews.
Of the 15 most-cited papers, two were published inJournal of Cell Biology,another two were published inJournal of Clinical Investigation, and the remaining 11 were published in 11 different journals.
Of the 15 most-cited papers, there were five published in 2004. In 2002 and 2005, there were three top-cited papers published. And in 2003 and 2006, there was two top-cited papers published.
Highly-cited papers on stem cell transplantation for Duchenne muscular dystrophy in Web of Science from 2007 to 2011 (Table 6)
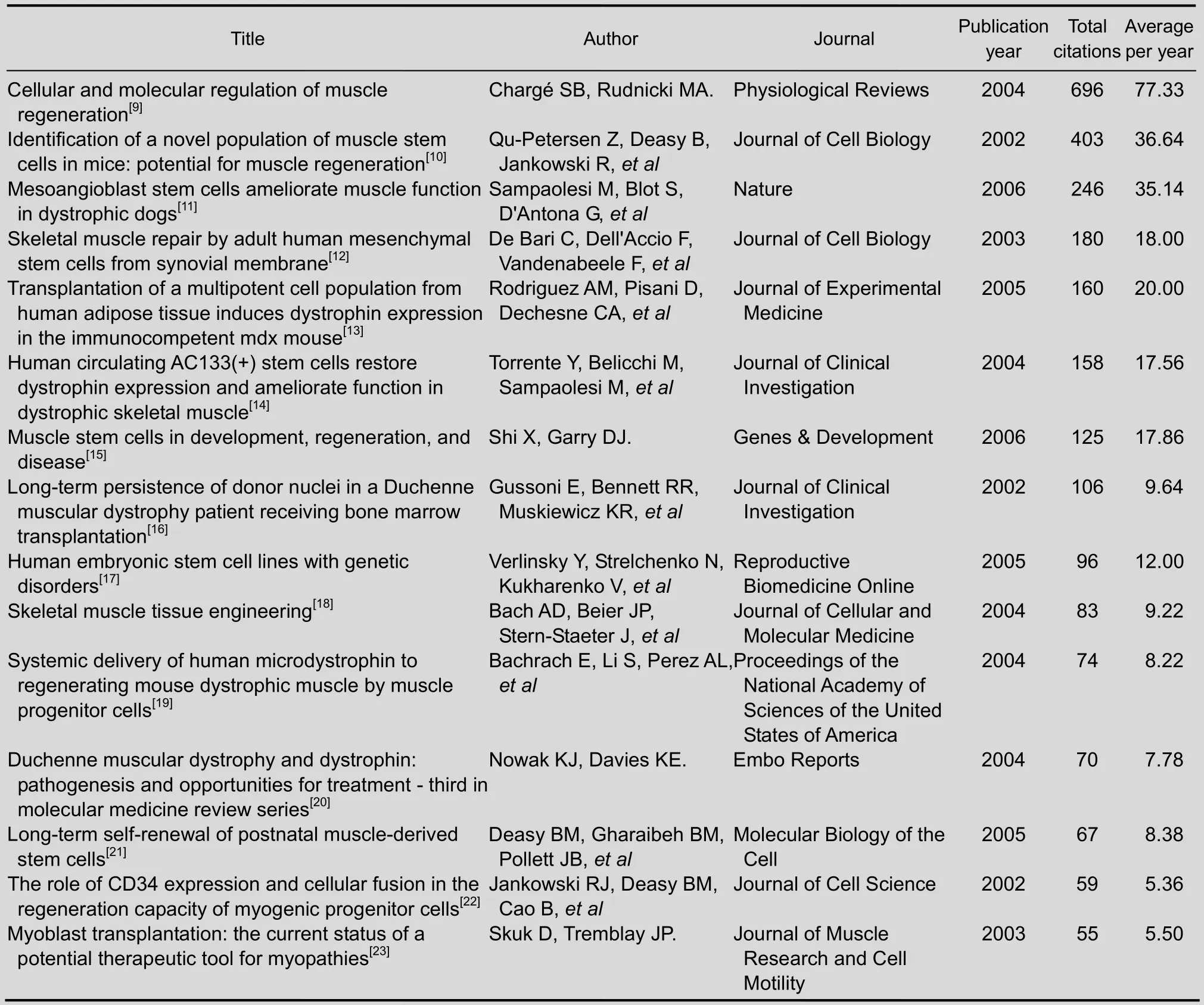
Table 5 The top 10 highly-cited papers on stem cell transplantation for Duchenne muscular dystrophy from 2002 to 2006
