Description of two new species of Hemiphyllodactylus(Reptilia: Gekkonidae) from karst landscapes in Yunnan, China, highlights complex conservation needs
Ade Prasetyo Agung, Ada Chornelia, L. Lee Grismer, Jesse L. Grismer, Evan S. H. Quah, Jian-Mei Lu,Kyle W. Tomlinson, Alice C. Hughes
1 Landscape Ecology Group, Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan 666303, China
2 University of Chinese Academy of Sciences, Beijing 101408, China
3 Herpetology Laboratory, Department of Biology, La Sierra University, Riverside, California 92515, USA
4 Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Jalan UMS, Kota Kinabalu, Sabah 88400, Malaysia
5 Lee Kong Chian Natural History Museum, National University of Singapore, Singapore 117377, Singapore
6 Community Ecology and Conservation Group, Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan 666303, China
7 School of Biological Sciences, University of Hong Kong, Pokfulam, Hong Kong SAR, China
ABSTRACT Karst habitats are hotspots of diversity and endemism. Their naturally fragmented distributions across broad geographic landscapes have led to the complex array of smaller evolutionary ecosystems that present unique challenges from a conservation perspective. Comprehensive biodiversity assessments of karst habitats have revealed that these ecosystems contain an almost unparalleled level of endemism, and many site-restricted species remain undescribed, thus posing considerable challenges for effective conservation management.Small rock-dwelling species, such as geckos, may be particularly prone to such isolation. In this paper, we discuss one such genus, i.e.,Hemiphyllodactylus, and explore its diversity across karst landforms in Yunnan Province, southwestern China. Based on morphological and genetic data, we describe two new species of Hemiphyllodactylus from karst habitats in Simao District and Yanshan County. A phylogenetic tree for Hemiphyllodactylus was constructed using 1 039 base pairs (bp) of the mitochondrial NADH dehydrogenase subunit 2 gene(ND2). The Simao and Yanshan specimens can be distinguished from all other congeners within their respective subclades based on uncorrected genetic pairwise distances greater than 6.3% and 4.3%respectively, as well as significant morphological differences. The discovery and description of these two new species brings the total number of described Hemiphyllodactylus species in China to 14 and indicates many more undescribed species from unsurveyed karst regions await discovery. Our findings suggest that karst ecosystems in Yunnan support a higher diversity of Hemiphyllodactylus than previously known. This study also highlights the importance of karst ecosystems as refugia for sitespecific endemic species and the need for heightened conservation efforts.
Keywords: Discovery; Endemism; Geckos;Reptiles; South China
lNTRODUCTlON
Karst landscapes are characterized by high endemism due to their distinct ecological niches, which allow for the diversification of a wide variety of species (Clements et al.,2006; Grismer et al., 2021). However, these naturally fragmented ecosystems are challenging, as each lone karst formation may host species found nowhere else. Almost every research expedition of karst landforms (both caves and outer rocky surfaces) has uncovered new species with localized distributions (Agung et al., 2021; Dittmar et al., 2005; Huang et al., 2019; Quah et al., 2021; Tian & Huang, 2015), thereby identifying karsts as hotspots of endemism and biodiversity and priorities for conservation. Karst species are also often highly specialized, with poor dispersal capabilities due to their adaptations to the unique abiotic environments of karst ecosystems, such as microclimate, light intensity, and topography (e.g., fissured cliffs) (Whitten, 2009). Thus,understanding the distributions and patterns of endemism and diversity is critical for developing effective conservation plans for the region as many cryptic herptiles may have been overlooked (Vieites et al., 2009).
As the largest family of geckos, Gekkonidae shows high levels of endemism in karst systems. For example, Grismer et al. (2014, 2018b, 2018c, 2020a, 2021) has reported over 100 gecko species endemic to karsts in Southeast Asia,underscoring the need for further work to gain a more complete understanding of the diversity and range of karstdependent taxa. Given their occurrence in fragmented karst hills and their limited dispersal capabilities, it is likely that more species are waiting to be described. In support of this, Grismer et al. (2018a) identified 12 new gecko species within two weeks in a single study of karsts in Myanmar, with similar patterns likely to exist in karsts across Southeast Asia.
HemiphyllodactylusBleeker, 1860 (commonly known as half leaf-fingered geckos, dwarf geckos, or slender geckos),belongs to the family Gekkonidae. Recently, many new species of this genus have been discovered in vegetated karst ecosystems (Do et al., 2020; Grismer et al., 2018b; Nguyen et al., 2020; Zhang et al., 2020), many of which are endemic to single karst hills. However, like other small organisms (e.g.,snails, millipedes, and other invertebrates), this genus is often overlooked, and given the highly endemic nature of the group,more targeted protection is clearly needed.
The genus is widely distributed across South Asia,Southeast Asia, South China, and the western Pacific islands(Agarwal et al., 2019; Grismer et al., 2013; Zug, 2010).Generally,Hemiphyllodactylusspecies are small in body size(snout-vent length<63 mm), nocturnal, scansorial, forestdwelling, and well camouflaged in their environments (Grismer et al., 2013; Zug, 2010), and thus easily overlooked unless specifically targeted. Most members of the genus are confined to tropical and subtropical montane regions in mainland Indochina, although some are also restricted to islands(Agarwal et al., 2019; Eliades et al., 2019; Grismer et al.,2013; Zug, 2010). To date,Hemiphyllodactylusconsists of two main groups, i.e.,hartertiandtypus. Thehartertigroup is composed of upland species from Peninsular Malaysia, while thetypusgroup is comprised of all other species from the entire range of the genus (Grismer et al., 2013). Many of these species are limited to a single site or a limited number of sites,and some “species” are suspected to represent complexes that require further work for accurate species delineation and description. This is especially important given the high rates of karst loss across Southeast Asia and South China, estimated to be 5.7% per year (Hughes, 2017). Thus, without information to ensure the identification of key sites, there is significant potential for species loss (Hughes, 2017).
In recent years,Hemiphyllodactylusresearch and discoveries have experienced a renaissance, with species descriptions increasing every year from 2013. Since then, the number of species has jumped from 14 to 52, mostly from karst regions in Myanmar, Laos, and Vietnam (Uetz et al.,2021). In contrast, gekkonid research in karst regions of South China continues to lag, highlighting the need for in-depth exploration of these areas. This deficiency in field research has resulted in an underestimation ofHemiphyllodactylusdiversity in China. For example, in Yunnan Province, all populations ofHemiphyllodactyluswere previously considered to be a single widespread species (H. yunnanensis), until integrative taxonomic study revealed multiple species under the nomenH. yunnanensis(Grismer et al., 2013).
At present, 12 described species ofHemiphyllodactylusare found in South China: i.e.,H. changningensisGuo, Zhou, Yan& Li, 2015;H. dupanglingensisZhang, Qian & Yang, 2020;H.dushanensisZhou & Liu, 1981;H. hongkongensisSung, Lee,Ng, Zhang & Yang, 2018;H. huishuiensisYan, Lin, Guo, Li &Zhou, 2016;H. jinpingensisZhou & Liu, 1981;H. longlingensisZhou & Liu, 1981;H. typusBleeker, 1860;H. yunnanensisBoulenger, 1903;H. zayuensisJiang, Wang, & Che, 2020,H.zhutangxiangensisAgung, Grismer, Grismer, Quah,Chornelia, Lu & Hughes, 2021; andH. zugiNguyen,Lehmann, Le Duc, Duong, Bonkowski & Ziegler, 2013.
Based on fieldwork in the karst areas of Yunnan, we identified several potential new species, including the recently described and published speciesH. zhutangxiangensis(Agung et al., 2021). Here, we describe two new species, with our preliminary work also suggesting several other species awaiting description. In the current study, we phylogenetically delimited new evolutionary lineages based on molecular evidence and diagnosed those lineages based on morphological evidence, with descriptions of the new species.We also discuss the relevance of mapping distributions such as these as a basis for conservation of range-limited, siteendemic species that may be particularly vulnerable to extinction without better inclusion in conservation and environmental impact assessments.
MATERlALS AND METHODS
Field sites and specimen collection
Surveys were conducted in August to September 2018 and June to July 2019 in karst landscapes across Yunnan.Permission to conduct surveys and collect samples was granted by relevant local protected area authorities and ethics approval for research was granted by the Ethics Committee of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, China. In total, 139Hemiphyllodactylusspecimens were collected by hand from 13 different karst areas (Table 1). Specimens were collected at night (between 2000h-2200h) as geckos are nocturnal and actively forage during this time. AllHemiphyllodactylusspecimens were photographed in the morning after capture to record coloration and patterns. The specimens were euthanized using Tricaine MS-222 solution injected into the intracelomic cavity (Conroy et al., 2009). Tissue samples were obtained from the liver of each individual and stored in 95% ethanol separately for further genetic analysis. The specimens were then preserved in 10% formalin and transferred to 70% ethanol for storage prior to morphological investigation. After investigation, the specimens were deposited in the Kunming Natural History Museum of Zoology, Kunming Institute of Zoology (KIZ),Chinese Academy of Sciences, China. All animal procedures were performed in accordance with the ethical standards of the institution at which the study was conducted(Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences).
