YAP activates pancreatic stellate cells and enhances pancreatic fibrosi s
Lennard Spanehl, Denis Revskij, Karen Bannert, Luise Ehlers, Robert Jaster
Department of Medicine II, Division of Gastroenterology, Rostock University Medical Center, Ernst-Heydemann-Str. 6, 18057 Rostock, Germany
Keywords:Hippo pathway Pancreatic stellate cells Fibrosis Pancreatic cancer Chronic pancreatitis
A B S T R A C T
Introduction
In chronic pancreatitis (CP), inflammation and destruction of exocrine and endocrine tissues induce and maintain a wound healing response that involves proliferation of pancreatic stellate cells(PSCs) [1] . Activated PSCs produce large amounts of extracellular matrix (ECM) proteins, thereby contributing to pancreatic fibrosis after injury. Under the persistent pathological conditions of CP, however, ECM proteins accumulate in the diseased organ,and a vicious cycle of inflammation, cell death and dysregulated wound healing develops. Eventually, exocrine and endocrine pancreatic tissues are largely replaced by connective tissue in a state termed fibrosis, resulting in organ failure [ 2 , 3 ].
In the past two decades, the role of PSCs in CP (as well as in fibrosis associated pancreatic ductal adenocarcinoma; PDAC) was studied extensively, and various extracellular and intracellular regulators of PSCs activity have been identified [ 2 , 4 ]. Nonetheless,specific therapies of pancreatic fibrosis are not applicable for clinical practice yet. The reasons include a lack of efficient antifibrotic drugs and remaining gaps in the molecular understanding of PSCs function.
The Hippo-YAP (for Yes-associated protein) signaling pathway is essentially involved in the control of tissue growth and organ size,but also acts as a regulator of cell proliferation and tissue regeneration [ 5 , 6 ]. YAP and the YAP-homolog TAZ (transcriptional coactivator with PDZ-binding motif) are transcriptional coactivators and active in a dephosphorylated state. Upon phosphorylation by LATS1/2 and Mst1/2, the proteins are subjected to proteasomal degradation, which is triggered by the formation of a stable cytoplasmic complex of YAP and TAZ with 14-3-3 proteins. Protein kinases upstream of YAP and TAZ are themselves controlled in their activity by diverse signals derived from cell surface receptors, adhesion molecules and tight junction proteins [ 5 , 6 ].
A growing body of evidence suggests the YAP pathway as an important determinant of cancer progression in PDAC patients,which exerts its effects by activating an oncogenic signaling network in the tumor cells [7] . However, the consequences of Hippo activation (or inhibition) in pancreatic fibrosis and PSCs biology remain incompletely understood. Morvaridi et al. [8] reported the first study to show that activated stellate cells in CP and PDAC patients exhibit high protein levels of YAP and TAZ. More recently,Hu et al. [9] implicated that YAP plays a central role in the process of PSCs activation. They also provided evidence for a role of YAP in platelet-derived growth factor-mediated PSCs growth and fibroinflammatory gene expression responses.
Here, we investigated the effects of a pharmacological inhibitor of YAP in PSCs and characterized upstream regulators of YAP activity. Furthermore, we employed rats with dibutyltin dichloride(DBTC)-triggered CP [ 10 , 11 ] to delineate YAP expression in the context of the disease. We aimed to explore the antiproliferative action of verteporfin in PSCs and the role of YAP in CP-associated fibrogenesis.
Materials and methods
Reagents
Reagents without specified distributor were purchased from Sigma-Aldrich (Deisenhofen, Germany).
Cell culture
PSCs were gathered from male Lewis inbred rats (approximately 3 months old; Charles River Laboratories, Sulzbach, Germany). The pancreas was digested using collagenase, and PSCs were collected by Nycodenz density gradient centrifugation [12] . Freshly isolated PSCs were resuspended in fetal calf serum (FCS; Bio&Sell, Feucht,Germany) supplemented with 10% dimethyl sulfoxide (DMSO) and stored as viable cells at -150 °C. Depending on the experimental requirements, cell aliquots were thawed and cultured in Iscove’s modified Dulbecco’s medium (IMDM; Biochrom, Berlin, Germany)supplemented with 17% FCS, non-essential amino acids (Biochrom)and antibiotics (penicillin and streptomycin; Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C in a humidified atmosphere with 5% CO 2 . For the experiments, only primary cultures and cells of the first passage were employed. For replating (typically on day seven after isolation), trypsinized cells were seeded at equal densities.Immortalized PSCs (LTC cells [ 13 , 14 ]) were grown under the same conditions, except of that the FCS content was reduced to 10%.Counting was performed with a NucleoCounter NC-200 and Via1-cassettes as suggested by the manufacturer (ChemoMetec, Kaiserslautern, Germany). Via1-cassettes were filled with immobilized acridine orange (AO) and 4 ′ ,6-diamidino-2-phenylindole (DAPI) for labeling of all cells and dead cell populations, respectively.
Cell proliferation assay
PSCs were seeded into 96-well half-area plates, allowed to adhere overnight, and treated for 48 h with the YAP-inhibitor verteporfin (Selleckchem, Houston, TX, USA) as indicated, and controls were exposed to solvent (DMSO) only. Afterwards, bromodeoxyuridine (BrdU) labelling was started by incubating the cells with labelling solution at 10μmol/L for 8 h. Finally, BrdU incorporation was measured according to the protocol provided by the manufacturer.
