Neuroprotective effects of bovine colostrum on intracerebral hemorrhage-induced apoptotic neuronal cell death in rats☆●
Sung Eun Kim, Il Gyu Ko, Mal Soon Shin, Chang Ju Kim, Young Gwan Ko, Hanjin Cho
1 Department of Physiology, College of Medicine, Kyung Hee University, Seoul 130-701, Republic of Korea
2 Department of Emergency Medicine, College of Medicine, Kyung Hee University, Seoul 130-701, Republic of Korea
3 Department of Emergency Medicine, Korea University Ansan Hospital, Ansan 425-707, Republic of Korea
Neuroprotective effects of bovine colostrum on intracerebral hemorrhage-induced apoptotic neuronal cell death in rats☆●
Sung Eun Kim1, Il Gyu Ko1, Mal Soon Shin1, Chang Ju Kim1, Young Gwan Ko2, Hanjin Cho3
1Department of Physiology, College of Medicine, Kyung Hee University, Seoul 130-701, Republic of Korea
2Department of Emergency Medicine, College of Medicine, Kyung Hee University, Seoul 130-701, Republic of Korea
3Department of Emergency Medicine, Korea University Ansan Hospital, Ansan 425-707, Republic of Korea
Brain cell death after intracerebral hemorrhage may be mediated in part by an apoptotic mechanism. Colostrum is the first milk produced by mammals for their young. It plays an important role in protection and development by providing various antibodies, growth factors and nutrients, and has been used for various diseases in many countries. In the present study, we investigated the anti-apoptotic effects of bovine colostrum using organotypic hippocampal slice cultures and an intracerebral hemorrhage animal model. We performed densitometric measurements of propidium iodide uptake, a step-down avoidance task, Nissl staining, and caspase-3 immunohistochemistry. The present results revealed that colostrum treatment significantly suppressed N-methyl-D-aspartic acid-induced neuronal cell death in the rat hippocampus. Moreover, colostrum treatment improved short-term memory by suppressing hemorrhage-induced apoptotic neuronal cell death and decreasing the volume of the lesion induced by intracerebral hemorrhage in the rat hippocampus. These results suggest that colostrum may have a beneficial role in recovering brain function following hemorrhagic stroke by suppressing apoptotic cell death.
intracerebral hemorrhage; organotypic hippocampal slice culture; bovine colostrum; apoptotic cell death; N-methyl-D-aspartic acid; caspase-3; hippocampus; memory
Research Highlights
(1)In vitroexperiment results confirmed that bovine colostrum can inhibit N-methyl-D-aspartic acid-induced neuronal cell death in the rat hippocampus. (2)In vivoexperimental results confirmed that bovine colostrum can inhibit intracerebral hemorrhage-induced neuronal cell death, decrease caspase-3 expression, and reduce the size of intracerebral hemorrhage-induced lesions in the hippocampus, and improve the cognitive function of rats with intracerebral hemorrhage.
Abbreviation
NMDA, N-methyl-D-aspartic acid
INTRODUCTION
Brain injury after intracerebral hemorrhage occurs through multiple mechanisms including direct tissue destruction, the space-occupying effect of hematomas, ischemic damage to adjacent tissue, clot-derived toxic factors, and edema[1-2].
Brain cell death after intracerebral hemorrhage may be mediated in part by an apoptotic mechanism[3]. Apoptosis, the process of programmed cell death, plays an important role in normal development and tissue homeostasis through functions in cell replacement, tissue remodeling, and theremoval of damaged cells[4-5]. However, inappropriate or excessive apoptosis is implicated in several types of neurodegenerative disorders, including stroke[3-6].
Inflammation contributes to secondary brain injury induced by intracerebral hemorrhage. Inflammation is characterized by the accumulation and activation of inflammatory cells and mediators within the hemorrhagic brain[7]. Therefore, it has been suggested that activation and regulation of inflammatory responses in the hemorrhagic brain could be a therapeutic target for intracerebral hemorrhage[8].