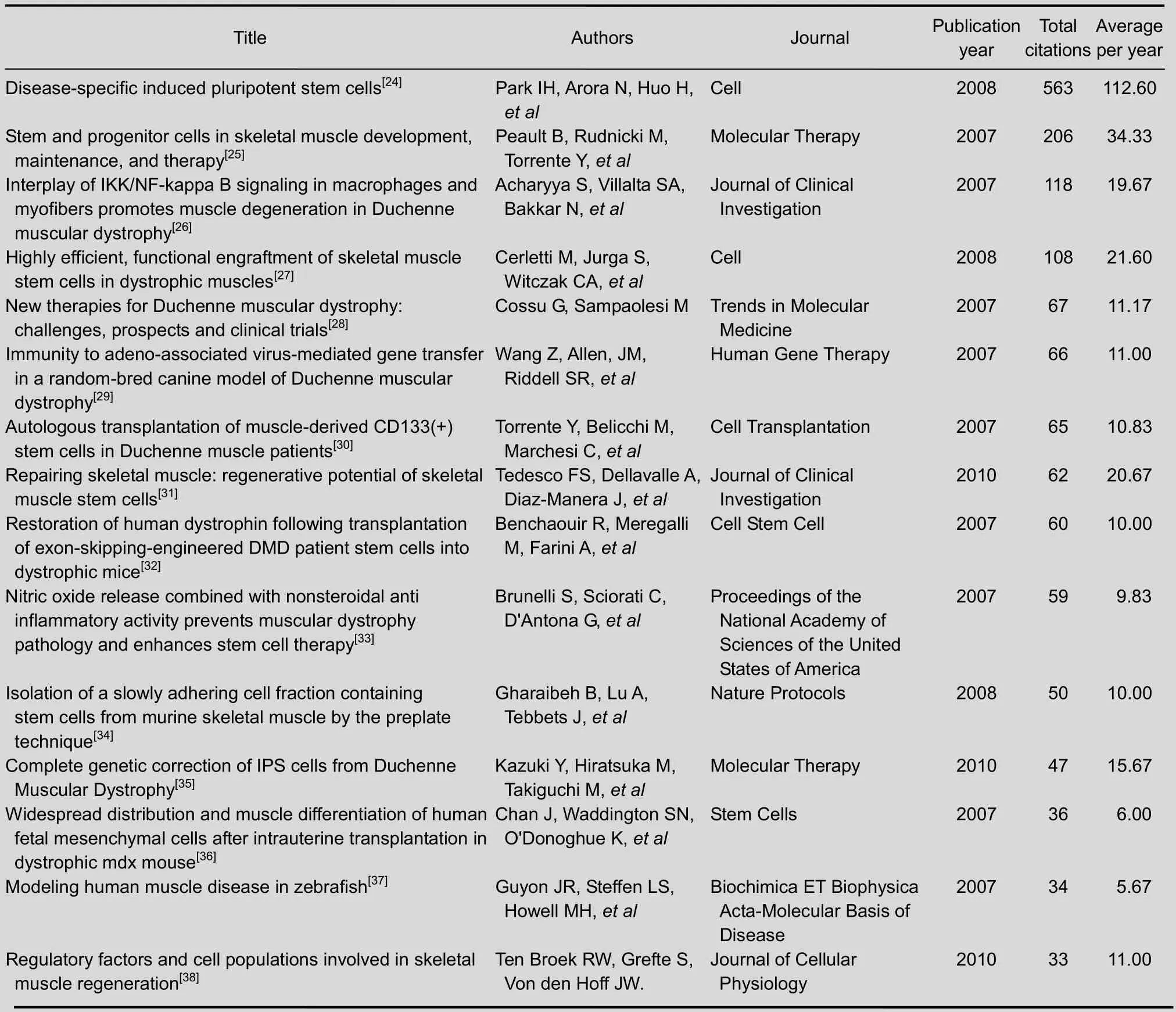
Table 6 The top 10 highly-cited papers on stem cell transplantation for Duchenne muscular dystrophy from 2007 to 2011
A total of 213 papers on stem cell transplantation for Duchenne muscular dystrophy were cited in Web of Science from 2007 to 2011. Most of the 15 most-cited papers were about new techniques of stem cell transplantation for treating Duchenne muscular dystrophy. The paper “Disease-specific induced pluripotent stem cells”[24]published in 2008 was cited 563 times, which was more times than any other paper. It was published by the journalCell.
