Micro-aluminum powder with bi-or tri-component alloy coating as a promising catalyst: Boosting pyrolysis and combustion of ammonium perchlorate
Cho Wng , Ying Liu , Mingze Wu , Ji Li , Ying Feng , Xinjin Ning , Hong Li ,Ningfei Wng , Bolu Shi ,*
a School of Aerospace Engineering, Beijing Institute of Technology, Beijing 100081, China
b School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, China
c State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China
Keywords:Micro-aluminum powder (μAl)Nano-sized alloy coating Combustion catalyst Ammonium perchlorate Pyrolysis behavior Ignition and combustion
ABSTRACT A novel design of micro-aluminum (μAl) powder coated with bi-/tri-component alloy layer, such as: Ni-P and Ni-P-Cu (namely, Al@Ni-P, Al@Ni-P-Cu, respectively), as combustion catalysts, were introduced to release its huge energy inside Al-core and promote rapid pyrolysis of ammonium perchlorate(AP)at a lower temperature in aluminized propellants.The microstructure of Al@Ni-P-Cu demonstrates that a three-layer Ni-P-Cu shell, with the thickness of ~100 nm, is uniformly supported by μAl carrier(fuel unit), which has an amorphous surface with a thickness of ~2.3 nm (catalytic unit).The peak temperature of AP with the addition of Al@Ni-P-Cu (3.5%) could significantly drop to 316.2 °C at hightemperature thermal decomposition, reduced by 124.3 °C, in comparison to that of pure AP with 440.5 °C.It illustrated that the introduction of Al@Ni-P-Cu could weaken or even eliminate the obstacle of AP pyrolysis due to its reduction of activation energy with 118.28 kJ/mol.The laser ignition results showed that the ignition delay time of Al@Ni-P-Cu/AP mixture with 78 ms in air is shorter than that of Al@Ni-P/AP (118 ms), decreased by 33.90%.Those astonishing breakthroughs were attributed to the synergistic effects of adequate active sites on amorphous surface and oxidation exothermic reactions(7597.7 J/g) of Al@Ni-P-Cu, resulting in accelerated mass and/or heat transfer rate to catalyze AP pyrolysis and combustion.Moreover, it is believed to provide an alternative Al-based combustion catalyst for propellant designer, to promote the development the propellants toward a higher energy.
1.Introduction
Solid composite propellants(SCPs)are considered as one of the most crucial propulsion energy sources and working medium of solid rocket motors (SRMs) via combustion in aerospace industry(rocket launching and space vehicles carrying etc.)[1-5].Generally SCPs are a solid heterogeneous mixture of metal fuel, binder,oxidizer,combustion catalyst and so on[6,7],such as a classical tripropellant formula of Aluminum (Al)/Hydroxyl-Terminated Polybutadiene (HTPB)/Ammonium Perchlorate (AP).Among these components in SCPs,AP is the most widely used oxidizer with the dosage of 65%-85%of the total mass of propellants[8],owing to the advantages of its high effective oxygen content and large combustion gas production.And it is important to note the thermal decomposition behavior [9], including high-temperature thermal decomposition (HTD) temperature, heat release, and apparent activation energy (Ea), of AP directly determines the combustion performance of the SCPs[2,10],that is,burning rate(r),burning rate pressure exponent (n) and Al agglomeration at burning surface[11-13].Thus,a vital problem is how to better regulate and control the r of SCPs via a small amount of (commonly, with ~1 wt%)combustion catalyst added in SCPs.
To meet the increasing demand, nano-size catalyst has been largely developed owing to its short contact distance and large specific surface area [2,4,8,10,14-19].The mostly widely used combustion catalysts toward thermal decomposition of AP focus on nano-size transition metal oxides(TMO)[8],such as Fe2O3[11,20],CuO[21-23],TiO2[19],ZnO[24],Co3O4[14],Mn3O4[25],CuCo2O4[14], ZnCo2O4/ZnO [26] and so on, which could reduce the temperature of AP by 80-160°C at HTD.Such excellent catalytic activity may be attributed to their high specific surface area and intriguing unfilled d-electron orbitals, which ensure a beneficial chemical environment to promote electron transfer and to accelerate the process of intermediates adsorption/desorption [9,13].However, there are several obvious shortcomings in TMO due to their nanoscale oxidation states,such as non-energetic feature[16],easy agglomeration [27], and poor formability [28], which make them difficult to achieve large-scale promotion in practical application.
Nowadays, aiming to higher energy density, there is a trend to develop high-energy solid composite propellants (HESCPs) to obtain higher specific impulse (Is) [3].Nanoscale non-oxidative pure metal [8,12,29-31], alloy [29,30,32], carbon based active catalysts[16,33-37],and AP-based composite particles[17,38,39]used as combustion catalysts in SCPs have also attracted the interest of solid propellant designers.Fig.S1 (see in the supplementary material) presents the detailed comparison results of the above catalysts for AP pyrolysis parameters.And the classification of substances, abbreviations as well as references were listed in Table S1.
Via the comparative analysis of Fig.S1, several findings are summarized as follows: (1) Compared with nano aluminum (nAl)powder, nano-sized pure transition metal (TM) particles can significantly reduce the temperature of AP at HTD, but the combustion enthalpy change (ΔH) of those composites with combustion catalyst is around 1000 J/g; (2) Compared with alloy particles without copper (Cu), nano-size Cu alloy particles can further dramatically reduce the temperature of AP by ~140°C at HTD, yet the ΔH for those is equivalent to that of pure TM particles in(1);(3)AP based composite energetic materials can greatly reduce the HTD temperature of AP,in which they are used as energetic materials via a small amount of micro-sized Al(μAl)powder additives.However,the ΔH of those compounds is unable to be significantly improved,resulting from that AP accounts for a large proportion of those compounds; (4) Although carbon-based active catalysts can synergistically reduce the HTD temperature of AP (40-135°C) and significantly increase the ΔH (1500-4000 J/g) when compared with those of pure TM in (1), alloy particles in (2) and AP based energetic composites in (3) as combustion catalyst, the easy agglomeration, poor formability, and low volumetric energy density still remain to be solved.
Hence,those advantages can be integrated,such as:the addition of nAl and/or μAl powder could be used as fuel,nano-sized Cu alloy is able to conduct as combustion catalyst, and the compounding method of carbon-based active catalyst can be adopted[2,40-45].A core-shell energetic combustion catalyst (CSECC), included μAlcore and Ni-P-Cu shell, can be designed cooperatively combined with μAl powders and Ni-P alloy shell doped with element Cu,which provides energetic feature and catalytic function for AP pyrolysis.Since μAl powder, commonly used as a metal fuel component in SCPs[46,47],has a higher combustion heat with 30.96 kJ/g and high thermal chemical reactivity,besides,excellent flowability,abundance in earth and so on.