Knockdown of YAP using siRNA
siRNA to YAP (5’-UUAUGUAGUAAACUUCUCCCCC-3’) and a nonsilencing control RNA (5’-UUGUACUACACAAAAGUGUAGUACAA-3’)were purchased from Riboxx (Radebeul, Germany). Transfection of LTC cells was performed according to the instructions of the manufacturer, employing RiboxxFECT transfection reagent and siRNA at 20 nmol/L. Protein levels of YAP were monitored for up to 72 h after transfection. BrdU incorporation assays were initiated by adding labelling solution 48 h after transfection and conducted as described above. Total RNA (for gene expression studies) was isolated from cells 48 h post transfection.
Immunoblot analyses
Protein extracts of PSCs were subjected to Western blotting analyses as previously described [12] , employing polyvinylidene fluoride (PVDF) membrane for protein transfer. Proteins were detected using the following primary antibodies: anti-β-actin(#4970), anti-YAP (#14074), anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, #2118) (all from New England Biolabs, Frankfurt am Main, Germany) and anti-α-SMA (#A2547; Sigma-Aldrich).The blots were developed as described previously [15] , using an Odyssey? CLx Near Infrared Imaging System and reagents provided by LI-COR Biosciences (Lincoln, Nebraska, USA). Signal intensities of protein bands were determined and analyzed using the Odyssey? software Image Studio Lite Version 5.2.5. GAPDH andβactin served as loading controls.
Histochemical detection of intracellular fat droplets
Primary cultures of PSCs were grown on glass coverslips in the presence or absence of verteporfin for up to 6 days as indicated.Afterwards, oil red O staining of intracellular lipids was conducted as previously described [ 16 , 17 ]. Assessment of blinded samples by light microscopy was performed by two independent investigators.The fat droplets were scaled from 0 (lack of lipid droplets) to 3(numerous, large lipid droplets).
Reverse transcriptase-polymerase chain reaction (PCR)
Cellular RNA was extracted using TriFast reagent (PEQLAB Biotechnologie, Erlangen, Germany). Reagents for sample processing and PCR were obtained from Thermo Fisher. Possible remnants of genomic DNA were eliminated with the DNA-free kit. Subsequently, reverse transcription of RNA into cDNA was initiated, using TaqManTMReverse Transcription Reagents, 250 ng of RNA per sample and random priming. Quantification of target cDNA levels was performed by real-time PCR with a ViiA 7 sequence detection system (Thermo Fisher Scientific). MasterMix for qPCR was obtained from Eurogentec (Seraing, Liège, Belgium). The following TaqManTMgene expression assays (rat-specific) with fluorescently labeled MGB probes were employed: Rn01759928_g1 (Acta2/α-SMA) Rn00561420_m1 (interleukin-6,Il-6), Rn01463848_m1 (α1 typeIcollagen,Col1a1), Rn00572010_m1 (Tgf-β1), Rn00573960_g1(connectivetissuegrowthfactor,Ctgf), Rn0 0580 055_m1 (Ccn1, also known asCyr61), Rn00627285_m1 (Axlreceptortyrosinekinase,Axl), Rn00574012_m1 (BaculoviralIAPRepeatContaining5,Birc5,also known assurvivin), Rn00432360_m1 (cyclinD1,Ccnd1),Rn00589996_m1 (cyclin-dependentkinaseinhibitor1A,Cdkn1a),Rn00580 6 64_m1 (cyclin-dependentkinaseinhibitor2A,Cdkn2a), and Rn01527840_m1 (hypoxanthineguaninephosphoribosyltransferase,Hprt, house-keeping gene control). PCR was started with an initial step of 95 °C for 5 min, followed by 45 cycles of 15 s at 95 °C/1 min at 60 °C. Relative amounts of target mRNA are expressed as 2?(ΔΔCt), where ΔΔCt sample = ΔCt sample - ΔCt control .
Transient transfection assays
Upstream regulators of Hippo pathway activity were studied in luciferase reporter gene assays, using the Stimulight Hippo Pathway trans-reporting system (Lifeome, Oceanside, CA, USA). The system includes the two plasmids pGAL4-TEAD (encoding for a fusion protein of the GAL4 DNA binding domain and the transactivation domain of TEAD4) and pHTS-GAL4. The first plasmid acts as a sensor of the activation of Hippo kinase cascade. The latter plasmid contains an expression cassette for the firefly luciferase under the control of a GAL4-binding element. The transcription coactivator YAP works together with GAL4-TEAD4 to activate luciferase expression from pHTS-GAL4. Upon activation of the Hippo kinase cascade, YAP will become phosphorylated, resulting in translocation to the cytoplasm and reduced luciferase expression. Elevated luciferase activity, on the other hand, indicates the presence of dephosphorylated/active YAP in the nucleus. Luciferase activities were determined using the reporter assay system Dual-Luciferase from Promega (Madison, WI, USA). This assay supports the normalization of transfection efficiencies by enabling the sequential measurement of the activities of the experimental firefly luciferase and a constitutively expressed renilla luciferase (encoded by a co-transfected plasmid) from a single sample. Therefore, LTC cells were grown to subconfluency in 96-well plates and transfected with 100 ng pHTS-GAL4, 6 ng pGAL4-TEAD4 and 10 ng pGL 4.70 per well, using FuGene HD Transfection Reagent (Promega) as suggested by the manufacturer. Subsequently, the cells were challenged with rat interferon-γ(IFN-γ; ImmunoTools, Frisoythe, Germany) and human TGF-β1 (Biotechne, Wiesbaden, Germany) as indicated. After 24 h, luciferase activities were measured according to the instructions of the vendor. Data are expressed as ratios of firefly and renilla luciferase activities.
