熱激脅迫可能通過降低脂肪與糖原的積累抑制渦蟲的發(fā)育
劉培黨,林興鳳,王大勇
(東南大學(xué)醫(yī)學(xué)院生物化學(xué)與分子生物學(xué)系、發(fā)育與疾病相關(guān)基因教育部重點(diǎn)實(shí)驗(yàn)室,江蘇南京 210009)
Freshwater free-living planarians,an important component of the aquatic ecosystem,are distributed worldwide in unpolluted streams.Planarians have a high regenerative capacity,and every fragment is capable of growing into a complete individual if an individual planarian is cut into small fragments.Traditionally,planarians have been a favored model animal in developmental biology.They can be easily collected in large numbers,require only low culture and test medium volumes,and can be kept inexpensively in laboratory for the study of stem cell biology[1],neuroregeneration[2],and toxicology[3].
So far,the characteristics of planarian make it a suitable organism for studying the effects of environmental stresses.The acute toxicities of eight widely used surfactants on oxidative stress and cholinesterase(ChE)activities were examined in planarian Dugesia japonica,and the differences in acute toxicity among these surfactants were at least in the range of three orders of magnitudes[3].The catalase activity was significantly induced by copper exposure at concentrations of 40,80,and 160μg·L-1,suggesting that the catalase levels in planarians could represent a biomarker for estimation of copper effects in freshwater ecosystem[4].The effects of UV-irradiation to two planarian species were also deter mined,and UV-rays caused obvious alterations of mortality,behavior,and morphology in each species[5]. In addition,the number of neoblasts extremely increased in the area of damaged tissue,immediately after UV irradiation[5].Hypertonic osmotic shock treatment could induce the temporarily increase of putrescine and spermidine levels[6].Moreover,the genes required for autophagy can function at the interface between survival and cell death during stress-inducing processes like regeneration and starvation in sexual and asexual races of planarians[7].
The effects of low temperature on reproduction and regeneration have been investigated in planarians. The regeneration of planarianswas significantly retarded for all levels by lowering the temperature[8].Vowinckel(1970)showed that exposure to low water temperatures stimulated the increase in germ cells associated in testicular primordial and that this activity could be transmittable via homogenates[9]. In addition,the putrescine and sper midine levels could be temporarily increased after cold-shock treatment in planarians[6].Nevertheless,the possible effects of heat-shock on the planarian development are still largely unclear.
Considering the fact that complex carbohydrates,such as lipid and glycogen,are involved in multiple biological processes in organis ms,the first objective of this study was to investigate the effects of heat-shock on development of planarians,and the second objective of this study was investigate the alterations of lipid and glycogen in heat-shock treated planarians.Moreover,the possible associations of developmentwith lipid or glycogen storage were examined in heat-shock treated planarians.
1 Materials and methods
1.1 An i mals
The planarians(Dugesia japonica)were collected from the stream in the ZijinMountain,Nanjing,in 2009.The planarians were asexually maintained in autoclaved water at 22℃,and fed with chicken liver twice a week.Planarianswere starved formore than 1 week prior to the experiments.The planarianswere treated with heat-shock at 35℃for 16,20,24,28,and 32 h.The planarians with the approximately same size(5 mm length)were used for assay of development and accumulation of lipid and glycogen.
1.2 Body weight
To calculate the average body weight of planarians,10 animalswere weighed after the water on the surface of animals was cleaned with filter paper.The experiments were repeated six times.
1.3 Body length
The midline length of planarians was examined to represent the body length.The experiments were repeated six ti mes.
1.4 Lipid staining
A modified sudan black B staining method was used to evaluate the lipid accumulation in parenchymal cells as previously described[10].Planarians were fixed in Bouin's solution for 8 h,and the frozen sections were cut at 8~12μm using freezing microtome(Leica CM1900,Germany).The sections were stained with 10 mg·ml-1sudan black B solution,and the stained lipid droplets were dark blue.The diameters of stained lipid droplets were calculated to reflect the size of lipid droplet,and the experiments were repeated at least three times.