DNA isolation, sequencing, and phylogenetic analyses
Genomic DNA of the 139 newly collected specimens was isolated from liver tissue following proteinase K DNA extraction protocols using a QIAGEN Genomic-tip 2500(www.qiagen.com) DNA extraction kit. We amplified the complete mitochondrial NADH dehydrogenase subunit 2 gene(mtDNA-ND2), totaling 1 039 bp, using primers L4437b and H5934 following Macey et al. (1997) (5'-AAGCAGT TGGGCCCATACC-3' and 5'-AGRGTGCCAATGTCTTTG TGRTT-3', respectively). The protocol for polymerase chain reaction (PCR) amplifications followed Agung et al. (2021).The PCR processes and sequencing were executed at the South China DNA Barcoding Center.
We constructed a dataset for phylogenetic analyses. We downloaded a total of 209ND2sequences from GenBank,containing 205 sequences of extantHemiphyllodactylusspecies and fourND2sequences of other outgroup taxa(Gehyra fehlmanni,G. mutilata,Hemidactylus frenatusandLepidodactylus lugubris), then added the 139 new sequences to the dataset. All downloaded sequences used in the analyses followed Agung et al. (2021), while the newly published sequences in this study are presented in Supplementary Table S1.
Phylogenetic relationships were analyzed using maximumlikelihood (ML) and Bayesian inference (BI) in IQ-TREE(Nguyen et al., 2015; Trifinopoulos et al., 2016) and MrBayes 3.2.7a (Ronquist et al., 2012) on XSEDE using the CIPRES Science Gateway (Cyberinfrastructure for Phylogenetic Research; Miller et al., 2010), respectively. Prior to ML analysis, the best substitution model (TIM+F+R5) was selected for the non-partitioned dataset based on Bayesian information criterion (BIC) in ModelFinder (Kalyaanamoorthy et al., 2017). The ultrafast bootstrap approximation algorithm(UFBoot) was used with 1 000 bootstrap pseudoreplicates(Hoang et al., 2018), where nodes bearing values ≥95 were considered strongly supported (Minh et al., 2013). For BI analysis, default priors were selected, and two independent Markov Chain Monte Carlo (MCMC) algorithms were applied,with four chains in each (three hot and one cold), 50 million generations sampled every 1 000 generations, and the first 25% of samples discarded. All parameters from the two runs were checked in Tracer v1.7.1 (Rambaut et al., 2018),confirming convergences and effective sample sizes (ESS)were >200. Post burn-in sampled trees from both runs were combined and a 50% majority-rule consensus tree was produced. Nodes bearing Bayesian posterior probabilities(BPP) ≥0.95 were considered strongly supported(Huelsenbeck et al., 2001; Wilcox et al., 2002). Uncorrectedpairwise distances among and within species were computed using MEGA 7 (Kumar et al., 2016).
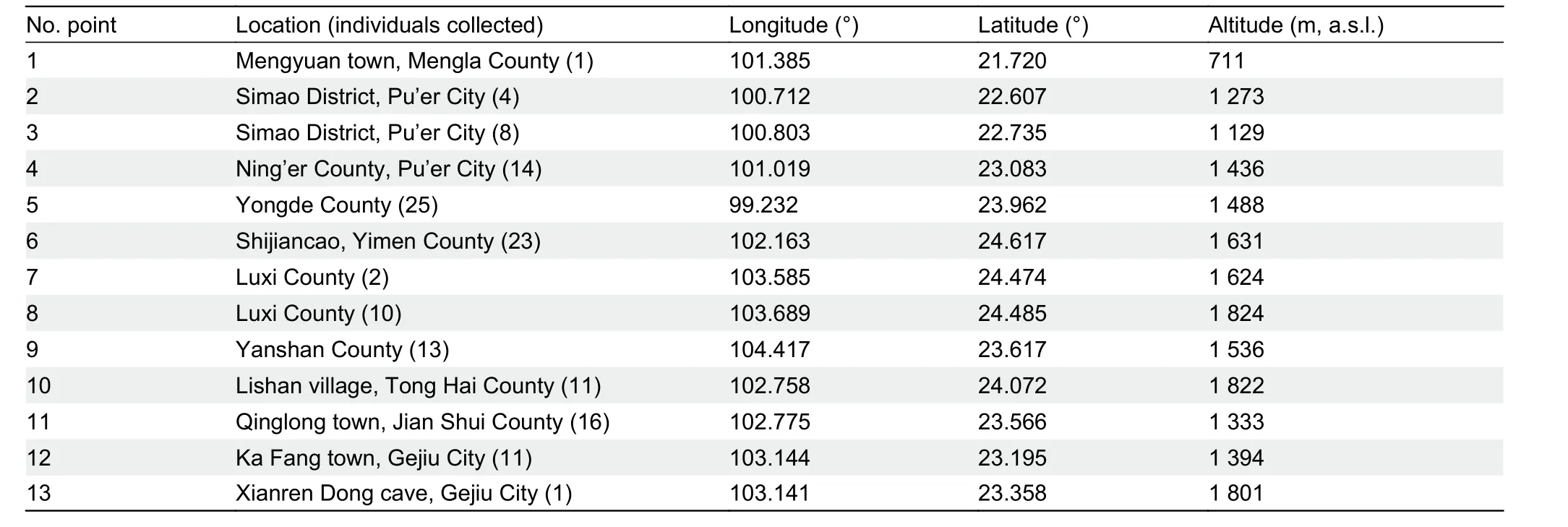
Table 1 Location of the 13 selected karst field sites in Yunnan, China
Morphological measurements and analyses
We measured mensural and meristic traits of the 139 collected specimens following Zug (2010) and Grismer et al. (2013),with slight modifications following Agung et al. (2021).Mitutoyo Absolute Series-500 digital calipers (accuracy 0.01 mm) were used to measure mensural traits under a dissecting microscope (Nikon SMZ 445), on the left side of the body when possible. Recorded traits included: snout-vent length(SVL), trunk length (TL), head length (HL), head width (HW),eye diameter (ED), snout-eye length (SnEye), naris-eye length(NarEye), and snout width (SnW). Recorded meristic traits included: circumnasal scales (CN), internasal scales (IS),supralabial scales (SL), infralabial scales (IL), chin scales(Chin), ventral scales (VS), dorsal scales (DS), subdigital lamellae wider than long on first finger (SL1F) and toe (SL1T),subdigital lamellae formula determined as number of Ushaped digital pads on digits II-V of hands and feet, number of femoroprecloacal pores, and number of cloacal spurs(CloacS) on each side of hemipenial swelling. We also noted coloration and pattern on the dorsum, presence or absence of dark postorbital stripes extending at least to neck, presence or absence of dorsolateral and ventrolateral stripes, and presence or absence of anteriorly projecting arms of postsacral markings.
We compared the morphology of each new lineage recovered from phylogenetic analysis against published morphological data for selected closely related species to establish significant differences between any of the measured traits. Prior to analysis, we corrected for the effects of body size on mensural traits in each new lineage combined with data from closely related species using the following equation:

whereXadj is the adjusted value;Xis the measured value; β is the unstandardized regression coefficient for each operational taxonomic unit (OTU); SVL is the measured SVL;and SVLmean is the overall average SVL of all OTUs(Lleonart et al., 2000; Thorpe, 1975, 1983; Turan, 1999).
All morphological analyses were computed in R v.4.0.1 (R Core Team, 2020). Each adjusted mensural trait was checked for equal variances across groups using Levene’s test. Traits with equal variances (P≥0.05) were analyzed using ordinary linear models, while traits with unequal variances (P≤0.05)were analyzed using generalized linear models with weighted least squares (gls) in the "nlme" package (Pinheiro et al.,2020). Error degrees of freedom (df) were calculated with the Satterthwaite approximation, applying theemmeansfunction in the package “emmeans” (Lenth, 2021), which compares appropriate estimates for uneven group variances. For meristic traits (count data), glm models were used. Quasi-Poisson errors were implemented in the glm models as all traits were under-dispersed when checked with thedispersiontestfunction in the “AER” package (Kleiber &Zeileis, 2008). For both mensural and meristic traits,significant differences were first evaluated with a variance test(analysis of variance (ANOVA) or Chi-square tests), and, if significant, subjected toposthocTukey’s HSD tests for mean comparisons involving three or more groups. We also used non-parametric permutation ANOVA (PERMANOVA) to determine whether the posited species differed from closely related species in multi-trait space using theadonisandpairwise.adonisfunctions in the “vegan” package (Oksanen et al., 2020) with 50 000 permutations. Lastly, principal component analysis (PCA) was run to test for group separation along the first two principal components using theprcompfunction.