In this way,the micro-sized catalyst is conducive to improve the process properties of propellant manufacturing, and avoid the agglomeration caused by the high specific surface area of nanosized combustion catalyst that may lead to poor mix of the numerous components of SCPs.Moreover, it will also prolong the curing time of SCPs, and contribute to the casting of SCPs [16,48].The μAl powders with nano-sized ternary alloy coating are able to improve the ignition and/or combustion properties of this catalyst,and furthermore,the triggered reactions by multi-interfaces would regulate the heat and mass transfer efficiencies during burning,and also elevate the energy release level and rate.Such composited catalyst would significantly reduce the HTD temperature and improve decomposition reaction rate of AP at HTD.Besides,it might inhibit the Al powders agglomeration at the burning surface of aluminized SCPs[1,49-52].It would further improve the density of SCPs as well as reduce the sensitivity of SCPs,such as impact and/or friction sensitivity, and improve the safety of propellant in manufacturing, storage, transportation and application [43,53].Thus, in this study an effort has been devoted to enhancing the catalysis of AP pyrolysis, as well as improving the ignition and/or combustion characteristics through the strategy of μAl powder with Ni-P-Cu coating.Thus,when the nano-sized ternary shell is used as catalyst, the energetic combustion catalyst not only meets the high catalytic reactive activity and easier ignition ability, but also yields violent 'micro-explosion' phenomenon during burning.
This work is organized through the following parts: firstly, a multi-component heterogeneous core-shell energetic combustion catalyst (CSECC), Al@Ni-P-Cu, was fabricated via a chemical method of ‘one-step’ in-situ electroless plating.After that, its micro-structured analysis, morphological and compositional characterizations was conducted to confirm the functional structure with catalytic activity.And the next, thermal analysis was used to estimate the catalytic properties,energy release characteristics,and then to obtain the activation energy of AP pyrolysis with the addition of catalysts.Finally, systematic on-line ignition/combustion diagnosis and off-line characterization were conducted to analyze the ignition/combustion behaviors, including ignition delay time,agglomeration of Al powders in AP burning gas flowing and pyrolysis rate of AP in the air atmosphere,as well as condensed combustion deposits, respectively.
2.Materials and experimental methods
2.1.Raw materials
The relevant raw materials for the preparation method of Al@Ni-P-Cu was same as our previous work [54].In this experimental investigation, other chemical reagents, such as: copper sulfate pentahydrate (CuSO4·5H2O), Hydrazine hydrate(N2H4·H2O), sodium hydroxide (NaOH), anhydrous ethanol, and acetone, were purchased from Aladdin.In addition, ammonium perchlorate(AP,class III,globular)with the size of ~150 μm,which is commonly used as oxidant in solid propellants,was supplied by Dalian Gaojia Chemical International Industry and Trade Co., Ltd.,China.The micromorphology of AP particles could be found in supplementary material (SEM image in Fig.S2).All chemicals and deionized water were pure analytes.
2.2.Fabrication process of control groups
The preparation method of Al@Ni-P-Cu had been detailly reported in our previous work [54].In brief, the Al@Ni-P-Cu (coreshell energetic combustion catalyst: CSECC) was fabricated via a pragmatic method of 'one-step' in-situ electroless plating.Here, a detailed introduction was given to the preparation method for obtaining control groups.On the one hand,to transform that CSECC composites into a more stable core-shell material, Al@Ni-P-Cu(Annealing) were obtained through a method of annealing,resulting from a slight intermetallic reaction at its interface,and the corresponding treatment method is described in Text S1.Thus, it could be used to better investigate the effect of surface crystalline transformation as well as interfacial intermetallic reactions on catalytic properties of AP pyrolysis.
On the other hand, to precisely compare with CSECC and find out the catalytic active sites,most hollow structured Air@Ni-P-Cu were prepared by etching Al-core of Al@Ni-P-Cu compounds in the 8.0 mol/L NaOH solution for 7 days at room temperature under magnetic stirring.In addition, to identify the catalytic active element that promotes thermal decomposition of AP, the nanocrystal Cu particles were prepared via a method of one step liquid phase reduction, labeled as nCu.In detail, N2H4·H2O (50 mL, as reducing agent) was quickly poured into CuSO4·5H2O solution(5.0 g/L, source of Cu), with volume of 500 mL, and reacted for 30 min under magnetic stirring.Then, the methods of drying and collection after filtration were consistent with the above mentioned.The morphology, composition, and structural characterization of the material are the same as previously reported methods [54,55].
2.3.Thermal analysis
Non-isothermal thermogravimetry (TG) and differential scanning calorimetric (DSC) tests were used to measure the mass loss and heat flow during AP pyrolysis with different as-prepared catalysts, as well as the oxidation process of prepared samples, conducted by thermal analyzer (TGA/DSC3+, METTLER TOLEDO).The DSC base lines were calibrated for 3 times without sample under the same condition.
Aiming to investigate the catalytic effect of the as-prepared catalysts on the thermal decomposition of AP, pure AP as well as mixtures of AP/catalysts with different weight ratios were accurately weighed via this thermal analysis instrument.And then mixed and ground carefully for 30 min, in a 70 μL corundum crucible with central hole on the cover.According to the practical application of solid propellant, the common mass fraction of combustion catalyst is 1.0% [2,10].Thus, among those binary mixtures of AP/catalyst,the mass content of non-energetic combustion catalyst is 1.0% in this work, including: raw μAl, nCu and hollow structured Air@Ni-P-Cu, yet that of energetic combustion catalysts is 3.5% to guarantee the ratio of catalyst to oxidizer in propellant is consistent with non-energetic case, due to their energetic constituent of Al-core, such as Al@Ni-P, Al@Ni-P-Cu and Al@Ni-P-Cu(Annealing).Here,based on our previous work about Al@Ni-P [55], Al@Ni-P-Cu and Al@Ni-P-Cu (Annealing) energetic composite[54],it exhibited outstanding advantages of shorter ignition delay time and higher heat release rate compared with that of raw μAl powders.Thus,this sample was also used to investigate the catalytic effect of element Cu doped in the Ni-P shell.
Typically, Al@Ni-P-Cu with 1.2000 mg (3.5 wt%) and pure AP with 33.0840 mg were accurately weighed by thermal analyzer and mixed them more thorough, since the thermal analysis (TGA/DSC3+,METTLER TOLEDO)experimental platform can yield precise mass measurement;then,this mixture(total mass of 34.2840 mg)was measured in the thermal analyzer to obtain TG/DTG-DSC curves; and the control group of pure AP without catalyst was obtained by this same method.The carrier gas was high-purity argon (Ar, 99.999%) under the flow of 50 mL/min, and the testing temperature range and heating rate were 30-500°C and 10°C/min,respectively.Besides,for the calculation of apparent activation energy (Ea) with introduction of combustion catalyst in AP particles,the heating rate was chosen as 5°C/min,10°C/min,15°C/min and 20°C/min.As for the oxidation process of prepared samples,this investigation is consistent with the previous work on Al@Ni-P composites[55].
2.4.On-line diagnosis of ignition and combustion performances
The ignition platform contained five parts: high-pressure combustion chamber noumenon (HPCC, volume of ~40 L, limiting pressure with 20 MPa)used in previous investigation[54];the laser ignition unit; atmosphere control suite; combustion diagnosis module; and data acquisition package, as illustrated in Fig.1.The 1064 nm diode pumped solid state laser (MFSC-10) generated a laser with energy of 50 W.Then,the mixing samples were directly heated by radiation until ignited in the HPCC via window,with the 1000 ms of the laser pulse.