1.5 Glycogen staining
The periodic acid-schiff(PAS)method for glycogen staining in parenchymal cells was performed as previously described[11].Planarians were fixed in Bouin's solution for 8 h,and the frozen sections were cut at 8~12μm using freezing microtome(Leica CM1900,Germany).Sections were treated with 0.5% periodic acid for 10 min,rinsed in water for 10 min,and stained with schiff reagent for 10 min.Moreover,the sections were rinsed with sodium pyrosulfite for 2 min,washed in water for 5 min,and dehydrated and mounted in neutral balsam.Control sections were pretreated with a-amylase.The stained glycogen was red color in the cytosol.The intensities of stained glycogen were examined,and the experimentswere repeated at least three times.
1.6 Statistical analysis
All data in this article were expressed as means±S.D.Graphs were generated using Microsoft Excel(Microsoft Corp.,Redmond,WA).One-way analysis of variance(ANOVA)followed by a Dunnett'st-test was used to deter mine the significance of the differences bet ween the groups.The probability levelsof0.05 and 0.01were considered statistically significant.The associations of bodyweight with accumulation of lipid or glycogen,as well as the associations of body length with accumulation of lipid or glycogen,were examined with the method of linear regression in heat-shock treated planarians.
2 Results
2.1 Effects of heat-shock on body weight of planari ans
In the present study,the development of planarians was evaluated by the endpoints of body weight and body length.We first investigated the effects of heat-shock on bodyweight.As shown in Fig 1,after heat-shock(35℃)treat ment for different ti me intervals(16,20,24,28,and 32 h),the body weights of heat-shock treated planarians were all significantly(P<0.01)decreased compared with those in control planarianswithout heat-shock treat ment.
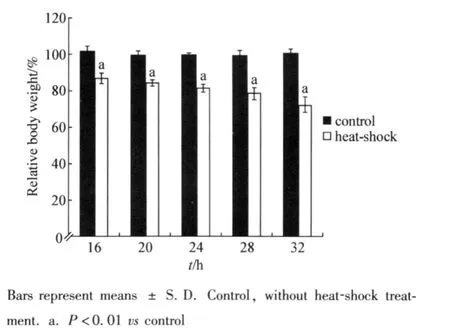
Fig 1 Effects of heat-shock on body weight of planari ans
2.2 Effects of heat-shock on body length of planari ans
We next examined the effects of heat-shock on body length.As shown in Fig 2,similarly,after heat-shock treatment for different time intervals,the body lengths of heat-shock treated planarians were also all significantly(16 h,P<0.05;20,24,28,and 32 h,P<0.01)decreased compared with those in control planarians without heat-shock treatment. Therefore, treatment with the stress of heat-shock can obviously suppress the development of planarians.
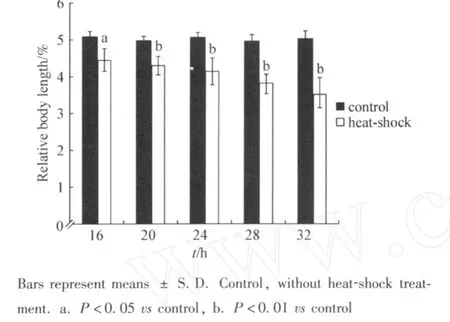
Fig 2 Effects of heat-shock on body length of planarians
2.3 Effects of heat-shock on lipid accumulation in planarians
Again,we examined the effectsof heat-shock treat ment on lipid accumulation in planarians.As shown in Fig 3A,compared with the lipid accumulation in control planarians without heat-shock,heat-shock treat ment resulted in the sharp decrease of number of lipid droplets in parenchy mal cells.Moreover,after heat-shock treat ment for different ti me intervals,the relative size of lipid droplets in parenchy mal cells of heat-shock treated planarianswere significantly(P<0.01)reduced compared with those in control planarians without heat-shock treat ment(Fig 3B).Therefore,treat ment with the stress of heat-shock will inhibit the lipid accumulation in parenchy mal cells of planarians.