Designation of species-level lineages
We used an integrated approach to delineate species-level lineages by consolidating phylogenetic position, genetic divergence, and morphological differences. We used three sequential criteria to designate species-level lineages: First,new lineages not clustered within the named species lineages in phylogenetic topology were marked as potential new species. Second, uncorrected pairwise genetic distances among the new lineages with either known species or other putative species lineages were measured, with a 3.0%difference in mtDNAND2considered minimal to define a potential new species. This cut-off was based on Zhang et al.(2020), who reported a 3.6% sequence divergence between
H. linnwayensisandH. ywanganensisafter uncorrected pairwise comparison of the 670 bp (partial)ND2gene across numerous species, with these two species known to be morphologically distinct (Grismer et al., 2018c). Third, those lineages showing >3.0% genetic difference from their nearest relatives were examined for morphological distinctiveness from closely related species. If this third criterion was also met, the lineage was considered a confirmed new species.Lineages satisfying all three criteria were described.Individuals not satisfying all criteria may also be distinct species, but further data and specimens are needed for this to be established.
RESULTS
Phylogeny
The ML and BI analyses recovered similar tree topologies with strong nodal support, but with a slight difference in the position ofH. khlonglanensis, i.e., sister species to clades 3 and 4 in the ML tree, but sister species to clade 4 only in the BI tree.Figure 1 shows the topology of the ML tree, including both nodal support values (UFBoot/BPP). Our inferred ML topology was highly consistent with the topology reported by Grismer et al. (2020b). We named all clades following Grismer et al.(2020b) and renamed the South Myanmar lineage, which consisted ofH. zwegabinensis,H. kyaiktiyoensis, andH.pinlaungensis, as clade 8.
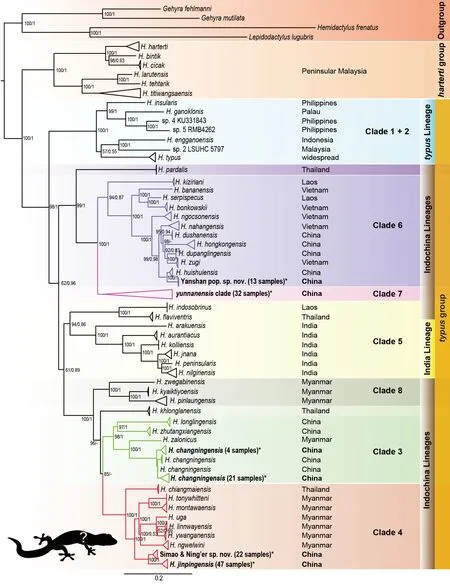
Figure 1 Maximum-likelihood topology illustrating phylogenetic relationships among Hemiphyllodactylus species
All 139 Yunnan karst specimens belonged to thetypusgroup and were placed into four clades: i.e., clades 3, 4, 6,and 7 (in bold in Figure 1). All specimens were nested and formed OTUs as follows: 25 specimens were from Yongde County, recovered asH. changningensis(clade 3); 47 specimens were from Jian Shui County, Yimen County, and Gejiu City, recovered asH. jinpingensisand sister to the newly recovered OTU comprised of 22 specimens from Simao District and Ning’er County (clade 4); 13 specimens were from Yanshan County, recovered as a new OTU (clade 6) and sister toH. huishuiensis; and 32 specimens were from multiple locations, including Mengla County, Simao District, Luxi County, Jianshui County, Gejiu City, and Tonghai County, and nested within theyunnanensisclade complex (clade 7). Theyunnanensisclade (clade 7) consisted of multiple distinct evolutionary lineages, and further analysis is needed to resolve their relationships and taxonomy.
The ML and BI trees recovered two distinct evolutionary lineages in our Yunnan samples, i.e., Simao and Ning’er County population (hereafter referred to as Simao population)in clade 4 and Yanshan County population in clade 6, as described below.
Genetic distance
Uncorrected geneticP-distances among and within theND2gene of the newly recovered OTUs (within clades 4 and 6) are presented in Tables 2, 3. The interspecific genetic distances within clade 4 ranged from 2.7% (betweenH. ywanganensisandH. linnwayensis) to 14.3% (betweenH. chiangmaiensisandH. jinpingensis), and the new Simao population showed at least 6.3% genetic distance to its sister speciesH. jinpingensis(Table 2).
In clade 6, interspecific genetic distances ranged from 4.3%(between new Yanshan population and sister speciesH.huishuiensis) to 23.0% (betweenH. kizirianiandH. banaensis;Table 3). The genetic distances of the two new populations(Simao and Yanshan) within their clades (clades 4 and 6,respectively) were above 3%, the threshold used to delineate a new species in this study (see Methods).
Morphological analysis of Simao population
We compared the Simao population to members within clade 4, i.e.,H. jinpingensis,H. montawaensis,H. ngwelwini, andH.tonywhitteni, as they were the closest relatives to the Simao population containing more than two individuals in each species (P-distance=6.3%, 10.2%, 10.5%, and 10.8%,respectively; Table 2). The rawH. jinpingensisdata used for statistical analysis were obtained from our specimens (45 individuals out of 47 collected from various locations in Yunnan) as no appropriate raw data were available from previous studies. The rawH. jinpingensisdata are provided in Supplementary Table S2. The raw data forH. montawaensisandH. tonywhitteniwere obtained from Grismer et al. (2018b),and forH. ngwelwiniwere obtained from Grismer et al.(2020a). Linear and general linear model analyses of each morphological trait in the Simao population and its congeners showed that seven mensural traits and eight meristic traits differed significantly among the five groups (Table 4).
Post hocmultiple comparison tests showed significant differences in two mensural and five meristic traits between theH. jinpingensisand Simao specimens, although it should be noted that there was overlap in the recorded range of values among individuals across species (see Supplementary Table S3). Of the two mensural traits,H. jinpingensishad greater ED, whereas the Simao specimens had greater HW.Furthermore,H. jinpingensishad higher values in five meristic traits (VS, SL, IL, SL1F, and SL1T) compared to the Simaospecimens. Two mensural and five meristic traits differed significantly between theH. montawaensisand Simao specimens. Of the two mensural traits,H. montawaensishad greater HL, whereas Simao specimens had greater HW. Of the five meristic traits,H. montawaensishad more VS, but fewer SL, IL, SL1T, and Chin. One mensural and three meristic traits differed significantly between the Simao specimens andH. ngwelwini, notably the Simao specimens had shorter HL and fewer VS and Chin, but more IL. Two mensural and seven meristic traits differed significantly between the Simao specimens andH. tonywhitteni.Of the two mensural traits, the Simao specimens had a shorter HL, but greater HW. For the meristic traits, the Simao specimens had more CN, SL, IL, SL1F, and Chin, but fewer DS and VS(Figure 2).

Table 2 Uncorrected genetic P-distances (%) in ND2 (1 039 bp) gene of Hemiphyllodactylus in clade 4
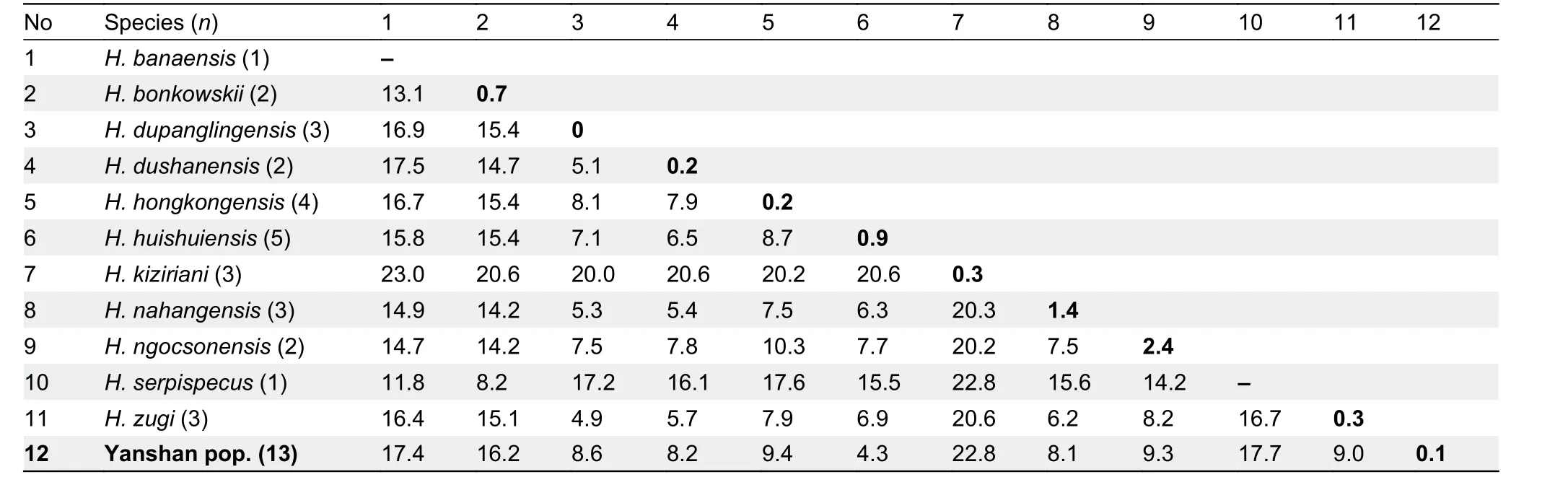
Table 3 Uncorrected genetic P-distances (%) in ND2 (1 039 bp) gene of Hemiphyllodactylus in clade 6

Table 4 Summary statistics for each trait of compared species in clade 4
Multi-trait spatial analysis using PERMANOVA pairwise comparisons indicated that the five groups (Simao specimens,
H. jinpingensis,H. montawaensis,H. ngwelwini, andH.tonywhitteni) differed significantly from each other (Table 5). In addition, clustering based on PCA showed that the Simao specimens were morpho-spatially distinct and clustered separately along PC1 with respect toH. montawaensisandH.tonywhitteni, but partially overlapped with respect toH.jinpingensisandH. ngwelwini, with 26.7% of the variation loaded most heavily for HL and IL (see Supplementary Table S4 for PCA scores on each trait). For PC2, all groups overlapped with each other, except forH. montawaensisandH. tonywhitteni(Figure 3). Details on statistical results for group separation along the two axes are presented in Table 6.