DBTC pancreatitis
Three months old male Lewis inbred rats received a single injection of DBTC (8 mg/kg body weight) into the tail vein as described previously [ 10 , 18 ]. Control rats were injected with solvent (mixture of 40% ethanol and 60% glycerol). At the indicated time points, the rats were sacrificed by an overdose of pentobarbital followed by cardiac puncture, and pancreata from at least 6 animals per time point (0, 7 and 28 days, respectively)were harvested and stored as cryoconserved samples and formalinfixed paraffin-embedded (FFPE) tissues. All animal experiments were approved by the local animal use and care committee (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern; LALLF numbers 7221.3-1.2-023/10 and 7221.3-1-070/16). The rats had access to water and chowadlibitumand received humane care according to the German legislation on protection of animals.
Histology and immunohistochemistry
For routine hematoxylin and eosin (HE) staining, sections of FFPE pancreatic tissue (4 μm) and for all other methods 6 μm thick cryosections were employed. Detection of collagen by staining with Direct Red 80 (Merck, Darmstadt, Germany) and staining ofα-SMA positive cells using the alkaline phosphatase antialkaline phosphatase (APAAP) technique were performed as described previously [ 11 , 18 ].
For the detection of YAP protein, cryosections were fixed with acetone and stained using the ImmPRESS-alkaline phosphatase as suggested by the vendor (Vector Laboratories, Burlingame, CA,USA). Primary antibody (anti-YAP) was from New England Biolabs(#14074). After counterstaining with Mayer’s hemalum solution,the slides were dehydrated by two short incubations in ethanol and xylene each. Finally, they were embedded in Pertex (MEDITE,Burgdorf, Germany).
Statistical analysis
Data were analysed with IBM SPSS Statistics 27.0 (IBM, Armonk,New York, USA) and expressed as median (interquartile range). The nonparametric Kruskal-Wallis test was used to check group differences. Pairwise testing of subgroup was performed with Mann-WhitneyUtest. Bonferroni-adjusted values ofP< 0.05 were considered statistically significant.
Results
DBTC pancreatitis is characterized by increased protein levels of YAP
We and others have demonstrated that intravenous injection of DBTC (at 8 mg/kg body weight) induces severe pancreatic inflammation and fibrosis [ 10 , 16 , 18 , 19 ]. Key findings of DBTC pancreatitis at days 0 (healthy rats), 7 (early-stage disease) and 28(advanced-stage disease) are presented in Fig. 1 . HE staining revealed the infiltration of inflammatory cells, cell death, acinar-toductal metaplasia (ADM) and progressive replacement of acinar tissue by fibrotic tissue; collagen staining indicated deposition of ECM in the diseased organ, and the increase ofα-SMA positive cells (with a peak on day 7) verified activation of PSCs. As also shown in Fig. 1 (upper panel, day 0), healthy rat pancreatic tissue stained negative for YAP. One week after DBTC-injection (middle panel), pronounced expression of YAP protein in inflamed pancreatic tissue and particularly in tubular structures characteristic of ADM was observed. After four weeks and with progressing fibrosis, YAP-positive areas extended to periductal fibrotic tissue (lower panel). The latter observation prompted us to ask if YAP might be directly involved in the activation of PSCs, which are considered the key cells in pancreatic fibrogenesis.
YAP is required for stellate cell proliferation
In an initial experiment, we evaluated the effects of the YAPinhibitor verteporfin on synthesis of DNA and survival of primary rat PSCs ( Fig. 2 ). Verteporfin diminished BrdU incorporation at nanomolar concentrations and in a dose-dependent fashion ( Fig. 2 A). Concentrations up to 100 nmol/L were not associated with significantly increased cell death. At 500 nmol/L, verteporfin exerted a strong cytotoxic effect ( Fig. 2 B). To further explore the function of YAP in stellate cell proliferation, immortalized LTC cells were transfected with siRNA to YAP, resulting in an efficient knockdown of the YAP protein ( Fig. 3 A). Simultaneously, a significant reduction of DNA synthesis was observed ( Fig. 3 B). Moreover, the YAP knockdown was associated with lower levels ofCcnd1and higher levels ofCdkn1a(but notCdkn2a) mRNA ( Fig. 3 C), compatible with an inhibition of cell cycle progression. Together, the data indicated an involvement of YAP in the stimulation of PSCs proliferation. In subsequent experiments, maximum verteporfin concentrations were restricted to 100 nmol/L.