2.4 Effects heat-shock on glycogen accumulation in planarians
Considering the fact that glycogen is another important carbohydrate in living organisms,we further investigated the effects of heat-shock treatment on glycogen accumulation in planarians.As shown in Fig 4,after heatshock treatment for different time intervals,the relative intensities of labeled signals for glycogen in parenchy mal cellswere significantly(P<0.01)decreased compared with those in control planarians without heat-shock treatment.Therefore,treat ment with the stress of heat-shock will also suppress the glycogen accumulation in parenchymal cells of planarians.
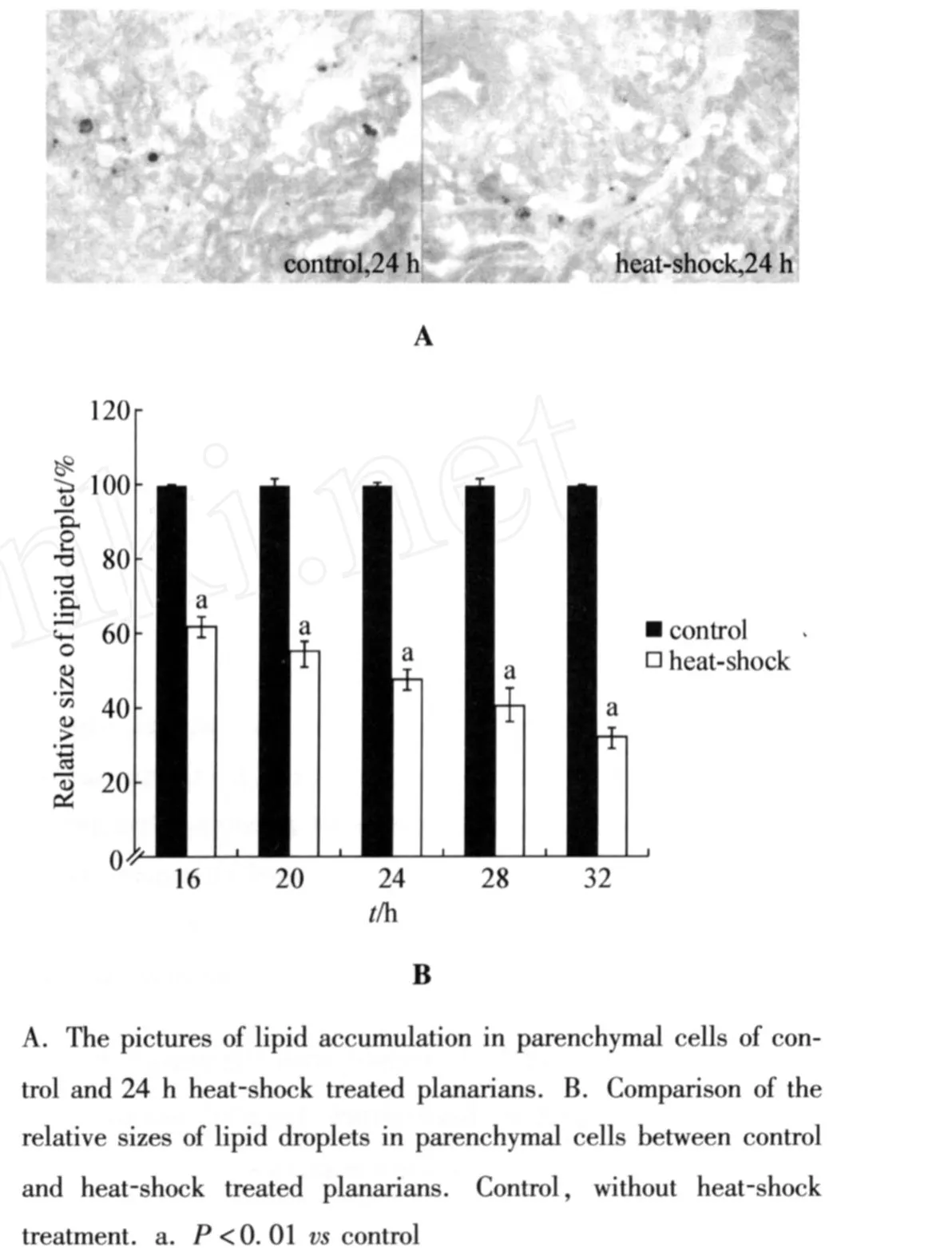
Fig 3 Effects of heat-shock on lipid accumulation in planar ian s
2.5 Associ ations of development with the accumulation of lipid or glycogen i n heat-shock treated planari ans
Linear regression analysis further suggests that the bodyweightwas significantly(P<0.01)correlated with the relative size of lipid droplet(R2=0.787),and the relative intensity of glycogen signal(R2=0.814)in heatshock treated planarians(Tab 1).Si milarly,the body length was significantly(P<0.01)correlated with the relative size of lipid droplet(R2=0.801),and the relative intensity of glycogen signal(R2=0.884)in heatshock treated planarians(Tab 2).Therefore,the close association bet ween the development and the accumulation of lipid or glycogen exists in heat-shock treated planarians.
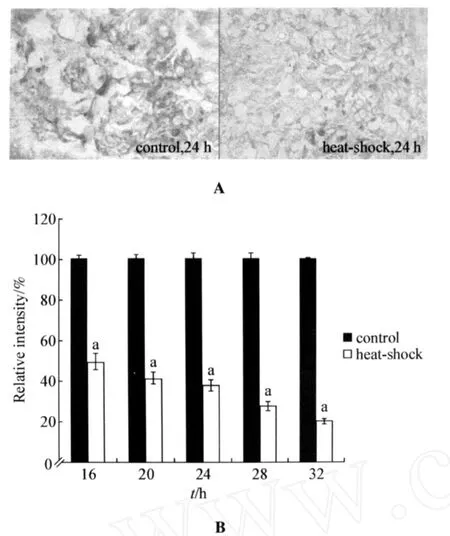
Fig 4 Effects heat-shock on glycogen accumulation i n planari ans
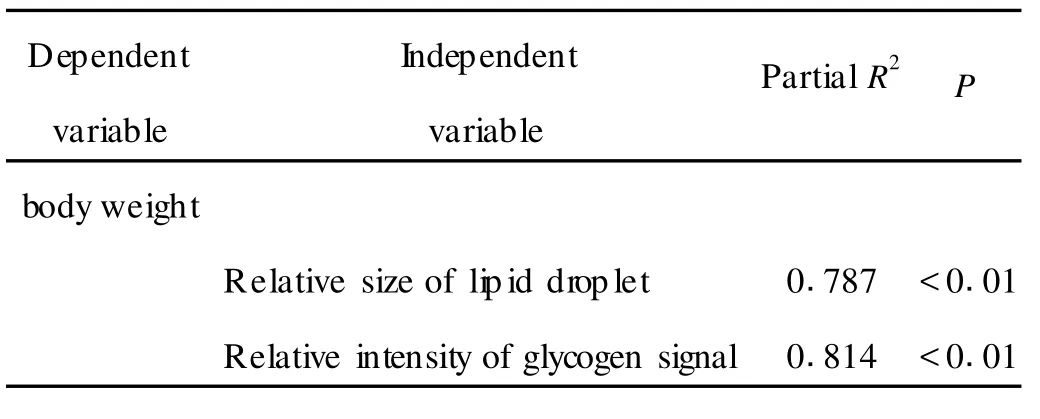