Morphological analyses of Yanshan population
We compared the Yanshan population toH. huishuiensis(raw data obtained from Yan et al., 2016) andH. nahangensis(raw data obtained from Do et al., 2020), as the latter two species were the closest relatives of the Yanshan population based on phylogeny and uncorrected genetic distance (Pdistance=4.3% and 8.1%, respectively; Table 3). Linear and general linear model analyses of each morphological trait in the Yanshan population and its two closely related species identified significant differences in seven mensural and seven meristic traits (Table 7).
Post hocmultiple comparison tests showed that six mensural and five meristic traits differed significantly between theH. huishuiensisand Yanshan specimens, although it should be noted that there was overlap in the recorded range of values among individuals across species (see Supplementary Table S5). Of the six mensural traits,H.huishuiensishad greater TrunkL and HL, whereas Yanshan specimens had greater HW, NarEye, SnEye, and SnW. Of the five meristic traits,H. huishuiensishad more IS, whereas Yanshan specimens had more CN, CloacS, SL1F, and SL1T.Seven mensural and five meristic traits differed significantly between the Yanshan specimens andH. nahangensis. Of the seven mensural traits, Yanshan specimens had greater HW,NarEye, SnEye, and SnW, whereasH. nahangensishad greater TrunkL, ED, and HL. Of the five meristic traits,Yanshan specimens had more CN, SL1F, and SL1T, whereasH. nahangensishad more DS and IS (Figure 4).
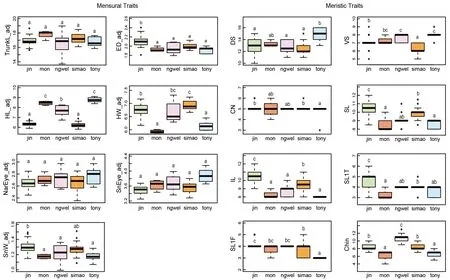
Figure 2 Differences in adjusted mensural (left) and meristic (right) traits between Simao population and closely related species
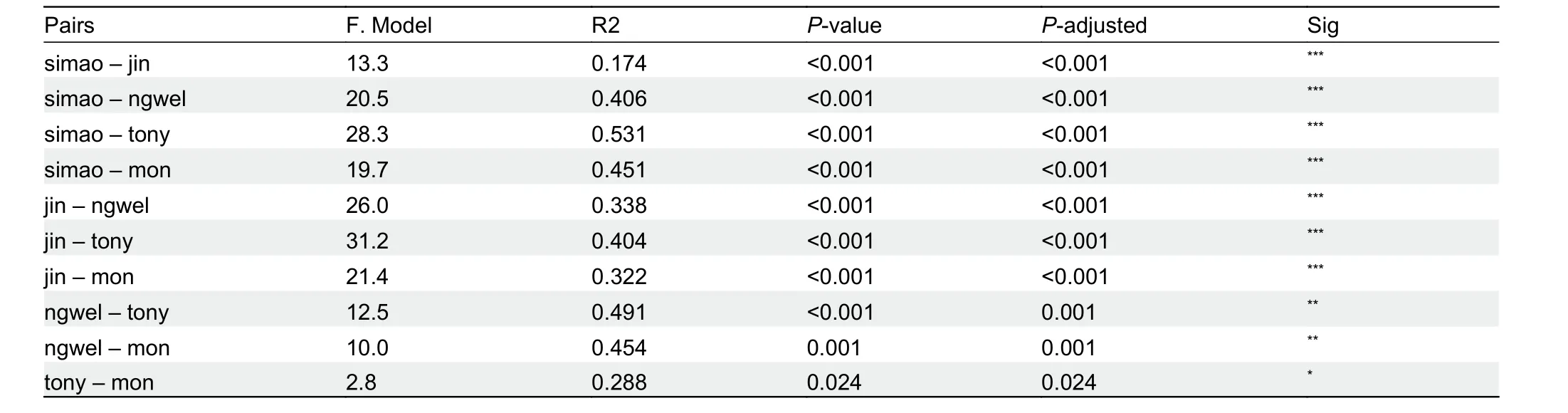
Table 5 PERMANOVA pairwise comparisons of multi-trait space of compared species within clade 4
Multi-trait spatial analysis using PERMANOVA pairwise comparison indicated that the three groups (Yanshan population,H. huishuiensis, andH. nahangensis) differed significantly from each other (Table 8). In addition, clustering based on PCA showed that specimens from Yanshan,H.huishuiensis, andH. nahangensiswere morpho-spatially distinct and clustered separately along PC1, which accounted for 45.7% of variation in the dataset and was loaded most heavily for SL1T, CN, and SnW (see Supplementary Table S6 for PCA scores on each trait). The Yanshan specimens overlapped withH. huishuiensisandH. nahangensisalong PC2, which accounted for 15.5% of variation in the dataset and was loaded most heavily for DS and VS (Figure 5).Details on statistical results for group separation on the two axes are presented in Table 9.
Systematics
Based on phylogenetic evidence, uncorrected genetic distance, and lineage distinction from significantly different morphological characters, the Simao and Yanshan populations likely constitute new evolutionary lineages, and thus represent two new species, as described below.
Hemiphyllodactylus simaoensis sp. nov. (Figure 6)
Suggested English name: Simao slender gecko Suggested Chinese name: 思茅半葉趾虎

Figure 3 Principal component analysis (left) and biplot (right) of compared species within clade 4
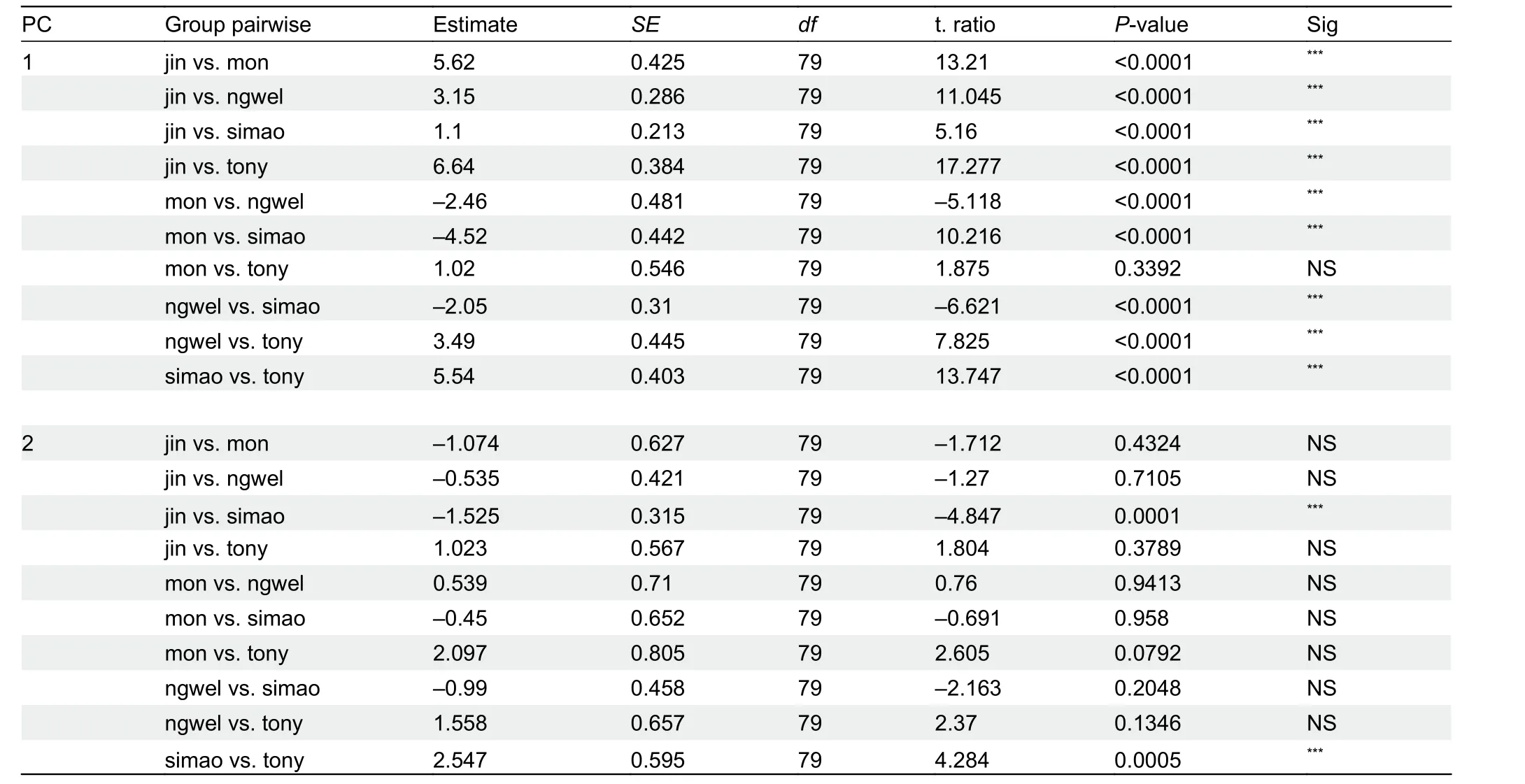
Table 6 Test results for group separation of compared species within clade 4 along first two PCA axes (PC1 and PC2)
Holotype: Adult male (KIZ 062064) collected on 6 July 2019 by Ade P. Agung, Jian-Mei Lu, and Zong-Bao Yang from forested karst hills in Simao District, Pu’er City, Yunnan Province, China (N22.735°, E100.803°; 1 129 m a.s.l.).