Inhibition of YAP does not reverse or prevent PSCs activation
Culture of quiescent rat PSCs, isolated from healthy pancreas,was associated with characteristic phenotypic changes known as PSCs activation. These changes included expression of the myofibroblastic proteinα-SMA ( Fig. 4 A) and loss of lipid droplets, which are typical for quiescent PSCs, within the first 6 days of primary culture ( Fig. 4 B). Treatment of activated PSCs with verteporfin at 20 and 100 nmol/L had no effect on the expression ofα-SMA( Fig. 4 A), suggesting that verteporfin was unable to re-induce a quiescent PSCs phenotype. Furthermore, the lipid droplet content of PSCs at day 3 and 6 of primary culture did not significantly differ between verteporfin-treated cells and corresponding controls ( Fig. 4 B), indicating that PSCs activation was verteporfinindependent.
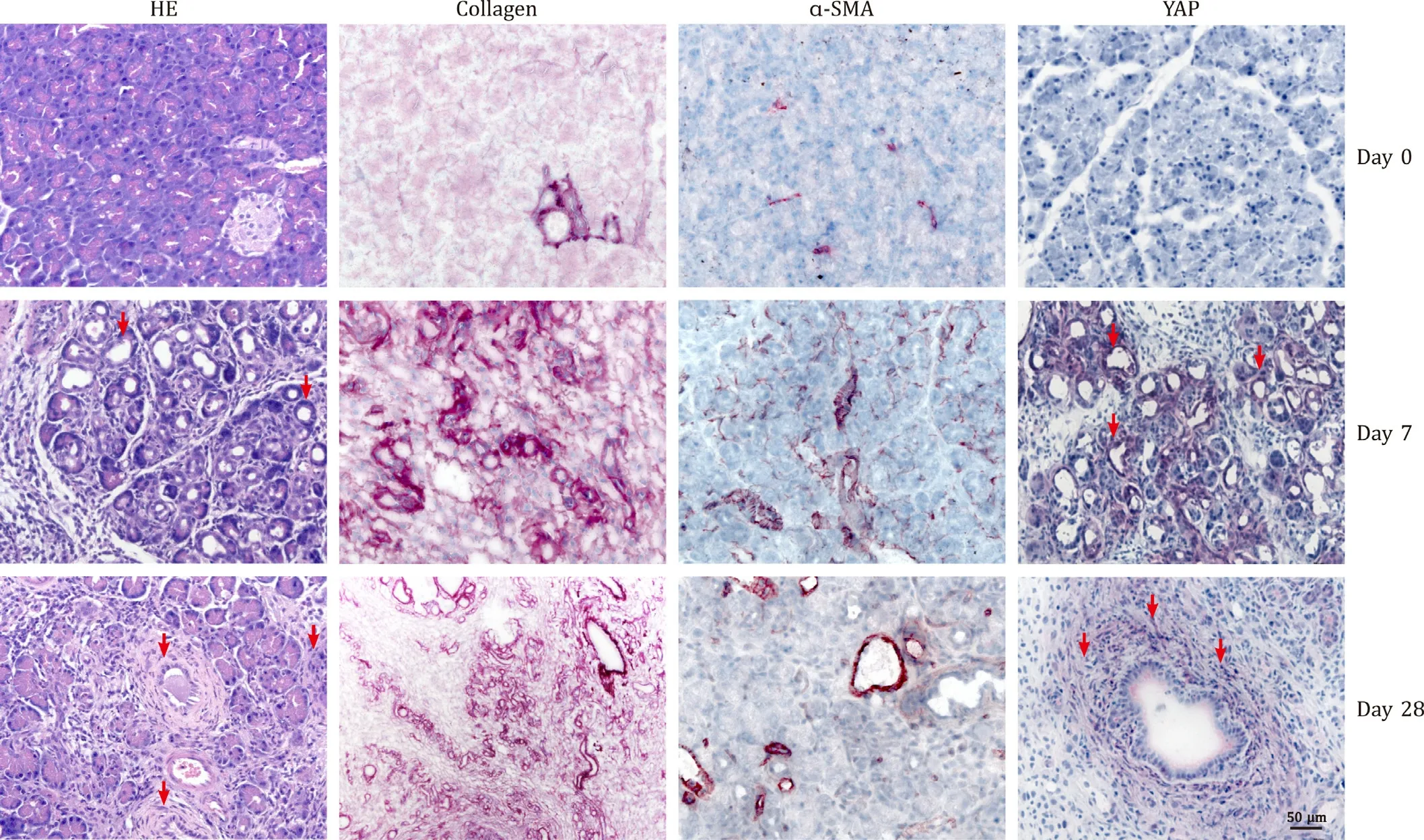
Fig. 1. Characteristics of DBTC pancreatitis and detection of YAP in pancreatic tissue. Rats were either left untreated (day 0), or injected with DBTC (8 mg/kg). Sections of pancreatic tissue were subjected to staining with HE, Direct Red 80 (collagen), anti- α-SMA and anti-YAP as indicated (original magnification, × 200). Arrows point to positively stained areas of tubular structures (day 7) and fibrosis (day 28), respectively. The findings are typical for all rats in the respective groups (day 0: n = 8; day 7:n = 6; day 28: n = 9).