Aiming to distinguish the delicate morphology of catalytic metal particles during binary mixing AP/catalyst burning, it was examined at an image resolution of 512×600 pixels via the high-speed camera(Phantom VEO 410L)viewing through a high magnification video microscope(Beijing PDV Instrument Co.,Ltd.,Beijing,China)with front installation of a narrow-band optical filter (532 nm),where the acquisition rate and exposure time of each frame were set to 10,000 frame per second (fps) and 4 μs, respectively.The emission spectrums were record simultaneously by an optical fiber spectrometer(Ocean Optics,USB 2000+spectrometer,USA)with a sampling interval time of 1 ms and an integration time of 5 ms.It is noted that the sample was fabricated by mixing raw Al or asprepared metal particles with AP to aid ignition (mass ratio of 1:1), adding an appropriate amount of acetone, and then fully,physically mixing them together.In each test, approximately 100 mg of the sample was stored in a 70 μL corundum crucible under air atmospheric (0.1 MPa),and then ignited by the laser.
To further evaluate the propulsion and AP pyrolysis rate of binary Al-based composites/AP mixtures during laser ignited burning,the mixed Al@Ni-P/AP or Al@Ni-P-Cu/AP samples were loosely packed into a greater volume of transparent reactor with 10 mL in the air atmosphere, which was vertically sticked on an upward force sensor (SBT904, Guangzhou Sparto Electronic Technology Co., Ltd., Guangzhou, China).The weight of mixtures was~200 mg with the mass ratio of 1:1, and the thrust with time was recorded by that force sensor.The sampling rate, resolution, and exposure time of high-speed camera with a lens magnification factor of two (Macro × 2; 100 mm; f/2.8), were set to 2000 fps,1280×600 pixels,and 50 μs,respectively.Here,the ignition delay time(tig)is defined as the time difference from the beginning of the laser signal to the initial high-speed video camera signal of bright spots due to the ignition of mixture.In addition, all experiments were synchronously operated via a home-written program in LabVIEW 2020(National Instruments),equipped with NI USB-6221.And all ignition and combustion experiments were repeated three times under each operating condition.
3.Results and discussion
3.1.Morphology and microstructure characterization
The schematic illustration of the fabrication process via the method of electroless plating and coating mechanism involving multi-step chemical reactions for CSECC (Al@Ni-P-Cu), the micromorphology, and surface elemental distributions of four asprepared samples, are presented in Fig.2.
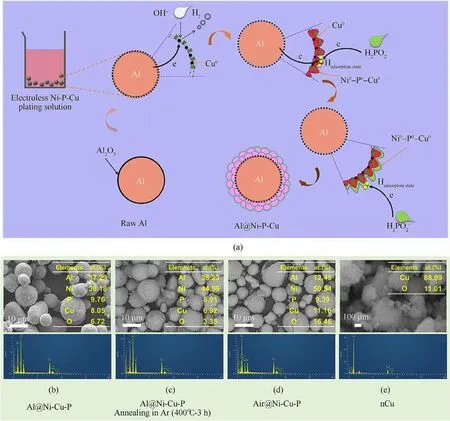
Fig.2.(a) Schematic of the coating process for CSECC; (b)-(e) SEM images ( × 2000) and the corresponding data from EDS spectrum of as-prepared samples.
The chemical reaction mechanism of μAl powder coated with Ni-P-Cu alloy might involve three steps(Fig.2(a)).In the first step,the outward dense alumina (α-Al2O3: amphoteric oxide) shell of μAl powder is chemically etched quickly, and then dissolved to yield‘wedge-shaped’Al2O3in a heated alkaline solution(T=80°C and pH = 9.0), due to the existence of crystal defects and thinner thickness in the alumina shell.Thus, the sectional exposed high reactive Al would react with OH-to release hydrogen,resulting in vast bubbles during the fabrication.Simultaneously, as for the mechanism of electroless plating[56],the Cu2+in the solution will be gradually reduced by that active Al atom,and then slowly coated on the residual 'wedge-shaped' alumina shell.It would form the innermost rich-Cu layer of the tri-shelled Ni-P-Cu anchored by Alcore, which also plays a role of catalytic nucleation sites.In the second step,with the decrease of Cu2+concentration in the plating solution as well as the increase of the thickness of rich-Cu layer,the electrons will be synergistically provided from adsorption state H(Hadsorptionstate) generated from NaH2PO2and active Al, and then,the Cu2+and Ni2+in the plating will be together reduced by that electron flowing to from initial co-deposited Ni-P-Cu layer[57].In the third step,due to the increased thickness of Ni-P-Cu layer and the further decreased concentration of Cu2+and Ni2+in the primary solution, the reactive Al atoms also no longer provides electron to reduce the Cu2+and Ni2+,and only the Hadsorptionstatein the NaH2PO2could provide electrons to form a co-deposited ternary Ni-P-Cu layer with higher content of Ni.
Finally, the outermost nano-sized amorphous oxide layer, as presented in the results of subsequent precise TEM characterization(Fig.4),is probably formed from the heated alkaline plating and the suddenly artificial termination of chemical reaction.The involved reactions of Cu2+and Ni2+reduced by active Al atom of Al-core(R1 and R2)as well as hypophosphite(R3)can be expressed as follows[57]:
The SEM images of Al@Ni-P-Cu,Al@Ni-P-Cu(annealing)and Air@Ni-P-Cu, as shown in Figs.2(b)-2(d) successively, indicate that the diameter of the Al coated with Ni-P-Cu alloy layer is still close to the size of the virgin Al powders with about 15 μm.The EDS results (Figs.2(b) and 2(c)) of Al@Ni-P-Cu and Al@Ni-P-Cu(annealing)demonstrates that the content of Al is relatively close to each other,which are 37.29 at%and 38.23 at%,respectively;and the contents of other elements, such as Ni, P, Cu and O, show a slight difference.Moreover, the EDS results (Fig.2(d)) of Air@Ni-P-Cu show that the content of Al significantly decreases to 12.45 at%,indicating that the internal Al-core almost disappears and a hollow structure has been formed, just like the empty shell observed in SEM morphology.Besides, SEM image of the prepared nano Cu particle(Fig.2(e)),shows that its size distribution is in the range of 100-500 nm and appears a phenomenon with partial agglomeration, due to the strong surface tension of particles.And the EDS results also manifests that there is a small amount of element O on the surface of Cu particles due to its oxidation in solution.