Paratypes:Four adult males (KIZ 062063, KIZ 062066-KIZ 062068) and three adult females (KIZ 062065, KIZ 062069-KIZ 062070), same data as holotype. Five adult males (KIZ 062072, KIZ 062073, KIZ 062077, KIZ 062079,KIZ 062087) and nine adult females (KIZ 062074-KIZ 062076,KIZ 062080-KIZ 062084, KIZ 062088) were collected on 16-17 August 2018 by Ade P. Agung, Ada Chornelia, Jian-Mei Lu, L. Lee Grismer, Jesse L. Grismer, Evan S.H. Quah, Brian Folt, and Myin Kyaw Thura from forested karst in Ning’er County, Pu’er City, Yunnan Province, China (N23.083°,E101.019°; 1 436 m a.s.l.).
Diagnosis:Hemiphyllodactylus simaoensissp. nov.can be distinguished from all congeners by a unique combination of the following characters: maximum SVL 40.87 mm; chin scales 7-10; enlarged postmentals; circumnasal scales 5-6;internasal scales 1-4; supralabial scales 8-12; infralabial scales 8-11; subdigital lamellae on fingers II-V (3 or 4)-(3-5)-(3-5)-(3 or 4); subdigital lamellae on toes II-V (3 or 4)-(3-5)-(3-5)-(3 or 4); dorsal scales 11-15; ventral scales 5-7; palegray base color on body, two lines of dark blotches running from neck to sacrum on dorsal side; dark postorbital stripe extending at least to base of neck; dorsolateral stripe indistinct or completely absent; ventrolateral stripe on trunk absent; dark postsacral markings bearing anteriorly projecting arms.
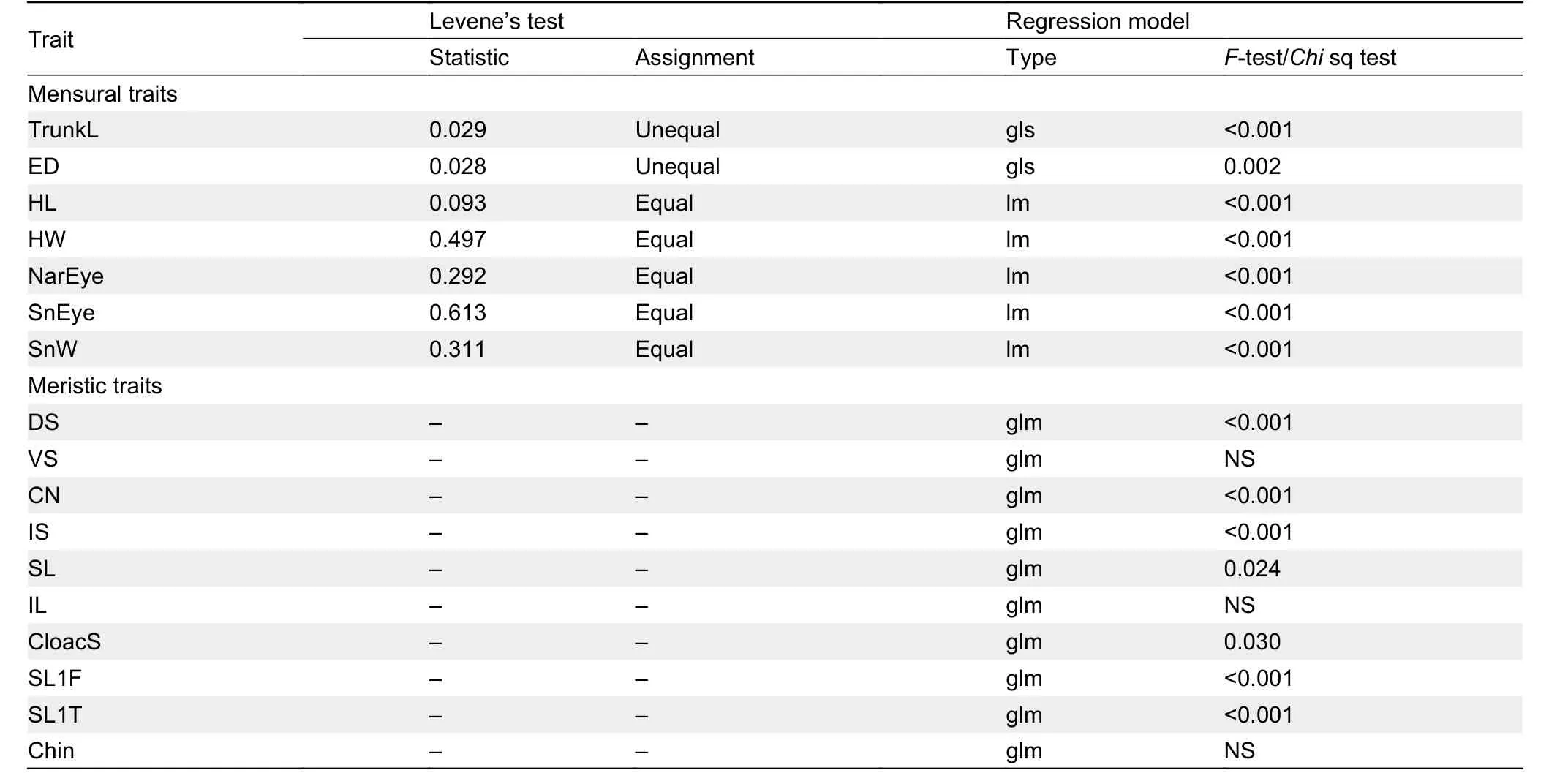
Table 7 Summary statistics for analysis of each trait of Yanshan population and selected congeners
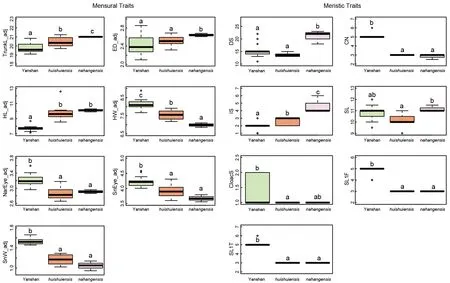
Figure 4 Differences in adjusted mensural (left) and meristic (right) traits between specimens from Yanshan population and closely related species (H. huishuiensis and H. nahangensis)

Table 8 PERMANOVA pairwise comparisons of multi-trait space of compared species within clade 6
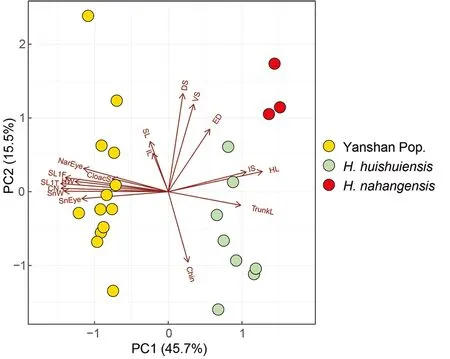
Figure 5 PCA of Yanshan population, H. huishuiensis, and H.nahangensis showing morphospatial relationships along first two components
Description of holotype: Adult male, SVL 34.06 mm; head triangular in dorsal profile, depressed, distinct from neck (HL 6.03 mm; HW 6.77 mm); lores flat; snout long (SnEye 3.48 mm; SnEye/HL 58%) and narrow (SnW 1.26 mm; SnW/HW 19%); eyes large (ED 1.96 mm; ED/HL 33%); rostral scale wider than high, bordered posteriorly by two large supranasals and three internasals (IS); nares bordered anteriorly by rostral scale, ventrally by first supralabial scale, dorsally by supranasal scale, posteriorly by three postnasals; supralabials square, 10/9 (left/right), tapering from rostral to point in line with posterior margin of orbit (SL); infralabials square, 10/9(left/right), tapering from mental to point in line with posterior margin of orbit (IL); scales on head small, rounded, largest on rostrum; mental triangular, bordered by first infralabials and posteriorly by two enlarged postmentals; each postmental bordered anterolaterally by first infralabial; eight chin scales touching internal edges of infralabials from juncture of 2ndand 3rdinfralabial scales on left of mental scale to same juncture on right (Chin); scales in gular region rounded, nonoverlapping, becoming larger and more ovoid on venter. Body type robust and small, (TrunkL/SVL 49%), dorsoventrally compressed; dorsal body scales small, granular, 12 dorsal scales at midbody contained within one eye diameter; ventral body scales smooth and flat, much larger than dorsal scales,subimbricate, seven ventral scales at midbody contained within one eye diameter; forelimbs relatively short, covered dorsally with granular, subimbricate scales, smaller smooth scales ventrally; palmar scales flat, unevenly shaped, nonoverlapping; finger I vestigial, clawless, fingers II-V well developed; proximal subdigital lamellae undivided,rectangular; distal subdigital lamellae divided and undivided,angular, U-shaped, except terminal lamellae rounded,undivided; lamellar formula on fingers II-V (3)-(4)-(4)-(3) on both hands; claws on fingers II-V well developed, unsheathed,strongly curved; hind limbs short, covered dorsally with granular, subimbricate scales, smaller smooth scales ventrally; plantar scales flat, unevenly shaped, nonoverlapping; toe I vestigial, clawless, toes II-V well developed;proximal subdigital lamellae undivided, rectangular; distal subdigital lamellae divided and undivided, angular, U-shaped,except terminal lamellae rounded, undivided; lamellar formula on toes II-V (3)-(4)-(4)-(4) on both feet; claws on toes II-V well developed, unsheathed, strongly curved; one cloacal spur(CloacS) on each side; tail long, original (TL 32.32 mm;TL/SVL 94%), round in cross-section, dorsal scales on taillarger than on body and head, smaller than subcaudals, no plate-like subcaudal scales.