Colostrum is the first milk produced by female mammals during the first few days postpartum. It provides various antibodies, growth factors, and nutrients such as proteins, carbohydrates, fats, vitamins and minerals for the neonate. Moreover, colostrum contains many biologically active constituents that play important roles in protection and development[9]. Bovine colostrum has been used for the treatment of various gastrointestinal disorders, intestinal inflammation, respiratory infections, rheumatoid arthritis, and the healing of injured tissues[10-12]. Recently, Schusteret al[13]reported that colostrinin, a class of proline-rich polypeptides derived from colostrum, had a protective effect on neuroblastoma cells by reducing fibril formation and cell death induced by beta-amyloid.
As mentioned above, many studies have reported on the beneficial effects of colostrum. However, these reports have mainly focused on its protective effects against various infectious microorganisms, and few studies have investigated the anti-apoptotic effect of colostrum. Therefore, in the present study, we investigated the anti-apoptotic effects of bovine colostrum in organotypic hippocampal slice cultures and in an intracerebral hemorrhage animal model.
RESULTS
Quantitative analysis of experimental animals
Inin vivoexperiments, thirty rats were initially included and randomly divided into three groups, with 10 rats in each group: sham-operation group, hemorrhage-induced group (induction of intracerebral hemorrhage plus intragastric administration of distilled water), and colostrum-treated group (induction of intracerebral hemorrhage plus intragastric administration of colostrum). During this study, there was no spontaneous death of rats, and 30 rats were included in the final analysis.
Effect of colostrum on N-methyl-D-aspartic acid (NMDA)-induced neuronal cell death in organotypic hippocampal slice cultures