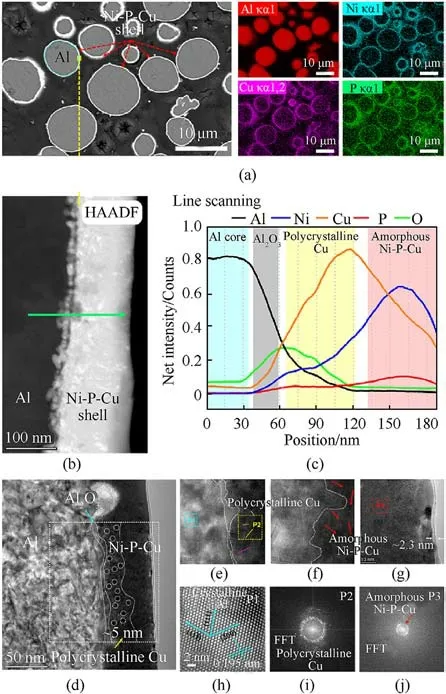
Fig.4.Microstructure characterizations of CSECC: (a) BSE-SEM image and the corresponding EDS mappings(Al,Ni,Cu and P);(b)STEM-HAADF image at the local edge of core-shell structure(Fig.4(a));(c)Line scanning profiles(Al,Ni,Cu,P and O)across the straight green line with an arrow (Fig.4(b)); (d)-(g) Detailed HRTEM micrographs at the interfaces/surface.(h)-(j) The fast Fourier transform (FFT) patterns located at P1,P2 (Fig.4(e)), and P3 (Fig.4(g)).
The XRD patterns of those samples (Fig.3) indicate that the Al@Ni-P-Cu is consists of virgin Al (PDF#04-0787) and slight amorphous Ni-P-Cu, due to a certain extent of broadening diffraction peak at 2θ ≈44°.And there are diversified intermetallic compounds in Al@Ni-P-Cu (annealing) after it annealed in Ar atmosphere,such as Al1·1Ni0.9(PDF#44-1187),Cu0·81Ni0.19(PDF#47-1406)and Ni3P(PDF#34-0501),due to the diffusion of atoms in the interfaces.Moreover, pure metals (Ni (PDF#04-0850) and Cu(PDF#04-0836)) are precipitated in the shell through crystallization transformation.In addition,it could be found that the intensity of the characteristic diffraction peak (2θ ≈44°), corresponding to Al,significantly decreases and the amorphous region widens more obviously when the Al-core of Al@Ni-P-Cu particles are etched in NaOH solution for 7 days,in accordance with the above SEM results.It indicates that their inner Al-core have been nearly completely corroded and the bonding is considerably tight between Ni-P-Cu coating and Al-core.Moreover, there is also small amount of Cu2O(PDF#34-1354) in the nano-sized Cu particles, which is consistent with the above EDS results.
To further analyze their internal detailed microscopic composition and structure,the cross-sectional morphology and elemental distribution of Al@Ni-P-Cu particle swarm were taken advantage of a method with the preparation of metallographic bulk sample(particles embedded by resin,grinded and then polished),as shown in Fig.4(a).Based on the above surface observation and phase composition of samples,The SEM results of multiple Al@Ni-P-Cu particles show that Al is uniformly and fully coated by elements of Ni,Cu and P,but the details inside the Ni-P-Cu shell could not be distinguished owing to the extremely thinner shell in comparison to the size of Al powder, which coincided with the results in Figs.2(b) and 2(d).Thus, it is indispensable to use precising and profound TEM characterizations to expound the microstructure and composition of the Al@Ni-P-Cu.
A slice sample, from randomly selected single Al@Ni-P-Cu particle prepared via focus ion beam (FIB), was used to deeply dissect the microscopic atomic arrangement and element distribution of Ni-P-Cu shell(Figs.4(b)-4(j)).The STEM-HAADF image(Fig.4(b)) of selected local core-shell area clearly shows that the Ni-P-Cu shell of Al-core is a multi-layered composite coating with the thickness of ~100 nm.The line scanning result (Fig.4(c)) indicates the concentration gradient distribution of five elements(Al,Ni, Cu, P and O) at the interfaces in the scanning direction with a green arrow(Fig.4(b)).Meanwhile,these elements are overlapping with each other in the direction of scanning in the STEM-HAADF image.
The high-resolution TEM (HRTEM) and fast Fourier transform(FFT) of Al@Ni-P-Cu were used to clarify the effects of surface morphology and valence state of elements on the catalytic AP pyrolysis.Overall, HRTEM (Figs.4(d)-4(g)) and FFT (Figs.4(h)-4(j))images demonstrate that the Al@Ni-P-Cu particle from inside toward outside are single crystal Al, discontinuous amorphous Al2O3, polycrystalline Cu, amorphous Ni-P-Cu alloy, and an amorphous oxide layer, successively.What's more, taking FFT at different positions of the core-shell Al@Ni-P-Cu, the ordered Al atoms (Fig.4(h)) at the position P1 (Fig.4(e)) are still harmlessly arranged inside raw Al powder due to the interplanar spacing with 0.195 nm in the crystal planes direction of (200), which is in good agreement with XRD result(Fig.3).According to the spots(Fig.4(i))with circumferential distribution as well as short straight lines marked by different colors at the position P2(Fig.4(e)),it indicates that this layer is polycrystalline Cu.Besides,the typical amorphous ring (Fig.4(j)) is characteristics of amorphous Ni-P-Cu, near the outermost layer at the position P3 (Fig.4(g)).
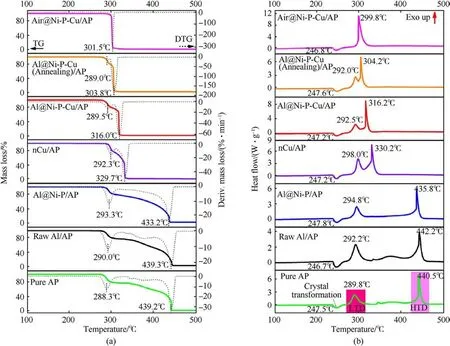
Fig.5.Thermal decomposition curves of pure AP with and without catalysts: (a) TG-DTG; (b) DSC.
Among them, polycrystalline Cu grains, with the white dotted circles in Fig.4(d) and the size of ~5 nm, are distributed at the interface of the two amorphous layers (Al2O3and Ni-P-Cu), as shown with the colored short straight lines in different orientations in Fig.4(e),which might be due to the rapid reduction reaction for electron provided by Al-core and NaH2PO2[57].Importantly, an amorphous oxide layer (thickness of ~2.3 nm) is exposed on the surface of the Ni-P-Cu shell due to the non-existent atomic arrangement in HRTEM image (Figs.4(d) and 4(h)), which is presumed that it is easily oxidized in the heated solution, in accordance with previous XPS results (see Fig.S3 in supplemental material) of Al@Ni-P compounds[55].
Simultaneously, the validity of Ni-P-Cu coating mechanism(Fig.2(a)) and XRD analysis(Fig.3) have also been verified by this in-depth TEM characterization.Those comprehensive crosssectional characterizations of the designed core-shell structure(Al@Ni-P-Cu) have clarified the phase composition and element distribution of this novel multi-functional material, which established a structure foundation of the following catalytic AP pyrolysis characteristics in subsection 3.2, and catalytic combustion performance in subsection 3.3.
3.2.Characteristics of catalytic AP pyrolysis with CSECC
Aiming to further investigate the positive catalytic effect of Al powders with Ni-P-(Cu)coating on the high-temperature thermal decomposition (HTD) of AP particles, TG-DTG/DSC curves of those mixed binary metal/AP powders were obtained by thermal analysis,as shown in Fig.5.Moreover,the apparent activation energy of AP pyrolysis was also analyzed via non-isothermal method.For comparison, two control groups, such as raw Al/AP and pure AP,were also conducted with the same analysis.