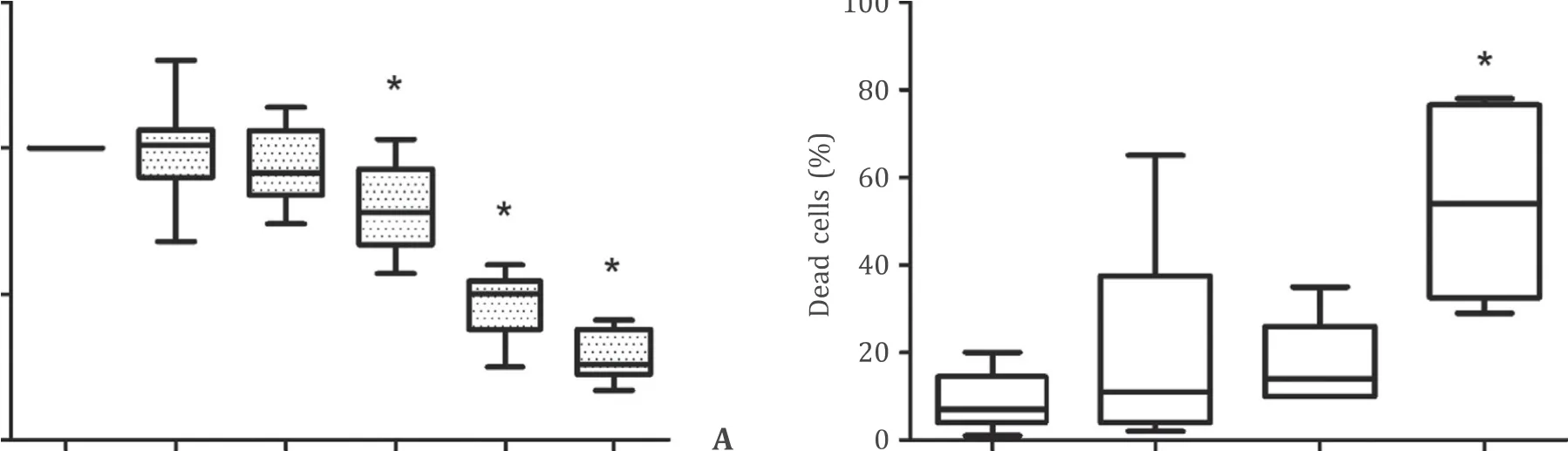
Fig. 2. Inhibition of YAP inhibits pancreatic stellate cells growth. A and B : PSC of the first passage were challenged with verteporfin as indicated. After 48 h, BrdU incorporation ( A ) and cell survival ( B ) were measured as detailed in the methods section. 100% BrdU uptake corresponds to untreated control cells (solvent exposure only). Box plots indicate the median, the 25th and 75th percentiles in the form of a box, and the 5th and 95th percentiles as whiskers ( n ≥9). ?: P < 0.05 versus untreated controls.
Effects of verteporfin on gene expression of PSCs
The gene expression of PSCs was studied by real-time PCR. We focussed on genes that encode for proteins secreted by activated PSCs and/or are involved in the activation process (Col1a1[ 20 , 21 ],α-SMA[ 20 , 21 ],Il-6[22] ,Ctgf[22] ). Furthermore, we included potential downstream targets of Hippo signaling (Axl[23] ,Birc5[24] ,Ccn1[25] and againCtgf[25] ) ( Fig. 5 ). Verteporfin, at 100 nmol/L,displayed a small but significant inhibitory effect onTgf-β1andCcn1gene expression. The mRNA levels ofAxl,Birc5,Col1a1,Il-6,Ctgfandα-SMAremained unaffected.
Regulation of Hippo pathway activity
TGF-β1 acts as a key mediator of PSCs activation (specifically, ECM protein synthesis [ 26 , 27 ]), while IFN-γdisplays the opposite effect [28] . Reporter gene assays in immortalized LTC cells ( Fig. 6 ) revealed that TGF-β1 enhanced activity of YAP in a dose-dependent fashion. In contrast, treatment of transfected cells with IFN-γcaused a decreased expression of luciferase, suggesting phosphorylation of YAP and inactivation of the protein.