Table 9 Test results for group separation of compared species within clade 6 along first two PCA axes (PC1 and PC2)

Figure 6 Hemiphyllodactylus simaoensis sp. nov.
Coloration in life(Figure 6): AllHemiphyllodactylusspecies can change the intensity and boldness of their coloration and patterns. The description below was taken when the holotype was photographed in the morning after capture. Base color of dorsal side of head, body, and limbs pale-gray, with two lines of dark blotches running from neck to sacrum on back; no dark marking on top of head; thin and indistinct dark pre- and postorbital stripes extending from external nares, through eye to just anterior of forelimb insertion on body; dark postsacral marking bearing anteriorly projecting arms; limbs with irregularly shaped, diffuse, and indistinct dark markings; dorsal side of tail with brown to black banded pattern; abdomen unicolor gray.
Variation: Coloration of this species varies considerably(Figure 6). Variations in mensural and meristic data are presented in Supplementary Table S7.
Distribution:Hemiphyllodactylus simaoensissp. nov.is currently only known from Simao District and Ning’er County,Pu’er City, Yunnan Province, China.
Natural history:The holotype and seven paratypes (KIZ 062063, KIZ 062065-KIZ 062070) were collected on the evening of 6 July 2019 from a wall of a vacant building rarely used by humans in a forested karst area. The left and right sides of the building were densely covered by shrubs and trees. The back of the building was bordered by forested karst hills. The front of the building faced an old basketball court with several artificial lights, with the main road located further in front (Figure 7). The other 14 paratypes from Ning’er County (KIZ 062072-KIZ 062077, KIZ 062079-KIZ 062084,KIZ 062087-KIZ 062088) were collected one year earlier, on the evenings of 16 and 17 August 2018, from a cement wall in a forested karst area.

Figure 7 Habitat of Hemiphyllodactylus simaoensis sp. nov.
Etymology:The specific epithetsimaoensisrefers to the name of the district where the holotype originates, Simao District, Pu’er City, Yunnan Province, China.
Morphological comparisons:A full list of trait comparisons is provided in Supplementary Table S3. Here, we describe morphological variations inHemiphyllodactylus simaoensis
sp. nov.and differences from its congeners for traits that differed between species. However, in terms of the range of trait values,Hemiphyllodactylus simaoensissp. nov.was indistinguishable from theH. jinpingensisspecimen examined but distinct fromH. jinpingensisreported in Guo et al. (2015)(Supplementary Table S3). We consider this to be an artifact of researcher bias in the way data were taken. In terms of body ratios,Hemiphyllodactylus simaoensissp. nov.differs fromH. jinpingensis(values obtained from Guo et al. (2015)),H.montawaensis,H. tonywhitteni, andH. ngwelwiniby shorter head (HL/SVL), wider head (HW/HL), greater SnEye distance(SnEye/HL), greater NarEye distance (NarEye/HL), larger eyes (ED/HL), and wider snout (SnW/HL) (Table 10).
In terms of scalation, the new species differs fromH.montawaensis,H. tonywhitteni, andH. ngwelwiniby fewer VS(5-7 vs. 7 or 8, 7-9, 7 or 8, respectively), and differs fromH.tonywhitteniby more CN (5 or 6 vs. 3-5). For body coloration and pattern, the new species differs fromH. montawaensisby presence of dark transverse blotches on dorsum (vs. reticulate pattern). The new species differs fromH. tonywhitteniby presence of dark transverse blotches on dorsum (vs. absent)and absence of light-colored dorsolateral spots on trunk (vs.present). The new species differs fromH. ngwelwiniby presence of dark transverse blotches on dorsum (vs. absent)(see Supplementary Table S3 for all comparative values).
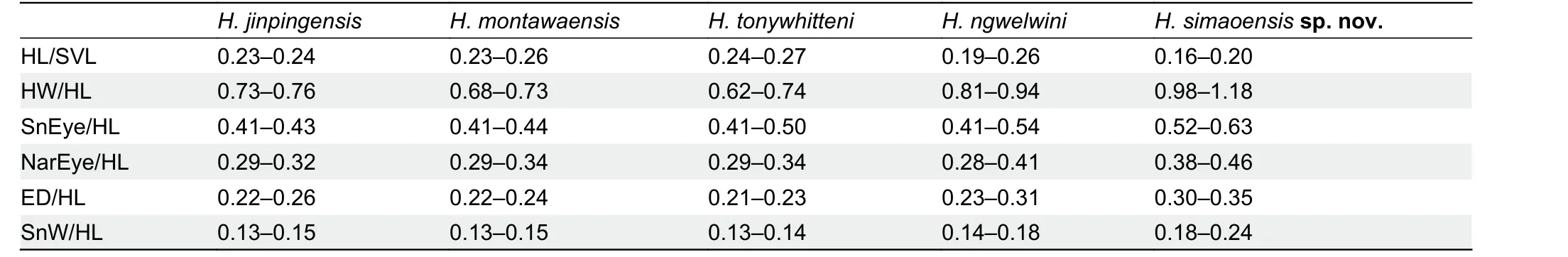
Table 10 Key trait differences between Hemiphyllodactylus simaoensis sp. nov. and its congeners

Figure 8 Variations in color and patterns of Hemiphyllodactylus yanshanensis sp. nov.
Hemiphyllodactylus yanshanensis sp. nov. (Figure 8)
Suggested English name: Yanshan slender gecko
Suggested Chinese name: 硯山半葉趾虎
Holotype: Adult male (KIZ 062090) collected on 1 July 2019 by Ade P. Agung, Jian-Mei Lu, and Zong-Bao Yang from forested karst hills in Yanshan County, Yunnan Province,China (N23.61680°, E104.41669°; 1 536 m a.s.l.).
Paratypes: Nine adult females (KIZ 062091-KIZ 062096, KIZ 062100-KIZ 062102) and three adult males (KIZ 062097-KIZ 062099), same data as holotype.