3.2.1.Catalytic thermal decomposition behavior of AP
As is known to propellant designer,the AP pyrolysis at HTD has an important influence on the flame propagation rate and the Al agglomeration on the SCPs’ burning surface [2,10,16].Thus, the thermal behaviors of AP with addition of above six as-prepared samples were evaluated by thermal analyzer to obtain TG-DTG/DSC curves (Fig.5) and detailed thermal decomposition parameters (Table 1).It is noted that the accuracy of mixing mass ratio would significantly affect the reliability of test results, where total mass ~35 mg of mixed samples would be used.
It could be clearly observed that the total mass loss of AP is-100%from the TG curve(Fig.5(a)),which indicates that it would have been completely decomposed after heating from 30 to 500°C.The DSC curve of pure AP shows an endothermic peak at 247.5°C(stage I in Fig.5(b)),resulting from the crystalline transformation of AP from orthorhombic to cubic with a endothermic peak of 56.76 J/g [8].This peak is then followed by two exothermic peaks appearing at 289.8°C and 440.5°C,which are corresponding to the low-temperature thermal decomposition (LTD) and HTD modes of pure AP particles, respectively, in consistent with that reported by Tang et al.[16].Between the two peaks,there is a slight exothermic peak around 350°C,which is consistent with the result in Ref.[22]and is somewhat different with Refs.[23,26,31]owing to relatively larger AP particle size in present study.According to DTG curves(Fig.5(a))of all binary metal/AP mixtures,they also undergo multistep mass loss similar to the AP pyrolysis during heating, and the total mass loss of those mixtures is in the range of -97.01%to-98.46%.Herein,the TG-DTG/DSC curves of the above six mixing samples maintain a similar pyrolysis process to that of AP as well.
It can be clearly found that AP with the introduction of raw Al or Al@Ni-P hardly reduces its the peak decomposition temperature(Tp2) at HTD, corresponding to 442.2°C and 435.8°C, respectively.Surprisingly,the Tp2of AP can be significantly reduced via Ni-P-Cu coating on the Al powders’ surface as well as nCu particles.According to the Tp2of AP at HTD(stage III),the order of those catalysts from high to low is nCu (330.2°C), Al@Ni-P-Cu (316.2°C),Al@Ni-P-Cu(Annealing)(304.2°C) and Air@Ni-P-Cu(299.8°C),which is reduced by 110.3°C, 124.3°C, 136.3°C and 140.7°C,respectively.Meanwhile,the maximum mass loss rate(Lmax)of AP mixed with the above four samples continuously accelerates at HTD, which are -47.36, -61.82, -178.29 and -296.12%/min,respectively.Thus,it indicates that Ni-P-Cu layer has a remarkable catalytic effect on the AP pyrolysis at HTD compared with single nCu particles.Since all the Lmaxare significantly faster than that ofAP pyrolysis (Lmax= -32.80%/min).It is speculated that nCu itself(Tp2= 330.2°C and Lmax= -47.36%/min) possesses an excellent catalytic effect on the thermal decomposition of AP at HTD in comparison to the raw Al and Al@Ni-P.Thus, it can cooperatively improve the thermal decomposition performance of AP at HTD when the element Cu is doped into the Ni-P alloy coating.The hollow Air@Ni-P-Cu also verify that speculation owing to the single exothermic peak at 299.8°C in the DSC curve (Fig.5(b)),resulting from the exposed adsorption/desorption sites on inner/outer surfaces of Ni-P-Cu shell to rapidly transfer intermediate products of AP decomposition.
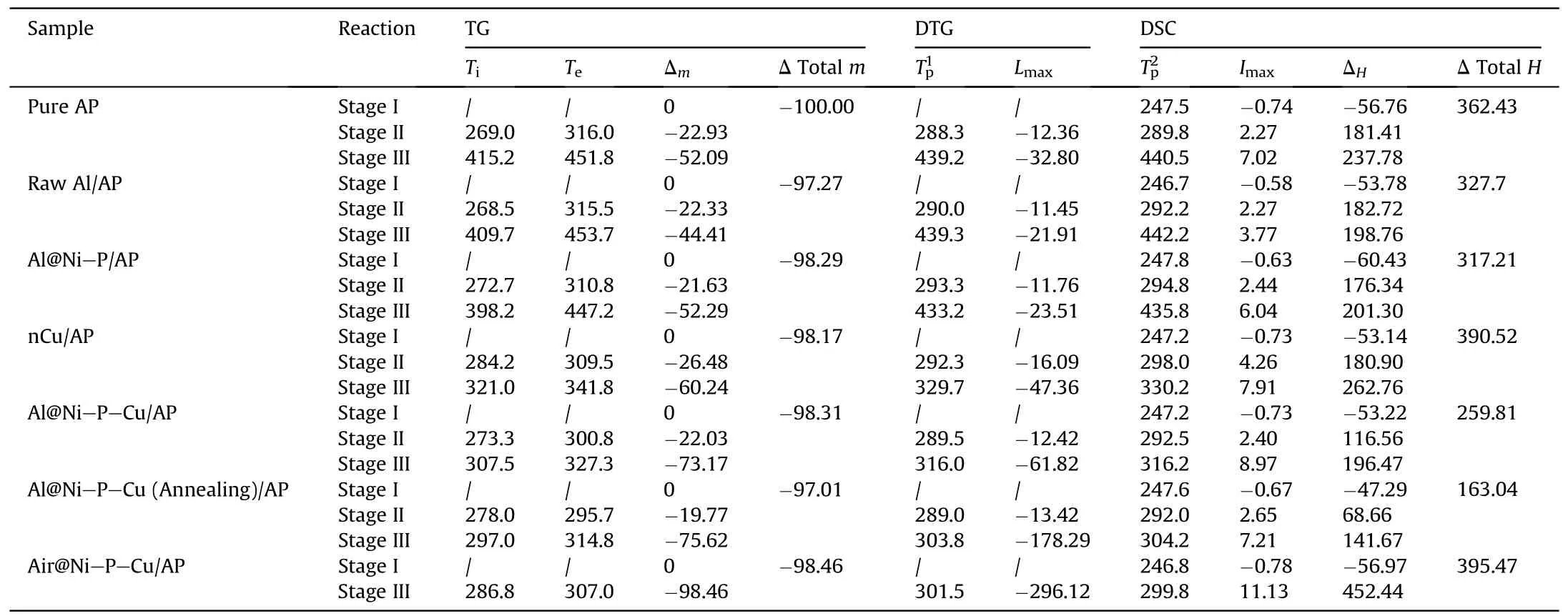
Table 1 The TG-DTG/DSC parameters of pure AP with and without catalystsa.