As shown in Figure 1, 100 μM NMDA treatment caused neuronal cell death in the hippocampus 24 hours after NMDA application. When the value of the NMDA-treated group was set as 100% damage, the value of slices incubated with bovine colostrum at a concentration of 0.1, 0.5, and 1.0 mg/mL was 75.66 ± 9.47%, 51.27 ± 3.58%, and 17.78 ± 5.55%, respectively. Pre-treatment with 0.5 and 1.0 mg/mL bovine colostrum significantly reduced the uptake of propidium iodide (Figure 1).
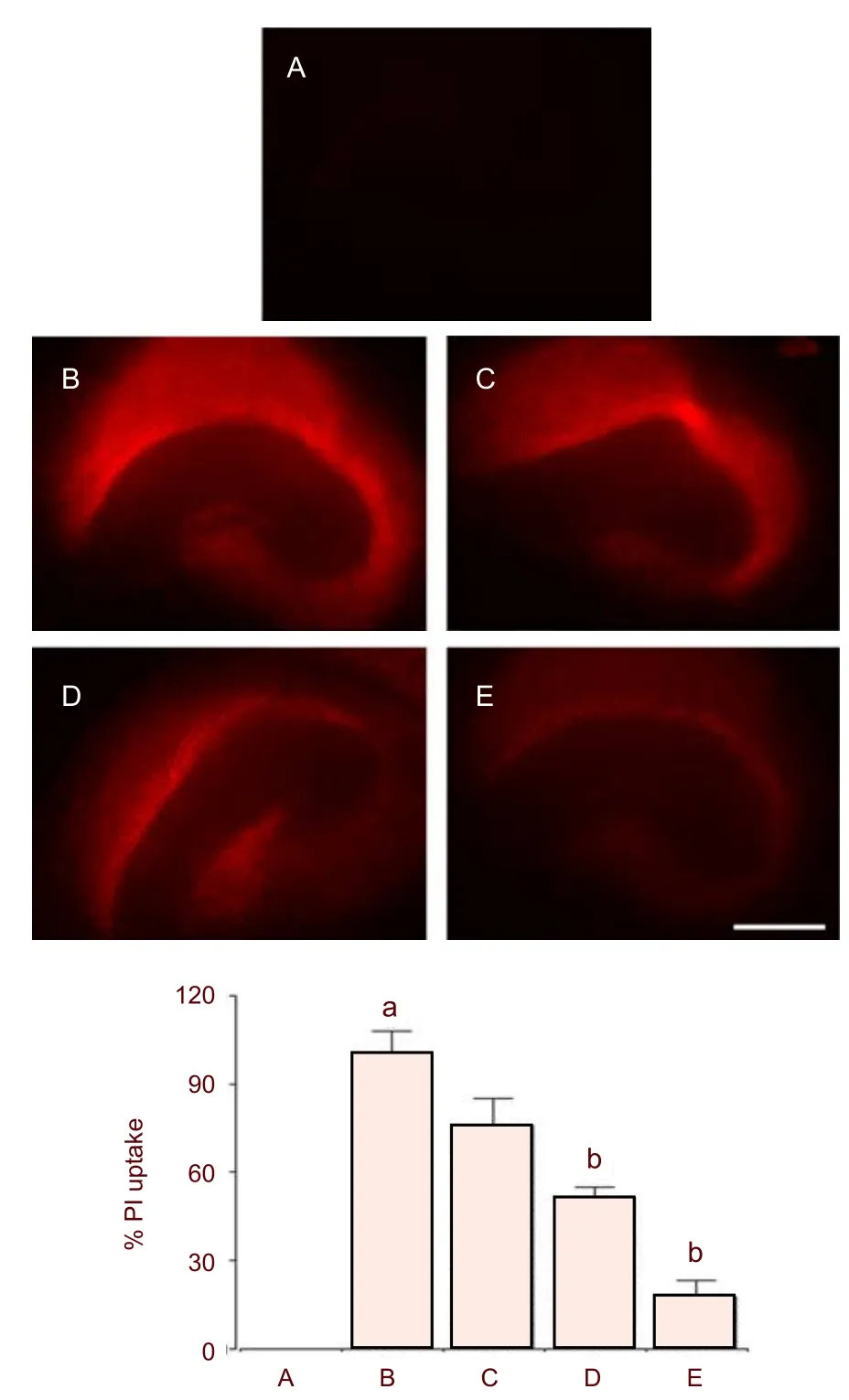
Figure 1 Effect of colostrum on N-methyl-D-aspartic acid (NMDA)-induced neuronal cell death in organotypic hippocampal slice cultures.
Body weight changes
The body weights of rats on the 22ndday of the experiment were 232 ± 4.14, 225 ± 5.32, and 238 ± 3.22 g in the sham-operation group, hemorrhageinduced group, and colostrum-treated group, respectively. There was no significant difference in body weight among the groups.
Effect of colostrum on short-term memory of cerebral hemorrhage rats
The short-term memory of rats was impaired by induction of intracerebral hemorrhage (P< 0.05). However, treatment with colostrum significantly alleviated hemorrhage-induced short-term memory impairment (P< 0.05; Figure 2).

Figure 2 Effect of colostrum on latency in the step-down avoidance task after intracerebral hemorrhage induction in rats.
Effect of colostrum on the size of the intracerebral hemorrhage-induced lesion
Photomicrographs of the lesioned area in the hippocampus are presented in Figure 3. The average neuronal lesion size in the hemorrhage-induced group was 56.68 ± 7.42% of the normal hippocampus area. No noticeable lesions were observed in the shamoperation group. However, the size of intracerebral hemorrhage-induced lesion was significantly reduced to 40.1 ± 2.45% following treatment with colostrums (Figure 3).
These results showed that lesion size was increased following intracerebral hemorrhage (P< 0.05), and treatment with colostrum significantly decreased hemorrhage-induced lesion size in the hippocampus (P<0.05).

Figure 3 Effect of colostrum on the size of the intracerebral hemorrhage-induced lesion.