Of the 15 most-cited papers, two were published inCell,two were published inMolecular Therapy,another two were published inJournal of Clinical Investigation, and the remaining nine papers were published in nine different journals.
Of the 15 most-cited papers, nine were published in 2007. In 2008, there were three top-cited papers published. And in 2010, there was three top-cited papers published too.
DlSCUSSlON
This bibliometric analysis based on Web of Science identified several research trends in studies of stem cell transplantation for Duchenne muscular dystrophy over the past 10 years. Of the 318 publications retrieved from Web of Science from 2002 to 2011, almost half derived from American authors and institutes. The number of publications gradually increased over the 10-year study period. Most papers appeared in journals with a focus on gene and molecular research, such asMolecular Therapy,Neuromuscular Disorders, andPLoS One.
From 2002 to 2006, the 10 most-cited papers were mostly about different kinds of stem cell transplantation for muscle regeneration, while from 2007 to 2011, the 10 most-cited papers were mostly about new techniques of stem cell transplantation for Duchenne muscular dystrophy.
It must be remembered that that technology relating to stem cell transplantation for treating Duchenne muscular dystrophy is still immature. While the aim of stem cell transplantation is to generate functional dystrophin protein, Duchenne muscular dystrophy patients lack dystrophin before treatment, and normal dystrophin generated after treatment may thus be recognized by the immune system and cause immune responses[39-41].
Nonetheless, stem cell therapy represents a groundbreaking treatment that could provide an effective clinical treatment for patients with Duchenne muscular dystrophy, if the relevant technologies can be improved. The technologies required for the cultivation, purification and isolation of embryonic stem cells are gradually maturing, and because the transplanted cells do not get rejected by the recipient’s immune system, no immunosuppressive agents are necessary[42]. However, the ability of this technique to increase dystrophin gene expression and thus improve muscle function remains to be determined, and considerable research is still required. Gradual improvements in the antenatal diagnosis of Duchenne muscular dystrophy can also reduce the chance of giving birth to affected individuals, and may thus further increase the opportunities for eradicating the occurrence of Duchenne muscular dystrophy.
Funding: The project was supported by the Key Technologies R & D Program of Liaoning Province, No. 2008225009.
Author contributions: Xiaofeng Yang retrieved the references, extracted the data, conceived and designed the study, and
wrote the manuscript.
Conflicts of interest: None declared.
[1] Chakkalakal JV, Thompson J, Parks RJ, et al. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. FASEB J. 2005;19(8):880-891.
[2] Skuk D, Goulet M, Roy B, et al. Efficacy of myoblast transplantation in nonhuman primates following simple intramuscular cell injections: Toward defining strategies applicable to humans. Exp Neurol. 2002;175(1):112-126.
[3] Quenneville SP, Chapdelaine P, Rousseau J, et al. Nucleofection of muscle-derived stem cells and myoblasts with phi C31 integrase: Stable expression of a full-length-dystrophin fusion gene by human myoblasts. Mol Ther. 2004;10(4):679-687.
[4] Erickson RP. Somatic gene mutation and human disease other than cancer. Mutat Res. 2003;543(2):125-136
[5] Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3(9):970-977.
[6] Huard J, Yokoyama T, Pruchnic R, et al. Muscle-derived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Ther. 2002;9(23):1617-1626.
[7] Anderson JE. The satellite cell as a companion in skeletal muscle plasticity: currency, conveyance, clue, connector and colander. J Exp Biol. 2006;209(Pt 12):2276-2292.
[8] Banks GB, Chamberlain JS. The value of mammalian models for duchenne muscular dystrophy in developing therapeutic strategies. Curr Top Dev Biol. 2008;84:431-453.
[9] Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004; 84(1):209-238.
[10] Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice:potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.
[11] Sampaolesi M, Blot S, D'Antona G, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444(7119):574-579.
[12] De Bari C, Dell'Accio F, Vandenabeele F, et al. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160(6):909-918.
[13] Rodriguez AM, Pisani D, Dechesne CA, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005; 201(9):1397-1405.
[14] Torrente Y, Belicchi M, Sampaolesi M, et al. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114(2):182-195.
[15] Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20(13):1692-1708.
[16] Gussoni E, Bennett RR, Muskiewicz KR, et al. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110(6):807-814.