Tab 1 Associations of body weight with the accumulation of lipid or glycogen in heat-shock treated planari ans as assayed by linear regression analysis
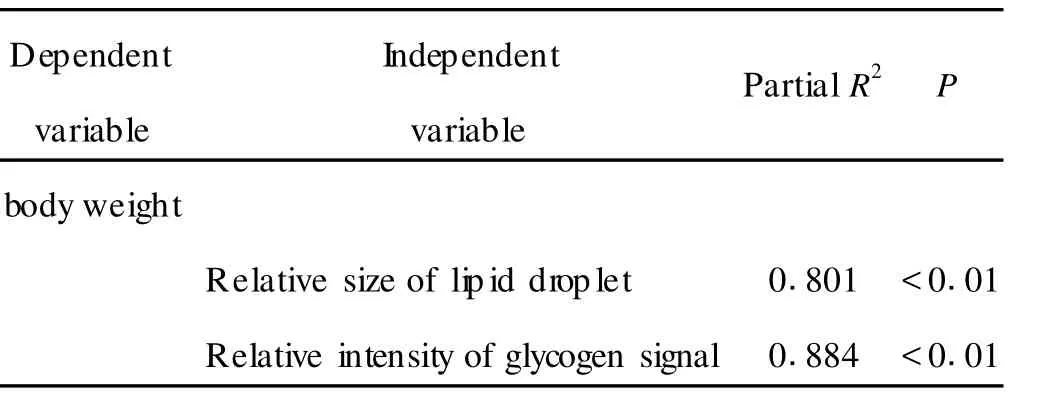
Tab 2 Associ ations of body length with the accumulation of lipid or glycogen in heat-shock treated planari ans as assayed by linear regression analysis
3 Discussion
Previous studies have shown that several kinds of stresses can induce the adverse alterations of development and reproduction in planarians[3-7].Our data in the currentwork further suggest that exposure to heat-shock can also cause the defects of development in planarians.Heat-shock treat ment for different time intervals caused the significant decrease of body weight compared with those in control planarians(Fig 1).Similarly,heat-shock treatment for different ti me intervals resulted in the significant decrease of body length compared with those in control planarians(Fig 2).These data demonstrated that the deficit in development will be for med in planarians exposed to severe heat-shock.That is,both the heat-shock and the cold-shockwill exert adverse effectson the development of planarians.Previous study has also demonstrated that the induction of stress protein HSP60 could be detected in planarians after 24 h treatment at 27℃[4],implying the severe stress response may be for med in heat-shock treated planarians.
Our data further demonstrated that exposure to the severe heat-shock will influence the accumulation of lipid and glycogen.We mainly examined the alterations of lipid and glycogen in parenchymal cells of planarians.The reason is that,in planarians,parenchymal cells are located between the epidermal and intestinal