Effect of colostrum on caspase-3 expression in the hippocampus
Photomicrographs of caspase-3-positive cells in the hippocampus are presented in Figure 4. The number of caspase-3-positive cells was 3.35 ± 1.51/mm2in the sham-operation group, 206.99 ± 9.31/mm2in the hemorrhage-induced group, and 147.65 ± 16/mm2in the colostrum-treated group (Figure 4).
These results showed that intracerebral hemorrhage increased caspase-3 expression in the hippocampus (P< 0.05) and treatment with colostrum significantly suppressed hemorrhage-induced caspase-3 expression (P< 0.05).
DISCUSSION
The excessive activation of postsynaptic NMDA receptors results in neurotoxicity[14]. In particular, activation of NMDA receptors causes the cellular influx of calcium ions and then intracellular accumulation of these ions, which leads to edema and cell death[15-16]. Along with the breakdown of the blood-brain barrier, edema and neuronal cell death are the main pathophysiologic changes induced by hematomas after intracerebral hemorrhage[17-18].
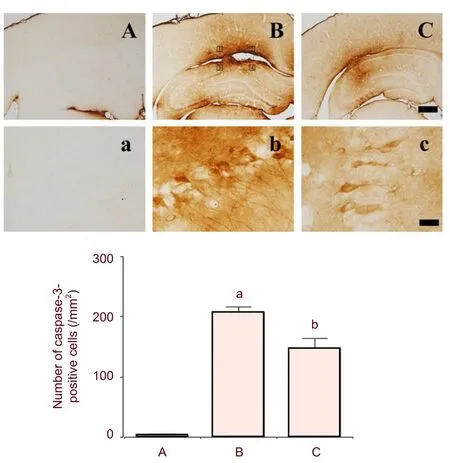
Figure 4 Effect of colostrum on caspase-3 expression in the hippocampus of intracerebral hemorrhage rats.
In addition, Ardizzoneet al[19]suggested that intracerebral hemorrhage mediates injury through activation of the protein phosphorylation of NMDA receptors. In the present study, bovine colostrum treatment prevented NMDA-induced cell death in hippocampal slices dose-dependently. Cognitive impairments evolve after stroke, with motor deficits. In particular, it was reported that hemorrhagic strokes had a 6 times greater frequency of cognitive impairment than ischemic stroke[20]. Recently, a neuropsychological study reported that the striatum plays key roles in some forms of learning and memory[21]. Lekicet al[22]reported that collagenase-induced intracerebral hemorrhage in the basal ganglia causes significant memory deficits.
Jeonet al[23]reported that subarachnoid hemorrhage significantly increased the number of apoptotic neurons in the hippocampus, the cerebral cortex and the cerebellum, and that cognitive and memory functions were impaired. However, they suggested that apoptosis in the hippocampus was not sufficient to cause neurobehavioral deficits, and other factors in the hippocampal cell death pathway may contribute to the impairment. The hippocampus, including the dentate gyrus, plays a pivotal role in learning and memory[24]. In the present study, we induced intracerebral hemorrhage by directly injecting collagenase into the hippocampal CA1 region. Consequently, intracerebral hemorrhage induction in the hippocampus shortened the latency in the step-down avoidance task, a measure of short-term memory. On the other hand, treatment with bovine colostrum significantly increased the latency. This result indicates that bovine colostrum improved short-term memory following hippocampal hemorrhage in rats. The beneficial effects of colostrum on cognitive function have been investigated previously. Popiket al[25]reported that colostrinin, one of the polypeptides derived from colostrum, facilitated the acquisition and retrieval of spatial memory and long-term memory in aged rats, and Bilikiewicz and Gaus[26]reported that colostrinin retarded the progression of Alzheimer’s disease.