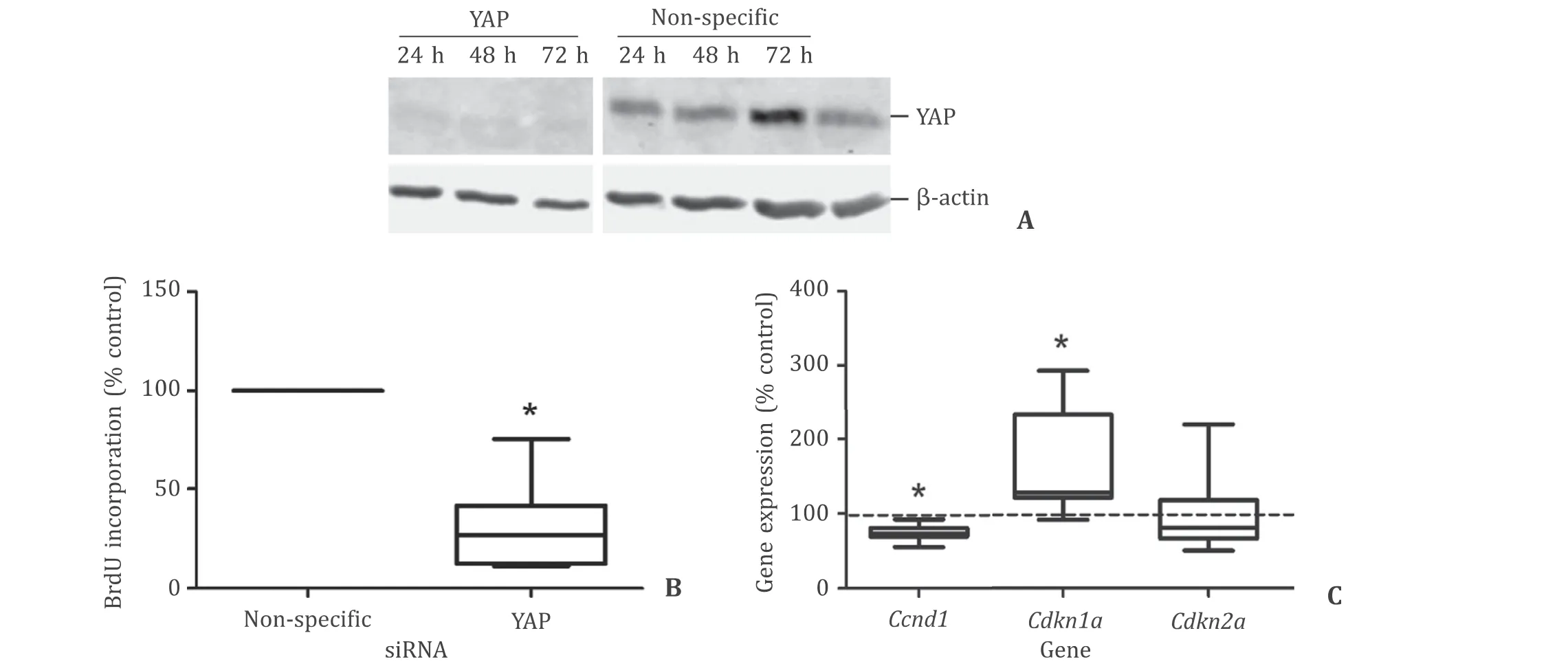
Fig. 3. Knockdown of YAP mitigates proliferation of immortalized pancreatic stellate cells. The effects of siRNA to YAP in LTC cells were studied by immunoblotting ( A ), BrdU incorporation assays ( B ) and gene expression analysis ( C ). Measurements were initiated 48 h post-transfection. Controls transfected with non-specific siRNA correspond to 100%. The mRNA expression of the indicated genes was quantified by TaqMan PCR and normalized to Hprt housekeeping gene expression. Box plots indicate the median, the 25th and 75th percentiles in the form of a box, and the 5th and 95th percentiles as whiskers ( n ≥9). ?: P < 0.05 versus controls.
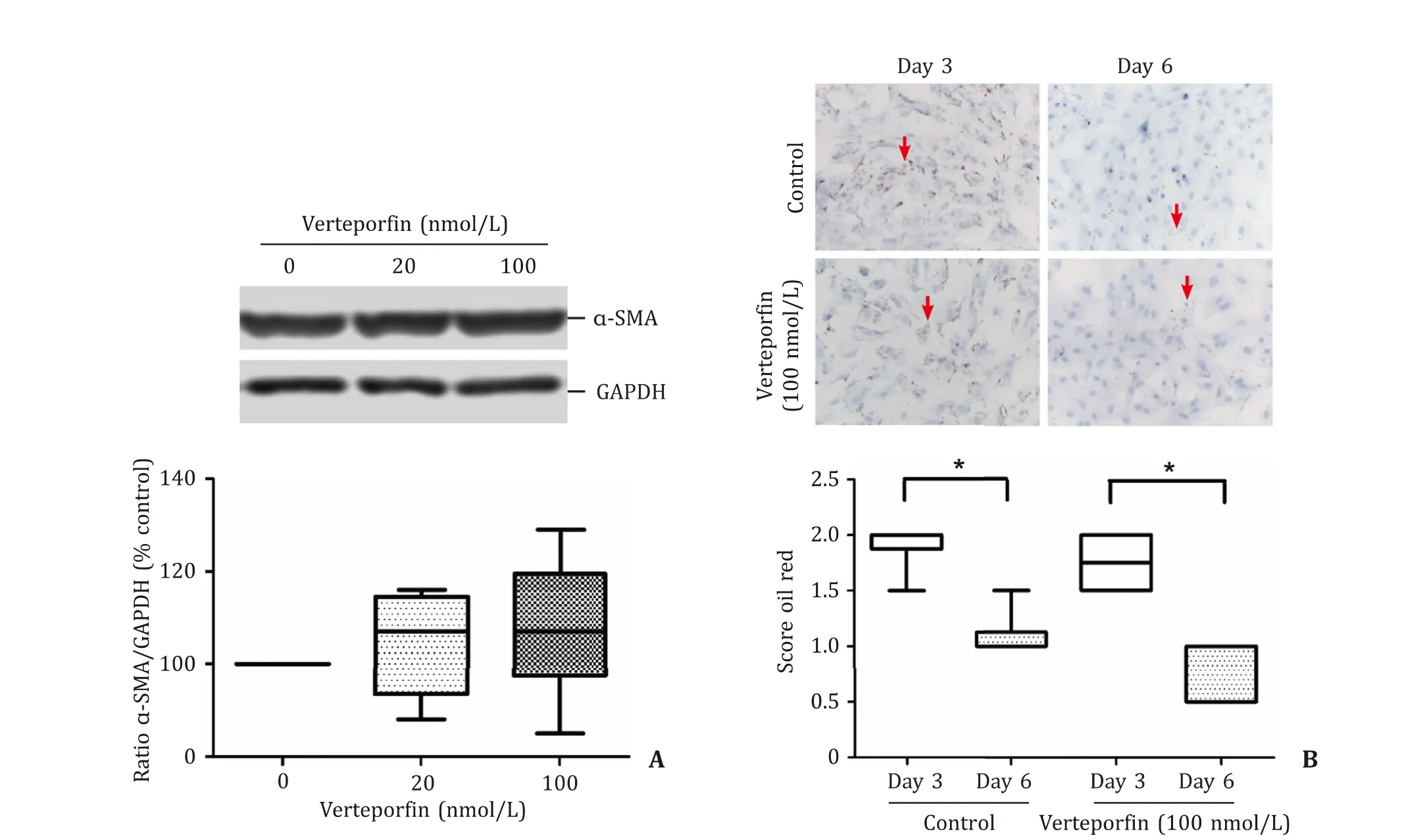
Fig. 4. Verteporfin has no effect on protein levels of α-SMA and storage of intracellular oil droplets. Pancreatic stellate cells of the first passage ( A ) and in primary culture( B ) were challenged with verteporfin as indicated. A: 48 h later, levels of α-SMA and GAPDH (housekeeping control) were assessed by immunoblot analysis (using the same membrane). Data were expressed as ratio α-SMA/GAPDH. Box plots indicate the median, the 25th and 75th percentiles in the form of a box, and the 5th and 95th percentiles as whiskers ( n ≥9). The blot shows a typical example of an individual experiment. B: On days 3 and 6 after application of verteporfin, samples were stained with oil red, and