[17] Verlinsky Y, Strelchenko N, Kukharenko V, et al. Human embryonic stem cell lines with genetic disorders. Reprod Biomed Online. 2005;10(1):105-110.
[18] Bach AD, Beier JP, Stern-Staeter J, et al. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8(4):413-422.
[19] Bachrach E, Li S, Perez AL. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A. 2004;101(10):3581-3586.
[20] Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment -Third in Molecular Medicine Review Series. EMBO Rep. 2004;5(9):872-876.
[21] Deasy BM, Gharaibeh BM, Pollett JB, et al. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16(7):3323-3333.
[22] Jankowski RJ, Deasy BM, Cao B, et al. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115(Pt 22):4361-4374.
[23] Skuk D, Tremblay JP. Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J Muscle Res Cell Motil. 2003;24(4-6):285-300.
[24] Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877-886.
[25] Péault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15(5):867-877.
[26] Acharyya S, Villalta SA, Bakkar N, et al. Interplay of IKK/NF-kappa B signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117(4):889-901.
[27] Cerletti M, Jurga S, Witczak CA, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134(1):37-47.
[28] Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13(12):520-526.
[29] Wang Z, Allen JM, Riddell SR, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18(1):18-26.
[30] Torrente Y, Belicchi M, Marchesi C, et al. Autologous transplantation of muscle-derived CD133(+) stem cells in Duchenne muscle patients. Cell Transplant. 2007;16(6):563-577.
[31] Tedesco FS, Dellavalle A, Diaz-Manera J, et al. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120(1):11-19.
[32] Benchaouir R, Meregalli M, Farini A, et al. Restoration of human dystrophin following transplantation of exon-skipping-engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell. 2007;1(6):646-657.
[33] Brunelli S, Sciorati C, D'Antona G, et al. Nitric oxide release combined with nonsteroidal anti inflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007;104(1):264-269.
[34] Gharaibeh B, Lu A, Tebbets J, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3(9):1501-1509.
[35] Kazuki Y, Hiratsuka M, Takiguchi M, et al. Complete genetic correction of iPS cells from Duchenne Muscular Dystrophy. Mol Ther. 2010;18(2):386-393.
[36] Chan J, Waddington SN, O'Donoghue K, et al. Widespread distribution and muscle differentiation of human fetal mesenchymal cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25(4):875-884.
[37] Guyon JR, Steffen LS, Howell MH, et al. Modeling human muscle disease in zebrafish. Biochim Biophys Acta. 2007;1772(2):205-215.
[38] Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224(1):7-16.
[39] Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293(2):C661-669.
[40] Vainzof M, Ayub-Guerrieri D, Onofre PC, et al. Animal models for genetic neuromuscular diseases. J Mol Neurosci. 2008;34(3):241-248.
[41] Pescatori M, Broccolini A, Minetti C, et al. Gene ex-pression profiling in the early phases of DMD: a con-stant molecular signature characterizes DMD muscle from early postnatal life throughout disease progres-sion. FASEB J. 2007;21(4):1210-1226.
[42] Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle: basic mechanisms and clinical im-plications. Curr Pharm Des. 2010;16(8):906-914.
Cite this article as:Neural Regen Res. 2012;7(22):1744-1751.
Xiaofeng Yang, Chief physician, Master’s supervisor, Cell Therapy Center, Chinese PLA 463 Hospital, Shenyang 110042, Liaoning Province, China
2012-04-14
2012-07-02
(N20120806005/MWJ)
Yang XF. Stem cell transplantation for treating Duchenne muscular dystrophy: A Web of Science-based literature analysis. Neural Regen Res. 2012;7(22):1744-1751.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.22.010
(Edited by Mu WJ/Song LP)
 中國(guó)神經(jīng)再生研究(英文版)2012年22期
中國(guó)神經(jīng)再生研究(英文版)2012年22期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Early hyperbaric oxygen therapy inhibits aquaporin 4 and adrenocorticotropic hormone expression in the pituitary gland of rabbits with blast-induced craniocerebral injury**★
- Transgene expression and differentiation of baculovirus-transduced adipose-derived stem cells from dystrophin-utrophin double knock-out mouse********☆
- Thrombospondins and synaptogenesis***★
- Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during murine neural stem/progenitor cell differentiation in vitro***☆
- Precision radiotherapy for brain tumors A 10-year bibliometric analysis☆
- ldentification of α7 nicotinic acetylcholine receptor on hippocampal astrocytes cultured in vitro and its role on inflammatory mediator secretion***★