cells,and these parenchymal cells are primarily composed of neoblasts,which will account for approximately 30%of the total planarians cells[12].Treatment with the stress of heat-shock inhibited the lipid accumulation in parenchymal cells of planarians as reflected by the sharp decrease of lipid droplet number and reduction of relative size of lipid droplets(Fig 3).In addition,treat ment with the stress of heat-shock also suppressed the glycogen accumulation in parenchymal cells of planarians as revealed by the decrease of relative intensitiesof labeled signals for glycogen(Fig 4).Considering the fact that the lipid and glycogen are main sources for energy supply in animals,our data strongly imply that the functions of parenchymal cellsmay be impaired,and the reduction of lipid and glycogen accumulation may be at least one of the main reasons for the induction of developmental defects in heat-shock treated planarians.
Our data further provide the evidence to support such a notion that the accumulation of lipid and glycogen in parenchymal cells is essential for the planarian development.As revealed by the linear regression analysis,the bodyweight was significantly correlated with the relative size of lipid droplet and the relative intensity of glycogen signal in heat-shock treated planarians(Tab 1).Moreover,the body length was also significantly correlated with the relative size of lipid droplet and the relative intensity of glycogen signal in heat-shock treated planarians(Tab 2).Therefore,heat-shock treatmentmay influence the planarian development by largely altering the accumulation of lipid and(or)glycogen in parenchymal cells.