size and number of intracellular oil droplets were scored on a scale from 0 to 3. Upper panel: typical stains for all experimental conditions (original magnification, × 200).The arrows point to intracellular fat droplets. Lower panel: oil red scores of verteporfin-treated cultures and controls ( n ≥6). ?: P < 0.05 (day 3 versus day 6). There was no statistically significant difference between controls and verteporfin at day 6.

Fig. 5. Gene expression of verteporfin-treated pancreatic stellate cells. Recultured cells were treated with verteporfin (100 nmol/L) for 48 h. The mRNA expression of the indicated genes was quantified by TaqMan PCR and normalized to Hprt housekeeping gene expression. The mRNA expression of untreated cells (solvent exposure only) was set as 100%. Box plots indicate the median, the 25th and 75th percentiles in the form of a box, and the 5th and 95th percentiles as whiskers ( n = 12). ?: P <0.05 versus untreated controls.
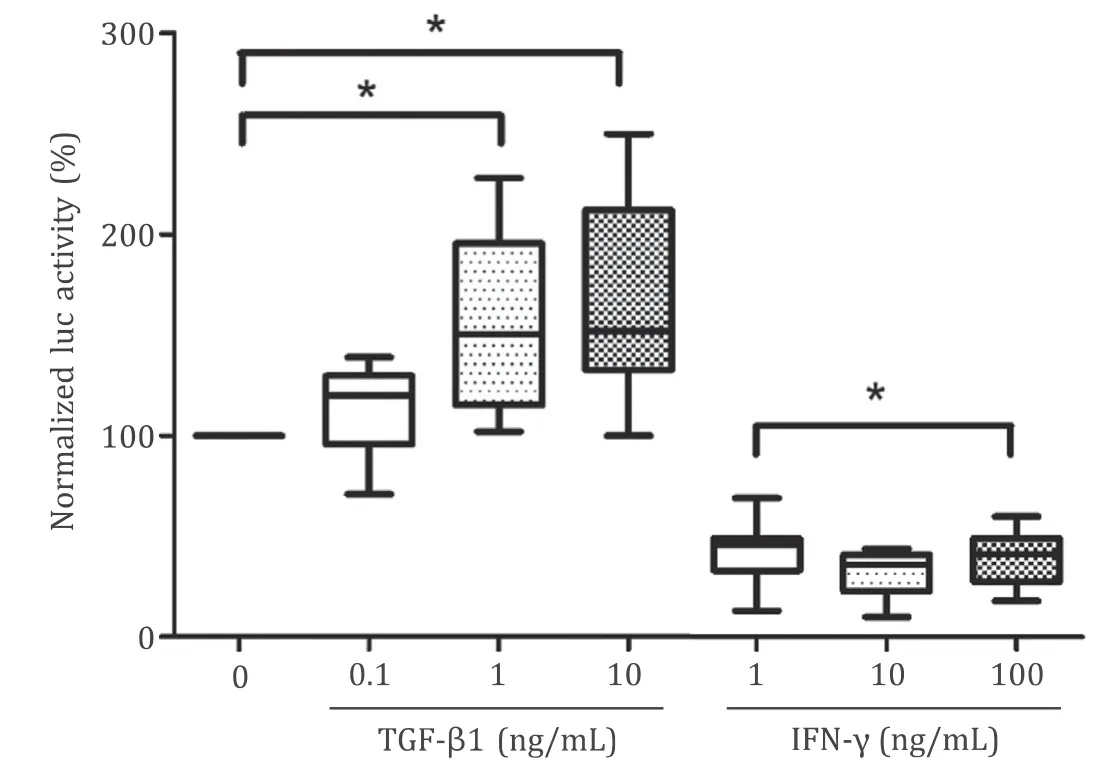
Fig. 6. Effects of TGF- β and IFN- γ on YAP activity. LTC cells were transfected with pHTS-GAL4, pGAL4-TEAD4 and pGL 4.70, before they were exposed to cytokines as indicated. After an incubation period of 24 h, luciferase activities were measured,and the ratio firefly/renilla luciferase was calculated (normalized luc activity). A ratio of 100% corresponds to untreated cultures. Box plots indicate the median, the 25th and 75th percentiles in the form of a box, and the 5th and 95th percentiles as whiskers ( n ≥11). ?: P < 0.05.