Apoptosis appears to play a key role in neuronal cell death following stroke[7,10]. Intracerebral hemorrhage injury induces the activation of caspase-3, which plays an important role in apoptotic cell death[6,27]. An increase in caspase-3 expression precedes DNA fragmentation, reaches its peak level at 24 hours after intracerebral hemorrhage induction, and then declines[28]. Our results showed that intracerebral injection of collagenase increased the lesion volume and the expression of caspase-3 in the hippocampal CA1 region, indicating that collagenase-induced intracerebral hemorrhage triggers apoptotic neuronal cell death in the hippocampus. On the other hand, treatment with bovine colostrum significantly reduced the lesion volume and suppressed the expression of caspase-3. Recently, Douraghi-Zadehet al[29]suggested that colostrinin may play a role in preventing Alzheimer’s disease through the inhibition of Fas-mediated apoptosis.
In conclusion, we demonstrated that treatment with bovine colostrum prevents apoptosis induced by NMDA, and improves short-term memory impaired by the induction of intracerebral hemorrhage by significantly reducing the lesion volume and suppressing the expression of caspase-3 in the hippocampal CA1 region. Although we investigated the anti-apoptotic effect of bovine colostrum in the present study, we could not give a detailed explanation of the mechanism by which bovine colostrum induces its anti-apoptotic effect. Moreover, bovine colostrum is suggested to have anti-inflammatory effects[3]. In fact, inflammation contributes to secondary brain injury induced by intracerebral hemorrhage[11], and colostrum has been shown to block interleukin-1βinduced proinflammatory gene expression and Cox-2 protein expression in intestinal epithelial cells through IκB-α degradation and inhibition of NF-κB signaling.
However, the precise inflammatory suppression mechanism bovine colostrum interacts with remains unknown[3]. Therefore, further studies are required to clarify the molecular and biological mechanisms by which colostrum exhibits its anti-apoptotic and anti-inflammatory effects. However, it is important to note that this study showed the therapeutic potential of bovine colostrum in the recovery of brain function following hemorrhagic stroke and its anti-apoptotic effect.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
This study was performed in the Physiology Laboratory of the College of Medicine, Kyung Hee University, Republic of Korea, between June and December 2010.
Materials
The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. Male Sprague-Dawley rats weighing 200 ± 10 g, aged 7 weeks, were used in the experiments. The rats were housed at 20 ± 2°C with a light cycle at 07:00-19:00, and had free access to food and water before and after surgery,
Methods
Intracerebral hemorrhage induction
To induce intracerebral hemorrhage, the rats were intraperitoneally anesthetized with Zoletil 50?(10 mg/kg; Vibac Laboratories, Carros, France) and placed in a stereotaxic frame as previously described[6]. Through a hole drilled in the skull, a 26-gauge needle was implanted into the hippocampal CA1 region at the following coordinates: 2.4 mm lateral to the midline and 4.2 mm posterior to the coronal suture at a depth of 2.4 mm from the surface of the brain. In total, 1 μL of saline containing 0.2 U collagenase (type 4; Sigma Chemical Co., St. Louis, MO, USA) was then infused over a 1-minute period. The needle remained in place for an additional 3 minutes following the infusion, and afterwards it was slowly withdrawn. The animals in the sham-operation group received 1 μL of physiological saline by the same method.
Colostrum treatment
Rats in the colostrum-treated group received bovine colostrum (0.4 g/kg)viaan orogastric tube once a day for 21 consecutive days beginning 1 day after surgery. The animals in the sham-operation and hemorrhage-induced groups received equal amounts of distilled water.
Step-down avoidance task
Short-term memory was evaluated by assessing the latency of the step-down avoidance task as previously described[30]. For training, the rats were placed on a 7 cm × 25 cm platform that was 2.5 cm in height and were allowed to rest on the platform for 2 minutes. The platform faced a 42 cm × 25 cm grid with parallel 0.1-cm caliber stainless steel bars spaced 1 cm apart. During the training session, the animals received a 0.3 mA scrambled foot shock for 2 seconds immediately after stepping down. The retention time was determined 2 days after the training session on the 20thday after starting the experiment. The interval between the time when the rats first stepped down and the time when they placed all four paws on the grid was defined as the latency of the step-down avoidance task. Latencies over 300 seconds were counted as 300 seconds.