Diagnosis:Hemiphyllodactylus yanshanensissp. nov. can be distinguished from all congeners by a unique combination of the following characters: maximum SVL 46.28 mm; chin scales 8-11; enlarged postmentals; circumnasal scales 5-6;internasal scales 1-3; supralabial scales 9-12; infralabial scales 9-12; ventral scales 7-13; dorsal scales 11-22;subdigital lamellae on fingers II-V (4 or 5)-(5-7)-(5-7)-(4 or 5);subdigital lamellae on toes II-V (4 or 5)-(5 or 6)-(5-7)-(5 or 6);subdigital lamellae wider than long on first finger (4 or 5);subdigital lamellae wider than long on first toe (5 or 6); pale brown color base on body with various transverse blotched patterns on dorsum; dark postorbital stripe extending at least to base of neck; dorsolateral stripe on trunk present;ventrolateral stripe on trunk absent; postsacral marking bearing anteriorly projecting arms.
Description of holotype: Adult male, SVL 40.03 mm; head triangular in dorsal profile, depressed, distinct from neck (HL 7.27 mm; HW 7.68 mm); lores flat; snout long (SnEye 4.07 mm; SnEye/HL 56%) and narrow (SnW 1.52 mm; SnW/HW 20%); eyes large (ED 2.27 mm; ED/HL 31%); rostral scale wider than high, bordered posteriorly by two large supranasals and two internasals (IS); nares bordered anteriorly by rostral scale, ventrally by first supralabial scale, dorsally by supranasal scale, posteriorly by three postnasals; supralabials square, 9/10 (left/right), tapering from rostral to point in line with posterior margin of orbit (SL); infralabials square, 10/10(left/right), tapering from mental to point in line with posterior margin of orbit (IL); scales on head small, rounded, largest on rostrum; mental triangular, bordered by first infralabials and posteriorly by two enlarged postmentals; each postmental bordered anterolaterally by first infralabial; nine chin scales touching internal edges of infralabials from juncture of 2ndand 3rdinfralabial scales on left of mental scale to same juncture on right (Chin); scales in gular region rounded, nonoverlapping, becoming larger and more ovoid on venter. Body type robust and small (TrunkL/SVL 47%), dorsoventrally compressed; dorsal body scales small, granular, 14 dorsal scales at midbody contained within one eye diameter; ventral body scales smooth and flat, much larger than dorsal scales,subimbricate, eight ventral scales at midbody contained within one eye diameter; forelimbs relatively short, covered dorsally with granular, subimbricate scales, smaller smooth scales ventrally; palmar scales flat, unevenly shaped, nonoverlapping; finger I vestigial, clawless, fingers II-V well developed; proximal subdigital lamellae undivided,rectangular; distal subdigital lamellae divided and undivided,angular, U-shaped, except terminal lamellae rounded,undivided; lamellar formula on fingers II-V (4)-(5)-(5)-(4) on both hands; claws on fingers II-V well developed, unsheathed,strongly curved; hind limbs short, covered dorsally with granular, subimbricate scales, smaller smooth scales ventrally; plantar scales flat, unevenly shaped, nonoverlapping; toe I vestigial, clawless, toes II-V well developed;proximal subdigital lamellae undivided, rectangular; distal subdigital lamellae divided and undivided, angular, U-shaped,except terminal lamellae rounded, undivided; lamellar formula on toes II-V (4)-(5)-(5)-(5) on left foot and (4)-(6)-(6)-(5) on right foot; claws on toes II-V well developed, unsheathed,strongly curved; one cloacal spur (CloacS) on each side; tail long, original (TL 34.01 mm; TL/SVL 85%), round in crosssection, dorsal scales on tail larger than on body and head,smaller than subcaudals, no plate-like subcaudal scales.
Coloration in life(Figure 8): AllHemiphyllodactylusspecies can change the intensity and boldness of their coloration and patterns. The description below was taken when the holotype was photographed in the morning after capture. Base color of dorsal side of head, body, and limbs pale-brown and densely mottled with darker markings; top of head overlain with indistinct blotches; indistinct pre- and postorbital stripes extending from external nares, through eye to just anterior of forelimb insertion on body; postsacral marking bearing anteriorly projecting arms; limbs with irregularly shaped,diffuse, dark markings; tail pale-gray, with several irregularly shaped dark markings on dorsal side, diffused on lateral sides;abdomen unicolor gray.
Variation: Coloration of this species varies considerably(Figure 8). Variations in mensural and meristic data are presented in Supplementary Table S8.
Distribution:Hemiphyllodactylus yanshanensissp. nov.is only known from the type locality in Yanshan County, Yunnan Province, China (Figure 9).
Natural history:All specimens were collected during the evening of 1 July 2019 on the surface of a forested karst hill in Yanshan County, Yunnan Province, China (Figure 10). There were several gravid females among the specimens collected,each containing two eggs. We also found two eggs placed together loosely in the crevices of the same hill, which we assumed were laid byHemiphyllodactylus yanshanensissp.nov.based on their size (Figure 10), indicating that its reproductive season extends into July (see reports on otherHemiphyllodactylusspecies in Cobos et al. (2016) and Grismer et al. (2015)). The karst hills were surrounded by paddy fields and several huts and houses were nearby.
Etymology:The specific epithetyanshanensisrefers to the name of Yanshan County where the specimens were found.
Morphological comparisons:A full list of trait comparisons is provided in Supplementary Table S5. Here, we describe morphological variations inHemiphyllodactylus yanshanensis

Figure 9 Map of Yunnan with type localities of Hemiphyllodactylus simaoensis sp. nov. (white circles) and Hemiphyllodactylus yanshanensis sp. nov. (black star)
sp. nov.and differences fromH. huishuiensisandH.nahangensisfor traits that differed between species. In terms of body ratios,Hemiphyllodactylus yanshanensissp. nov.differs fromH.huishuiensisandH. nahangensisby shorter head (HL/SVL), wider head (HW/HL), greater SnEye distance(SnEye/HL), greater NarEye distance (NarEye/HL), larger eyes (ED/HL), and wider snout (SnW/HL) (Table 11).
In terms of scalation, the new species differs fromH.huishuiensisandH. nahangensisby more circumnasal scales(5 or 6 vs. 3 and 2 or 3, respectively) and more subdigital lamellae wider than long on first finger and toe (SL1F=4 or 5 vs. 3; SL1T=5 or 6 vs. 3). Furthermore, the new species also differs fromH. nahangensisby fewer internasal scales(IS=1-3 vs. 4-6) and fewer femoroprecloacal pores or pitted scales in females (10-16 vs. 22-24). In coloration, the new species differs fromH. huishuiensisby presence of lightcolored dorsolateral spots on trunk (vs. absent). It also differs fromH. nahangensisby presence of dark postorbital stripe and dark transverse dorsal blotches (vs. absent) (see Supplementary Table S5 for all comparative values).
DlSCUSSlON
Diversity of Hemiphyllodactylus in southern Chinese karsts
Most Chinese specimens ofHemiphyllodactyluswere previously identified asH. yunnanensisBoulenger 1903. In 2013, however, Grismer et al. (2013) conducted an integrative taxonomic study and revealed that this taxon is much more diverse than previously reported. A number of species have since been described in China. Most recently,H.zhutangxiangensiswas described from Lancang Lahu Autonomous County (Agung et al., 2021) andH.zayuensiswas described from Zayu County, Tibet (Che et al., 2020),with both species considered likely members of clade 3, given that all members are distributed in western Yunnan and northern Myanmar. The population in Dao County, Hunan Province, was also recently identified asH. dupanglingensis(Zhang et al., 2020) and as sister toH. zugi, a species that occurs on the border of Vietnam and Guangxi Province, China(Nguyen et al., 2013). Both are members of clade 6, together withH. hongkongensis(Sung et al., 2018),H. dushanensis(Zhou et al., 1981), andH. huishuiensis(Yan et al., 2016),which occur in southeastern China. Thus, together withH.yunnanensis,H. longlingensis,H. jinpingensis,H.changningensis(Guo et al., 2015), andH. typusBleeker (Uetz et al., 2021), a total of 12 species are currently known in China. However, given the extensive and unsurveyed karst habitat across southern China, many more species are likely to be discovered.

Table 11 Key trait differences between Hemiphyllodactylus yanshanensis sp. nov. and its congeners
MitochondrialND2analysis indicated thatHemiphyllodactylussimaoensissp.nov.andHemiphyllodactylus yanshanensissp. nov.are members of clades 4 and 6, respectively, and are embedded within thetypusgroup.Hemiphyllodactylus simaoensissp. nov.appears to be most closely related toH. jinpingensis(6.3%; Table 2) in clade 4, with most members of this clade distributed on Shan Plateau, eastern Myanmar.Hemiphyllodactylus yanshanensis
sp. nov.appears to be most closely related toH. huishuiensisfrom southeastern China (4.3%; Table 3) in clade 6. These distributions and relationships highlight the complex biogeography of Yunnan. The discovery of these two new species increases the total number ofHemiphyllodactylusspecies in China to 14, four of which have been described in the last five years. Thus, further research is required as many species likely remain to be discovered in China and other parts of Asia.