Previous study has further suggested that the autophagy plays an essential role in the remodeling of the organis m that occurs during regeneration,providing the necessary energy and building blocks to the neoblasts for cell proliferation and differentiation[7]. In addition,evidence have been raised that the accumulation of lipid and glycogen reserves in the parenchymal and gastrodermal cells precedes the aggregation of undifferentiated mitotically active neoblasts beneath the wound after regeneration[13].Our study strongly imply that the autophagy may be involved in the regulation of both the accumulation of lipid and glycogen in parenchymal cells and the aggregation of undifferentiated mitotically active neoblasts during regeneration in planarians.
Acknowledgements This work was supported by the grants from the National Natural Science Foundation of China(No.30870810)and the Program forNew Century Excellent Talents in University.
[1]SáNCHEZ-ALVARADO A,TSON IS PA.Bridging the regeneration gap:genetic insights from diverse animal models[J].Nat Rev Genet,2006,7:873-884.
[2]ZHANG Y F,YEB P,WANG,D Y.Molecular actions guiding neural regeneration in planarian[J].Neurosci Bull,2008,24:329-337.
[3]L IM.Effects of nonionic and ionic surfactants on survival,oxidative stress,and cholinesterase activity of planarian[J].Chemosphere,2008,70:1796-1803.
[4]GUECHEVA T N,ERDT MANN B,BENFATO M S,et al.Stress protein response and catalase activity in freshwater planarian Dugesia(Girardia)schubarti exposed to copper[J].Ecotoxicol Environ Safety,2003,56:351-357.
[5]KALAFATIC’M,KOVACEV IC’G,FRANJEV IC’D.Resistance of two planarian species to UV-irradiation[J].Folia Biol,2006,54:103-108.
[6]HAMANA K,HAMANA H,SH I NOZAWA T.Alterations in polyamine levels of nematode,earthwor m,leech and planarian during regeneration,temperature and osmotic stresses[J].Comp Biochem Physiol B Biochem Mol Biol,1995,111:91-97.
[7]GONZALEZ-ESTEVEZ C,FEL IX D A,ABOOBAKER A A,et al.Gtdap-1 promotes autophagy and is required for planarian remodeling during regeneration and starvation[J].Proc Natl Acad Sci USA,2007,104:13373-13378.
[8]AGNES,BR?NDSTED H V.Influence of temperature on rate of regeneration in the time-graded regeneration field in planarians[J].J Embryol Exp Morph,1961,9:159-166.
[9]VOW I NCKEL C.The role of illumination and temperature in the control of sexual reproduction in the planarian Dugesia tigrina(Girard)[J].BiolBull,1970,138:77-87.
[10]ACKERMAN GA.A modification of the sudan blackB technique for the possible cytochemical demonstration of masked lipids[J].Science,1952,115:629-631.
[11]HOLMES J H G,ASHMORE C R,ROB I NSON D W.Effects of stress on cattle with hereditary muscular hypertrophy[J].J Ani m Sci,1973,36∶684-694.
[12]KOBAYASH I K,HASH IGUCH I T,ICH IKAWA T,et al.Neoblast-enriched fraction rescues eye for mation in eye-defective planarian‘menashi'Dugesia ryukyuensis[J].Dev Growth Differ,2008,50:689-696.
[13]BOWEN ID,DEN-HOLLANDER J E,LEW IS G H.Cell death and acid phosphatase activity in the regenerating planarians Polycelis tenuis Iijima[J].Differentiation,1982,21:160-167.
 東南大學(xué)學(xué)報(bào)(醫(yī)學(xué)版)2010年1期
東南大學(xué)學(xué)報(bào)(醫(yī)學(xué)版)2010年1期
- 東南大學(xué)學(xué)報(bào)(醫(yī)學(xué)版)的其它文章
- 汞暴露導(dǎo)致秀麗線蟲后代中出現(xiàn)可傳遞的表型和行為缺陷