Brain tissue preparation
Brain tissue preparation was made as previously described[31]. The rats were sacrificed on the 22ndday of the experiment immediately after determination of the latency. The animals were weighed and then given an overdose of Zoletil 50?(10 mg/kg, i.p.; Vibac Laboratories). After a complete lack of response was observed, the rats were transcardially perfused with 50 mM phosphate buffered saline and fixed with a freshly prepared solution consisting of 4% (w/v) Paraformal dehyde in 100 mM phosphate buffer (pH 7.4). The brains were dissected and post-fixed in the same fixative overnight and then transferred to a 30% (w/v) sucrose solution for cryoprotection. Serial coronal sections (40 μm thick) were made using a freezing microtome (Leica, Nussloch, Germany). On average, four hippocampal tissue sections were collected from each rat for Nissl staining and immunohistochemistry.
Determination of lesion size by Nissl staining
To determine lesion size, Nissl staining was performed as previously described[6]. A digital image of the Nissl stained cells was obtained from the field of view under a light microscope (Olympus, Tokyo, Japan). The lesion area of the collagenase injection site was determined using an Image-Pro?Plus image analyzer (Media Cybernetics Inc., Silver Spring, MD, USA). The lesion size in the hippocampal CA1 region (%) was calculated as follows: the hemorrhage-induced lesion size (collagenase injection side)/intact CA1 region size (contralateral side) × 100%.
Caspase-3 immunohistochemistry
For visualization of caspase-3 expression in the hippocampal CA1 region, caspase-3 immunohistochemistry was performed. Eight sections on average were selected from each brain region spanning from Bregma -3.80 mm to -4.50 mm. Free-floating tissue sections were incubated overnight with mouse anti-caspase-3 antibody (1:1 000, sc-7272; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in order to visualize caspase-3 expression. The sections were then incubated for 1 hour with biotinylated anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA, USA). The sections were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 hour at room temperature.
Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% (w/v) 3,3-diaminobenzidine and 0.01% (v/v) H2O2in 50 mM Tris buffer (pH 7.6) for 3 minutes. The number of caspase-3-positive cells was quantitatively assessed in four fields (250 μm × 250 μm in each field) within the hippocampal CA1 region adjacent to the hematoma according to a previously described method[6]. The data analysis for caspase-3-positive cells was performed in a blinded fashion.
Organotypic hippocampal slice cultures and quantification of cell damage
Organotypic hippocampal slice cultures were prepared from the hippocampi of 7-8 day-old Sprague-Dawley rats (n= 3) using the method of Stoppiniet al[32]. Briefly, transverse slices at a thickness 350 μm were cut from the hippocampi using a McIlwain tissue chopper (Mickle Laboratory Engineering Ltd., Surrey, UK). Five slices were placed on Millicell culture inserts (Millipore, Billerica, MA, USA). The slices were cultured for 10 daysin vitrowith culture medium consisting of 50% (v/v) minimum essential medium, 25% (v/v) Hank’s balanced salt solution, and 25% (v/v) heat-inactivated horse serum (Gibco BRL, Grand Island, NY, USA) supplemented with 6.5 g/L glucose. The slices were incubated at 37°C in a 5% CO2, 95% O2humidified incubator, and culture media were changed twice a week.
The slices were incubated for 10 days, and then bovine colostrum (Alpha Laboratories, Auckland, New Zealand) at concentrations of 0.1, 0.5, and 1.0 mg/mL were added to independent wells 1 hour before treatment with 100 μM NMDA. Thereafter, the slices were incubated for 24 hours in a humidified incubator. Control slices were treated with only 100 μM NMDA, which resulted in cell death.