3.2.2.Kinetic parameters of AP pyrolysis with CSECC
The DSC curves of CSECC were carried out at different heating rates to obtain their peak temperatures (Tp2) at the stage of high temperature oxidation(HTO),as shown in Figs.S4 and S5,and the oxidation reaction properties were listed in Table S2.Meanwhile,TG-DTG/DSC curves of pure AP mixed with CSECC were also conducted at different heating rates to unravel their peak temperatures(Tp2) during thermal decomposition of those mixtures at HTD, as shown in Fig.S6.
Firstly,the Tp2of thermal oxidation(HTO)and AP pyrolysis(HTD)obtained from DSC (Figs.S5 and S6) were substituted into the Kissinger equation[8,16,55].Then,the activation energy(Ea)can be resolved by linear regression via this equation, which represents the height of the energy barrier of the chemical reaction,including CSECC oxidation at HTO as well as solid phase AP pyrolysis at HTD.And the formula (Eq.(1)) for the calculation of Eais as follows[24,34]:
where β is the heating rate (°C/min); Tpis the peak temperature(HTO or HTD) at β (K); A is pre-exponential factor; R is ideal gas constant, 8.314 J/(mol·K); Eais the apparent activation energy (J/mol).
According to Eq.(1), the term ln(β/Tp2) varies linearly with 1/Tp,yielding the kinetic parameters of Eafrom the slope of the straight line.The linearity of the plots (Figs.S5(c) and S7(a)-S7(d)) of all samples means that they have similar reaction order.Since all the linear correlation coefficients(Tables S3 and S4)are close to 1,it is indicated that the solution method is dependable.Owing to Al@Ni-P-Cu is a representative sample, linear fittings of its Ea(thermal oxidation and AP pyrolysis)were comprehensively drawn on the same diagram at HTO and HTD, where also included two control groups:oxidation of raw Al and pure AP pyrolysis(Fig.6(a)).Thus, it can better interpret the reaction characteristics (oxidation and catalysis) of Al@Ni-P-Cu as a dual role of fuel and catalyst.
Furthermore, Figs.6(b) and 6(c) mainly showed that the comparisons of heat release and activation energy of energetic unit oxidation containing Al-core, respectively.It demonstrates that Al@Ni-P-Cu has the highest heat release(Table S2),up to 7597.7 J/g,under flowing O2atmosphere;and it also possesses a lower Eaof oxidation with 239.69 kJ/mol compared with raw Al(Table S3).This indicates that the heat release is significantly increased by 6648.5 J/g and Eaof Al@Ni-P-Cu from oxidation is dramatically dropped by 159.6 kJ/mol, compared with those of raw Al (949.2 J/g and 399.29 kJ/mol, respectively).
More importantly,based on thermal decomposition of pure AP,it is synthetically revealed the comparisons of conversion(Fig.6(d))as well as activation energy(Fig.6(e))of AP pyrolysis with catalytic units(Ni-P-Cu alloy coating),which reflects the catalytic effect of CSECC on the AP pyrolysis.According to the extent of conversion versus temperature curves at same heating rate of 10°C/min under flowing Ar atmosphere,it indicates that the temperature of fastest conversion rate of AP pyrolysis with Ni-P-Cu coating as catalyst at HTD can considerably retreat to a lower temperature compared with that of pure AP.It can also find that Al@Ni-P-Cu has the lowest activation energy with 116.48 kJ/mol at HTD among those samples,which decreases by 118.28 kJ/mol compared with pure AP of 234.76 kJ/mol (Table S4).And the Eaof pure AP pyrolysis in this work is slightly lower than that reported by Li et al.[34] with 271.8 kJ/mol due to the different size of AP particles.
Based on the Eaof Al@Ni-P-Cu during oxidation and the Eaof AP pyrolysis with addition of Al@Ni-P-Cu, it can find that both process of oxidation and AP pyrolysis have extremely low Ea,due to its energetic unit Al-core as the carrier of catalytic unit Ni-P-Cu containing active site.Thus,it can not only meet the requirements of high energy release, but also significantly reduce the peak temperature of AP pyrolysis at HTD.It is referred that the Al-core with Ni-P-Cu coating can easier break through the barrier of intrinsic Al2O3shell of raw Al, due to its diffusion reactions at the multi-interfaces;and then,can quickly react with oxygen to release its huge potential energy.More important, the outermost layer of Al@Ni-P-Cu particles would accelerate proton and electron transfer during thermal decomposition reaction of AP [16], owing to its very thinner amorphous oxide surface with a thickness of 2.3 nm.
3.3.Catalytic combustion behavior of binary metal/AP mixtures
For the sake of distinguishing the mesoscopic flame structure and variety morphology of binary metal/AP mixtures during combustion, the flame emission spectrum, particle outline during combustion, and micromorphology of CCPs were conducted by HPCC (Fig.1) under atmospheric pressure, and the schematic of local details is illustrated in Fig.7(a).Meanwhile, the abovementioned corresponding results are shown in Figs.7(b)-7(j) and scene 1 in Video S1.
The flame emission spectrum of four mixtures (Fig.7(b)) indicates that there are two emission peaks (NiO: 502.4 nm or CuO:606 nm) of some transition metal elements and emission peak of(P) at 520 nm in the luminescence process of combustion [58].It demonstrates that the Ni-P or Ni-P-Cu shell are participated in burning with AP gas stream as well.In addition, the typical characteristic AlO emission peaks of raw Al or Al-core are at 486.6 nm(strong), 435.5 nm (weak), 453.8 nm (weak) and 512.3 nm (weak)[6,10], respectively.Certainly, there would also be emission peaks produced by impurity polluting elements doped in those samples at 589 nm(Na),670 nm (Li) and 766 nm (K) [6,10,59].
Compared with serious agglomeration of Al during combustion(Fig.7(c)), it can be observed that the agglomeration degree of asprepared three composites(Figs.7(d)-7(f))is obviously weakened,due to their violent vapor and slightly micro-explosion in the flame.Flame with violent Al vapor and micro-explosion represents a faster energy release and stronger combustion, which is partially attributed to the effect of Al@Ni-P-Cu on AP powder as AP pyrolysis has an important influence on the flame propagation rate and the Al agglomeration on the SCPs’burning surface.Both of Al/AP and Al@Ni-P/AP present a similar yellow flame, yet both of Al@Ni-P-Cu/AP and Al@Ni-P-Cu(Annealing)/AP have an obvious nattier blue flame due to the flame emission peak of CuO at 606 nm.Thus,those results indicate that Ni-P shell doped with element Cu would transform the combustion mode of Al@Ni-P owing to the Cu involved in the combustion as well.
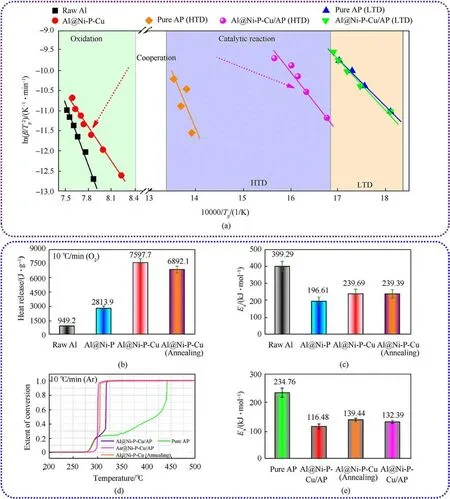
Fig.6.(a) Linear fit of plotted ln(β/Tp2) against 1/Tp for the calculation of activation energy (Ea) via non-isothermal Kissinger method; (b) Comparisons of the heat release during oxidation; (c)Comparisons of the Ea at HTO;(d)Extent of conversion versus temperature curves of AP pyrolysis with different catalyst;(e)Comparisons of Ea of AP pyrolysis with different catalyst at HTD.