Discussion
In this study, we used a model of severe pancreatic injury with necroinflammation, fibrosis and incomplete regeneration, DBTCinduced CP in rats, to investigate YAP expression in the course of the disease. Besides putative ADM structures, especially the periductal fibrotic tissue stained positive for the protein. In contrast, in healthy pancreatic tissue YAP expression was low or absent.
To further investigate possible links between the Hippo pathway and pancreatic fibrogenesis, we cultured PSCs. Within the first week of primary culture, PSCs (isolated from healthy pancreatic tissues) undergo phenotypic changes that mimic PSCs activation in CP and PDAC [ 2 , 3 ]. The process involves cell proliferation, expression ofα-SMA, loss of vitamin A-containing fat droplets, synthesis of ECM proteins and secretion of various cytokines [ 2 , 3 ]. Studies on PSCs activation and on functions of activated PSCsinvitromay thus provide insights into basic mechanisms of pancreatic fibrosis [2–4] . Our results showed that the YAP-inhibitor verteporfin significantly diminished PSCs proliferation at nanomolar concentrations. Under these experimental conditions, there was no effect of the drug on YAP phosphorylation (data not shown), compatible with its action as an inhibitor of TEAD-YAP association [29] . An essential role of YAP in PSCs proliferation was also suggested by the results of knockdown studies in immortalized cells (which were chosen because of their better transfectability).
Since synthesis ofα-SMA and loss of intracellular lipid droplets were not prevented by the drug, the process of PSCs activation itself progressed in a YAP-unrelated manner.
At the mRNA level, inhibitory effects of verteporfin were observed forTgf-β1andCcn1.Tgf-β1acts as an autocrine mediator and enhancer of PSCs activation that efficiently triggers ECM synthesis [ 26 , 27 ].Ccn1(also known asCyr61) has previously been shown to enhance the progression of hepatocellular carcinoma by increasing hepatic stellate cell (HSC) activities [30] . Both genes are therefore of potential interest as targets of antifibrotic therapies.
Together, our findings point to a contribution of YAP signaling to PSCs proliferation and activity. Our data are in line with previous results [ 8 , 9 ], which demonstrated an antifibrotic efficiency of a clinically available YAP-inhibitor, verteporfin,invitro. It has to be noted that most of the effects of the drug, despite of their statistical significance, were nonetheless rather small. This smallness of the effects confirmed that induction and maintenance of PSCs activation occurred through a network of interacting signal transduction pathways (including different mitogen-activated protein kinase pathways [ 2 , 12 , 31 ]), which may have to be targeted simultaneously to achieve full antifibrotic efficiency. Noteworthy, YAP activity was stimulated by TGF-β1 but blocked by IFN-γ. The two cytokines exert antagonistic effects on PSCs by enhancing (TGF-β1)and inhibiting (IFN-γ) biological activities of the cells [26–28] .
Preclinical investigations [ 32 , 33 ] and pilot clinical studies [ 34 , 35 ] have suggested the benzoporphyrin derivative verteporfin as a potential candidate for photodynamic treatment of PDAC. The results of this study implicated, for the first time,that verteporfin had inhibitory effects on profibrogenic PSCs functions. The effects of the druginvivoshould be addressed in follow-up investigations using patient-derived samples and animal models of CP and PDAC.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Mrs. Katja Bergmann from Department of Medicine II, Division of Gastroenterology, Rostock University Medical Center, Rostock, Germany.
CRediT authorship contribution statement
Lennard Spanehl: Data curation, Formal analysis, Investigation,Methodology, Validation, Writing – original draft, Writing – review& editing. Denis Revskij: Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing –review & editing. Karen Bannert: Data curation, Formal analysis,Investigation, Methodology, Supervision, Validation, Writing – review & editing. Luise Ehlers: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing.Robert Jaster: Conceptualization, Data curation, Formal analysis,Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
None.
Ethical approval
All animal experiments were approved by the local animal use and care committee (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern; LALLF numbers 7221.3-1.2-023/10 and 7221.3-1-070/16).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2022年6期
Hepatobiliary & Pancreatic Diseases International2022年6期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Combined analysis of imaging tumor capsule with imaging tumor size guides the width of resection margin for solitary hepatocellular carcinoma
- Prediction of early recurrence of hepatocellular carcinoma after liver transplantation based on computed tomography radiomics nomogram
- Complications of modern pancreaticoduodenectomy: A systematic review and meta-analysis
- Technical aspects in pancreaticoduodenectomy and therapeutic strategies for pancreatic cancer: History, current status, and future perspectives
- Contrast-enhanced ultrasound predicts microvascular invasion in patients with hepatocellular carcinoma
- Safety and immunogenicity of COVID-19 vaccination among liver transplant recipients in China