Cell damage in organotypic hippocampal slice cultures was evaluated with densitometric measurements of cellular uptake of propidium iodide (Sigma Chemical Co., St. Louis, MO, USA), used as a marker of dead or dying cells[33]. Propidium iodide (5 μg/mL) was added to the culture medium 24 hours after NMDA treatment and then incubated for 2 hours.
All images were captured with a digital camera under a fluorescence microscope (AMG, Bothell, WA, USA), and quantified using Image-Pro?Plus image analyzer (Media Cybernetics Inc., Silver Spring, MD, USA). The value obtained from the control slices was set as 100% damage and was then compared with values obtained from the slices treated with bovine colostrum.
Statistical analysis
Statistical analysis was performed using IBM SPSS (version 20.0; IBM Corp., Armonk, NY, USA). For the comparison between groups, one-way analysis of variance and Duncan'spost-hoctest were performed. The results were expressed as mean ± SEM.Pvalues <0.05 were considered statistically significant.
Author contributions: Hanjin Cho and Young Gwan Ko designed this study. Sung Eun Kim and Il Gyu Ko performed the experiments. Sung Eun Kim wrote the manuscript. Mal Soon Shin analyzed the data. Chang Ju Kim supervised laboratory procedures.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Kyung Hee University Institutional Animal Care and Use Committee in
Republic of Korea.
[1] Lee KR, Colon GP, Betz AL, et al. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84(1):91-96.
[2] Lee HH, Kim H, Lee MH, et al. Treadmill exercise decreases intrastriatal hemorrhage-induced neuronal cell death via suppression on caspase-3 expression in rats. Neurosci Lett. 2003;352(1):33-36.
[3] Matsushita K, Meng W, Wang X, et al. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab. 2000;20(2):396-404.
[4] Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456-1462.
[5] Woodle ES, Kulkarni S. Programmed cell death. Transplantation. 1998;66(6):681-691.
[6] Johnson EM Jr, Greenlund LJ, Akins PT, et al. Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma. 1995;12(5):843-852.
[7] Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010; 92(4):463-477.
[8] Teng W, Wang L, Xue W, et al. Activation of TLR4-mediated NFkappaB signaling in hemorrhagic brain in rats. Mediators Inflamm. 2009;2009:473276.
[9] Davis PF, Greenhill NS, Rowan AM, et al. The safety of New Zealand bovine colostrum: nutritional and physiological evaluation in rats. Food Chem Toxicol. 2007;45(2):229-236.
[10] Thapa BR. Therapeutic potentials of bovine colostrums. Indian J Pediatr. 2005;72(10):849-852.
[11] An MJ, Cheon JH, Kim SW et al. Bovine colostrum inhibits nuclear factor kappa B-mediated proinflammatory cytokine expression in intestinal epithelial cells. Nutr Res. 2009;29(4):275-280.
[12] Bodammer P, Maletzki C, Waitz G et al. Prophylactic application of bovine colostrum ameliorates murine colitis via induction of immunoregulatory cells. J Nutr. 2011; 141(6):1056-1061.
[13] Schuster D, Rajendran A, Hui SW, et al. Protective effect of colostrinin on neuroblastoma cell survival is due to reduced aggregation of beta-amyloid. Neuropeptides. 2005;39(4):419-426.
[14] Lees KR. Cerestat and other NMDA antagonists in ischemic stroke. Neurology. 1997;49(5 Suppl 4):S66-S69.
[15] Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330(9):613-622.
[16] Qureshi AI, Ali Z, Suri MF, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31(5):1482-1489.
[17] Qureshi AI, Ling GS, Khan J, et al. Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Crit Care Med. 2001;29(1):152-157.
[18] Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450-1460.
[19] Ardizzone TD, Zhan X, Ander BP, et al. Src kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38(5):1621-1625.