Moreover, as for the BSE-SEM images of the CCPs deposited at the bottom of the corundum crucible after those mixtures burned(Figs.7(g)-7(j)), there are unburned large-sized AP particles(150-200 μm) with some cracks and small-sized agglomerated particles(10-30 μm),which are presented in BSE-SEM images with light gray and white color, respectively.Thus, the above analysis confirms that Ni-P-(Cu)coating on the Al surface is contributed to improving the energy releasing characteristics of Al,which reflects the advantages of reactivity, ignition, and combustion via this strategy with design of core-shell structure.This binary metal/AP mixtures might be regarded as loose grain without mechanical properties due to its no addition of HTPB binder.This test method will also provide rough and simple data prediction in the next study about investigating the effect of adding the as-prepared composite fuels as a catalyst substitute on the controllable combustion of SCPs.
Based on the above preliminary evaluation of ignition and combustion performance, then, in order to further investigate the in-depth catalytic effects of as-prepared samples on the AP pyrolysis, the force versus time curves (F-t) of mixtures filled with Al@Ni-P/AP or Al@Ni-P-Cu/AP were collected and recorded by using a home-made micro-thrust measuring device equipped in the HPCC (Fig.8(a)).The micro-thrust, i.e., the transparent reactor packed with mixture,is put on the thrust transducer firstly,which is defined as loading process.Thereafter,the mixture in the reactor is ignited, and the hot gas is ejected from the outlet of reactor, by which thrust is generated and acts on the transducer.When the mixture is burned out,the thrust gain is no longer in existence,after which the reactor is taken away from the transducer corresponding to unloading process.This F-t curve during burning of mixture can reveal the decomposition rate of AP under the condition of burning.Meanwhile, their flame frames were captured by high-speed camera during ignition and combustion(Figs.8(b) and 8(c)).
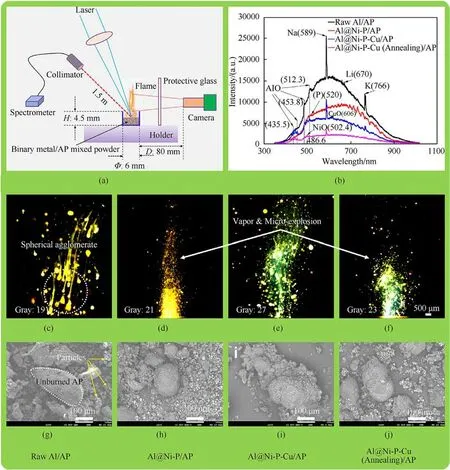
Fig.7.(a)Experimental setup of the laser ignition test under atmospheric pressure;(b)Typical flame emission spectra at full wave during combustion;(c)-(f)Flame snapshots of metal particles in AP gas stream; (g)-(j) BSE-SEM images of the CCPs for four mixtures shown in (c)-(f), respectively.
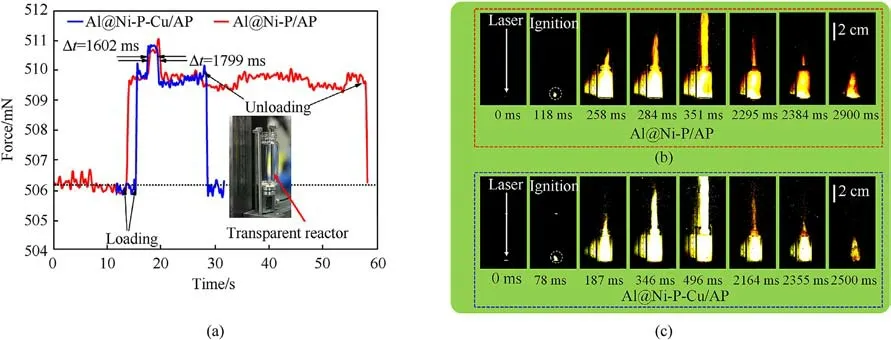
Fig.8.(a) F-t curves of mixtures during combustion; and the inset graph shows the experimental setup.(b) and (c) Side view snapshots of mixtures burning in the transparent reactor: (b)Al@Ni-P/AP; (c) Al@Ni-P-Cu/AP.
The total time required for complete combustion of Al@Ni-P-Cu/AP loaded in the transparent reactor is 1602 ms,which is about 197 ms lower than that of Al@Ni-P/AP (1799 ms)from the semi-quantitative F-t curves.In addition, the flame intensity of Al@Ni-P-Cu/AP with luminous flame is stronger than that of Al@Ni-P/AP.And the tigof the former(78 ms)is also shorter than that of the latter(118 ms),markedly reduced by 40 ms(scene 2 in Video S1).It should be worth noting that pure AP could not be ignited and maintained self-sustaining combustion with the same laser condition.Thus, those results demonstrate that Al-core with Ni-P-Cu coating is contributed to shorting the tigand enhancing the combustion intensity of Al@Ni-P-Cu/AP mixture; and importantly,to accelerating the decomposition rate as well as improving the reaction depth of AP.This can be attributed to thinner shell thickness, larger specific surface area and more chemical active sites of Al@Ni-P-Cu, accelerating the transfer rate of proton and electron in the AP decomposition reaction.
It is further simulated that the more realistic ignition and combustion process with increasing the mass of mixtures, as shown in Figs.7 and 8.According to the above thermal analysis and laser ignition test,it can be found a fact that energetic combustion catalyst Al@Ni-P-Cu not only has a higher energy density and a much faster potential energy release rate when it reacts with oxidizer (eg., O2(g) or AP(s)), but also provides with a beneficial function for catalyzing the rapid decomposition of AP.
3.4.The physicochemical mechanism of catalytic AP combustion via CSECC
In the light of the comprehensive discussion with microstructure and chemical compositional characterization, thermal analysis, ignition and combustion behaviors, a possible synergetic mechanism was put forward to explain the introduction of Al@Ni-P-Cu effects on the AP pyrolysis and its catalytic combustion, as shown in Fig.9.
Firstly,it is speculated that pure AP with the addition of Al-core with nano-sized Ni-P-Cu coating (thickness of ~100 nm),including elements P and Cu, has an advantage of being easily ignited than the mixture of Al@Ni-P/AP and pure AP, due to its lower ignition temperature and easy oxidation of trace solution P atoms in this Ni-P-Cu alloy shell [55,60].Meanwhile, Ni-P-Cu alloy shell is more beneficial to accelerate conduction of heat from the outside to the inside of Al@Ni-P-Cu,due to its higher thermal conductivity of metal [60,61].Thus, it demonstrates that the tigof Al@Ni-P-Cu is shorter than that of Al@Ni-P/AP mixtures.