Our Yunnan samples were nested in four different clades(clades 3, 4, 6 and 7; Figure 1), suggesting that Yunnan may have been colonized on at least four separate occasions, as the sister species to clades 3 and 4 (H. khlonglanensis) and clades 6 and 7 (H. pardalis) are only known from Thailand.Nevertheless, more population data are needed to determine if the splits between clades 3 and 4 and clades 6 and 7 occurred before or after colonization of Yunnan.
The description ofHemiphyllodactylus yanshanensissp.nov.in this paper is the first evidence of colonization in Yunnan by a member of clade 6, with most clade members occurring in eastern Indochina (Vietnam and eastern Laos)and southeastern China (Guizhou, Hunan, and Hongkong SAR). These species discoveries emphasize thatHemiphyllodactylusdiversity within China and neighboring regions is underestimated.
Trait reliability
The newly described species (Hemiphyllodactylus simaoensis
sp. nov.andHemiphyllodactylus yanshanensissp. nov.)differed significantly in several morphological traits from their sister species (see Results). Nevertheless, these morphological traits are challenging to use in the field without specimen comparisons, and thus, obtaining tissue samples for genetic analysis is crucial to corroborate what is noted in the field. The use of integrated taxonomic approaches with genetic and morphological data, coupled with robust statistical analyses, will increase the accuracy of species delimitation.Therefore, given that many species inHemiphyllodactylusare morphologically convergent and cryptic (Grismer et al., 2013),single morphological evidence may not be sufficient for species identification inHemiphyllodactylus.
Conservation implications
Our study suggests that karst regions in Yunnan, which cover nearly 44% of the province (Nester, 2021), are likely to harbor additional undescribed species ofHemiphyllodactylus.However, despite the recent delineation of geoparks (e.g.,Shilin, Dali-Cangshan), these hotspots of endemism are disproportionately under-protected. Furthermore,H.yunnanensismay be a species complex (clade 7) and require further taxonomic revision. Based on this preliminary analysis,we hypothesize thatHemiphyllodactylusin the karst regions of Yunnan has had a complex colonization history, given that we potentially found multiple species in one site as well as one species distributed in multiple karst hills (Agung et al.,unpublished data).
The continuation of species discoveries with increasing field surveys may clarify the status of isolated populations ofHemiphyllodactylusspecies groups. Karst outcrops provide biodiversity arks and focal points for speciation (Clements et al., 2006; Grismer et al., 2021), with a high degree of isolation forHemiphyllodactyluspopulations. Due to their poor dispersal abilities, different populations in different karst areas may have evolved into separate lineages, and thus could be recognized as distinct species (Grismer, 1999). Considering the allopatric populations ofHemiphyllodactylus, with presumably no or low rates of gene flow among them, they may have become phenetically and genetically distinguishable over time (as reviewed in de Queiroz, 2007). In the last 10 years, approximately 31 new species have been identified throughout Asia, with each species usually described from a single karst area or complex (i.e., Grismer et al., 2020a),highlighting the high rate of localHemiphyllodactylusendemism in each karst region.Hemiphyllodactylus simaoensissp. nov.andHemiphyllodactylus yanshanensis
sp. nov.exhibited clear genetic and phenotypic differences from their sister lineages,H. jinpingensis(located more than 200 km away) andH. huishuiensis(located more than 350 km away), respectively, thus supporting the assumption of high local endemism for each karst region, even though some areas may have multiple sympatric species. High endemism in karst geckos is not only reported forHemiphyllodactylus, but also forCyrtodactylus(Davis et al., 2019; Grismer et al., 2021;Luu et al., 2016; Nazarov et al., 2018; Nguyen et al., 2017;Pauwels et al., 2016) andCnemaspis(Grismer et al., 2014;Wood et al., 2017). In addition, high numbers of endemic flora and invertebrates have also been reported from limestone forest habitats (Clements et al., 2006; Marzuki et al., 2021;Nguyen et al., 2021). However, the genusHemiphyllodactylusremains understudied and increased survey efforts with broader geographic coverage will likely increase the number of new species discovered. Notably, many karsts still lack inventories and given the high rate of endemism, much work is needed to map taxa across the region and develop appropriate plans for protection and representative coverage of species across this naturally fragmented system.
Neglected habitats, such as karst ecosystems, require indepth investigation and survey. Quantifying the biodiversity of a region also requires accurate taxonomy, as species are the fundamental units of conservation planning (Mace, 2004).Conserving karst landscapes and the communities that depend upon them cannot be achieved without adequate knowledge of existing species. Major threats to gecko species,including the two described here, are habitat destruction from quarrying and deforestation. Furthermore, with the rapid rate of karst loss across much of Southeast Asia, failure to identify and map the ranges of species may ultimately mean species become extinct without formal description. Therefore,appropriate conservation management plans are needed to reduce the loss of key habitats and risk of potential invasion by non-native species. Without identifying species,understanding their ranges, and increasing systematic surveys of these diverse and complex regions, the extinction of species may occur before they are described, or we may only observe specimens whose habitats have already been destroyed. Mapping the distributions of range-limited herptiles and understanding their vulnerabilities are crucial factors for targeted management and conservation. For example,research on the Chinese salamander (Andrias jiangxiensis,Chai et al., 2022) has highlighted how few sites retain the species, underscoring the need for the identification and protection of these sites. Our study and that of Chai et al.(2022) demonstrate the need for an integrated approach,including genetics and morphology, to identify species and form the basis for management and conservation. Therefore,further work is urgently needed to understand cryptic distributions and enable targeted management.
Synthesis and future directions
Prior to 2021, only 12 species ofHemiphyllodactyluswere known in southern China. Recent studies have transformed our knowledge regarding the group and added new species based on a variety of different traits and molecular data.However, these recent studies also highlight the need to better understand regional diversity patterns and biogeography and provide a basis for future management and conservation given the high rates of habitat loss across the range. The description of multiple species from Yunnan in the last 10 years also emphasizes the need to redouble our efforts to ensure sufficient representation in sampling. Furthermore, given the lack of any single trait to differentiate between species (and the need to analyze multiple traits simultaneously), integration of morphological and genetic analyses is required to better identify species, although field identification may continue to be a challenge. Key regions where we expect high complexity in species populations (due to high environmental heterogeneity forming barriers to gene flow) include the Yuanjiang valley and Ailao mountain range in Yunnan. Studies in countries such as Malaysia and Myanmar have demonstrated high levels of regional endemism within the group, indicating that more work is needed to sample and describeHemiphyllodactylusin Yunnan. Without knowledge of where species are found, developing effective and efficient management will remain difficult. Given the rate of species description in this neglected taxon, as well as the high rates of habitat loss, further research is needed to describe species and map their ranges before they can be protected, especially for genera with high levels of site-specific endemism in inventory-poor areas.
NOMENCLATURAL ACTS REGlSTRATlON
The electronic version of this article in portable document format will represent a published work according to the International Commission on Zoological Nomenclature (ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5-8.6 of the Code). This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/.
Publication LSID: urn:lsid:zoobank.org:pub:763DE16C-BA54-41ED-910A-F0423AA62A98.
Nomenclatural act LSID forHemiphyllodactylus simaoensissp. nov.: urn:lsid:zoobank.org:act:A863C059-F959-4450-BC D9-6DF3B92D5E68.Nomenclatural act LSID forHemiphyllodactylus yanshanensissp. nov.: urn:lsid:zoobank.org:act:02D7C54D-EFEC-4225-86 49-BC1D7FED3617.
SClENTlFlC FlELD SURVEY PERMlSSlON lNFORMATlON
The Ethics Committee of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, approved the study and provided ethics permission. All local regulations were followed in the collection of samples and specimens for the study.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETlNG lNTERESTS
The authors declare that they have no competing interests
AUTHORS’ CONTRlBUTlONS
A.P.A., L.L.G., J.L.G., A.C.H. designed the study. A.P.A., A.C.,L.L.G., J.L.G., E.S.H.Q., and J.M.L. collected data. A.P.A.,A.C., and L.L.G. conducted phylogenetic analyses. A.P.A.examined morphology. A.P.A and K.W.T conducted statistical analyses. A.P.A. and E.S.H.Q. wrote the manuscript. A.P.A.,L.L.G., and A.C.H. revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Brian Folt, Myint Kyaw Thura, Zhong-Bao Yang, and Shi-Fu Pu for help during fieldwork, Tuanjit Sritongchuay for loaning equipment for specimen measurements, and Yan-Hua Chen and Zi-Nan Ding for help with administrative work.
- Zoological Research的其它文章
- Diversity of reptile sex chromosome evolution revealed by cytogenetic and linked-read sequencing
- Coevolutionary insights between promoters and transcription factors in the plant and animal kingdoms
- Deficiency of transmembrane AMPA receptor regulatory protein γ-8 leads to attention-deficit hyperactivity disorder-like behavior in mice
- Global cold-chain related SARS-CoV-2 transmission identified by pandemic-scale phylogenomics
- The Hippo pathway and its correlation with acute kidney injury
- Genomics and morphometrics reveal the adaptive evolution of pikas