[20] Nys GM, van Zandvoort MJ, de Kort PL, et al. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23(5-6):408-416.
[21] El Massioui N, Chéruel F, Faure A, et al. Learning and memory dissociation in rats with lesions to the subthalamic nucleus or to the dorsal striatum. Neuroscience. 2007;147(4):906-918.
[22] Lekic T, Hartman R, Rojas H, et al. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma. 2010;27(3):627-637.
[23] Jeon H, Ai J, Sabri M, et al. Learning deficits after experimental subarachnoid hemorrhage in rats. Neuroscience. 2010;169(4):1805-1814.
[24] Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278-294.
[25] Popik P, Galoch Z, Janusz M, et al. Cognitive effects of Colostral-Val nonapeptide in aged rats. Behav Brain Res. 2001;118(2):201-208.
[26] Bilikiewicz A, Gaus W. Colostrinin (a naturally occurring, proline-rich, polypeptide mixture) in the treatment of Alzheimer's disease. J Alzheimers Dis. 2004;6(1):17-26.
[27] Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157(5):1415-1430.
[28] Gong C, Boulis N, Qian J, et al. Intracerebral hemorrhage-induced neuronal death. Neurosurgery 2001;48(4):875-882.
[29] Douraghi-Zadeh D, Matharu B, Razvi A, et al. The protective effects of the nutraceutical, colostrinin, against Alzheimer's disease, is mediated via prevention of apoptosis in human neurones induced by aggregated beta-amyloid. J Nutr Health Aging. 2009;13(6):522-527.
[30] Kim H, Lee SH, Kim SS, et al. The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int J Dev Neurosci . 2007;25(4):243-249.
[31] Kang JO, Hong SE, Kim SK, et al. Adaptive responses induced by low dose radiation in dentate gyrus of rats. J Korean Med Sci. 2006;21(6):1103-1107.
[32] Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173-182.
[33] Macklis JD, Madison RD. Progressive incorporation of propidium iodide in cultured mouse neurons correlates with declining electrophysiological status: a fluorescence scale of membrane integrity. J Neurosci Methods. 1990; 31(1):43-46.
Cite this article as:Neural Regen Res. 2012;7(22):1715-1721.
Sung Eun Kim☆, Ph.D., Department of Physiology, College of Medicine, Kyung Hee University, #1 Hoigi-dong, Dongdaemoon-gu, Seoul 130-701, Republic of Korea
Hanjin Cho, M.D., Ph.D., Assistant professor, Department of Emergency Medicine, Korea University Ansan Hospital, #516 Gojan-dong, Dan
won-gu, Ansan 425-707, Korea
chohj327@hotmail.com
2012-04-05
2012-06-30
(NY20120326004/H)
Kim SE, Ko IG, Shin MS, Kim CJ, Ko YG, Cho HJ. Neuroprotective effects of bovine colostrum on intracerebral hemorrhage-induced apoptotic neuronal cell death in rats. Neural Regen Res.
2012;7(22):1715-1721.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.22.006
(Edited by Wang J, Sun M, Maslarov D, Shah Z/ Song L)
- 中國神經(jīng)再生研究(英文版)的其它文章
- 5-hydroxymethyl-2-furfural prolongs survival and inhibits oxidative stress in a mouse model of forebrain ischemia******☆
- Breaking News in Spinal Cord Injury Research FDA Approved Phase I Clinical Trial of Human, Autologous Schwann Cell Transplantation in Patients with Spinal Cord Injuries☆●
- Sonic hedgehog elevates N-myc gene expression in neural stem cells***★
- ldentification of α7 nicotinic acetylcholine receptor on hippocampal astrocytes cultured in vitro and its role on inflammatory mediator secretion***★
- Precision radiotherapy for brain tumors A 10-year bibliometric analysis☆
- Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during murine neural stem/progenitor cell differentiation in vitro***☆