Secondly, it clearly indicates that CSECC can rapidly release considerable heat,due to its the surface oxidation of elements(Ni,P and Cu) and formation of intermetallic compounds (IMCs) via diffusion reaction in the tight interfaces(Al/Ni,Al/Cu,and Ni/P)at a lower temperature;and its generated properly greater temperature gradient near the surface of Al@Ni-P-Cu, then forms concentration of thermal stress [62,63].The mass and heat diffusion might cause the fracture of tris-shelled Ni-P-Cu and internal residual Al2O3layer in the CSECC.Thus, this fracture would accelerate the evaporation of internal aluminum droplets and then makes Al burn more violently with outside oxidant than virgin Al powder.

Fig.9.A possible synergetic mechanism of ignition and combustion of Al@Ni-P-Cu as energetic combustion catalyst, and its catalytic effect on AP pyrolysis.
Finally, to clarify the in-depth effect of CSECC as energetic combustion catalyst on thermal decomposition of AP, a possible sophisticated multi-stage reaction mechanism is proposed, which are on basis of the proton and electron transfer mechanism accredited by most researchers[8,9,16,64-66],the thermal analysis and laser ignition experimental results in this work.Therein, one possible catalytic mechanism of AP with addition of Al@Ni-P-Cu are teased out as follows:
(1) A complex [NH3-H-ClO4] is firstly emerged at a low temperature (LTD: 300-330°C) from initial solid phase AP,resulting from the local electron transfer from anion to cation, and then it would decompose into adsorbed state NH3(ads.)and HClO4(ads.)with proton transfer from NH4+to ClO4-[8,16].Thus, it infers here that the outermost amorphous oxide layer with thickness of ~2.3 nm on the surface of Ni-P-Cu shell might be acted as proton-hopping sites to promote the initial thermolysis of the [NH3-H-ClO4].
(2) After the process of AP thermolysis in(1),the resultants(NH3and HClO4)are adsorbed on the residual perchlorate crystals surface (Figs.9(g)-9(j)), resulting in hindering their further chemical reactions in the adsorbed layer as well as desorption process due to the interaction in the gas phase stream[8], which is in good agreement with the adsorption/desorption of reaction products in chemical reaction kinetics of solid particles surface [56].Thus, the above multistep reactions might be only triggered when the temperature rises to a certain value, depended on the sublimation rate of the NH3(ads.)and HClO4(ads.).According to the results reported by Tang et al.[16],it is the HClO4(ads.),rather than NH3(ads.)that leads to the cessation of AP at HTD, owing to only pyrolysis products(CO,CO2,HCl,O2and O·)of HClO4detected at high temperature range (450.0-480.0°C).Thereby, the surface layer of residual perchlorate crystals will be saturated with HClO4,which is also regarded as the physical essence of reaction controlling steps of AP at HTD without the addition of catalysts.
(3) When the temperature of Al@Ni-P-Cu/AP mixtures increases to ~316°C, the complete decomposed gas phase intermediates(NH3and HClO4)of AP will come into gas stream and then generate the ClO3, ClO, and H2O, as well as an important super-oxide radical(O2-)from the HClO4pyrolysis[16,36].In this process, as a result of the electron transfer occurring locally in controlling step of HTD, the smaller distance among those ions,the higher reaction probability of controlling step will be arisen [24].
Thus,the catalytic unit of Al@Ni-P-Cu,served as both efficient electron acceptor as well as adsorption site of the reaction intermediates,would accelerate the process of electron transfer from O2to O2-.The outermost nano-sized Ni-P-Cu amorphous oxide layer on the surface of the energetic unit Al-core carrier is such catalytic unit of the AP pyrolysis, due to its high surface area and high electron conductivity [34,66].Moreover, the resultant intermediates will be reacted with NH3to form more robust resultants (N2O, NO, NO2, CO, CO2, HCl), accompanied by a large amount of heat release[16].The reaction heat from secondary of AP pyrolysis at HTD and oxidation of Al@Ni-P-Cu will be together feed back to the whole complex multi-step reaction system,and to further accelerate the reaction rate of the mixture Al@Ni-P-Cu/AP.
Thus,it is of great significance that lowering the decomposition temperature of AP at HTD is to technologically tune the combustion efficiency of AP-based SCPs.In this strategy, the amorphous oxide layer formed by in-situ oxidation of electroless plating, which can be served as an efficient catalyst for thermal decomposition of AP via promoting the process of proton and electron transfer.
4.Conclusions
In summary, Al@Ni-P-Cu, as energetic combustion catalyst,was prepared via a facile and inexpensive method of‘one-step’insitu electroless plating in this work.The preparation and characterization of micro-sized Al-core coated with Ni-P-Cu shell(CSECC) were firstly introduced.Both the morphology and constituent illustrated that the spherically distributed three-layer Ni-P-Cu alloy catalyst with the thickness of ~100 nm was uniformly coated on the μAl powder.Then,the TG-DTG/DSC and laser ignition/combustion experiments manifest that the CSECC particle has more obvious advantages in improving energy release rate and thermal decomposition of AP.In comparison to the oxidation of virgin Al,the heat release can reach up to 7597.7 J/g of Al@Ni-P-Cu particles, and its activation energy significantly decreases by 159.6 kJ/mol.Such improvement is caused by synergistic effects of nano-sized trinary-shell and intermetallic reaction in the surface/interface with exothermic process.Meanwhile, the amorphous oxide layer on Al surface with a thickness of 2.3 nm could promote the electrons transfer from ClO4-to NH4+in the secondary thermolysis of AP at HTD.That is,the peak temperature(316.2°C)of AP at HTD,with the addition of Al@Ni-P-Cu(3.5%),could be reduced by 124.3°C compared with that of pure AP(440.5°C);in addition,compared with the thermal decomposition of pure AP, the activation energy of Al@Ni-P-Cu/AP decreases by 118.28 kJ/mol.And the Al@Ni-P-Cu/AP mixtures exhibits extraordinarily shorter ignition delay time (tig) with 78 ms under atmospheric pressure, reducing by 33.90% in comparison to Al@Ni-P/AP mixtures (118 ms).The shortened tigand increased energy release rate may greatly accelerate the pyrolysis reaction kinetics of AP.Overall,the Al@Ni-P-Cu with high reactivity could effectively advance the application of energetic combustion catalysts in AP-based propellant due to its fascinating advantages of increased burning rate by catalytic effect,reduced non-energetic mass and increased volumetric energy density as well as improved manufacturing formability via the micro-sized higher energetic Al-core.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China, China (Grant Nos.U20B2018, U21B2086,11972087).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.06.001.
- Defence Technology的其它文章
- Explosion resistance performance of reinforced concrete box girder coated with polyurea: Model test and numerical simulation
- An improved initial rotor position estimation method using highfrequency pulsating voltage injection for PMSM
- Target acquisition performance in the presence of JPEG image compression
- Study of relationship between motion of mechanisms in gas operated weapon and its shock absorber
- Data-driven modeling on anisotropic mechanical behavior of brain tissue with internal pressure
- The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin

