Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor
SUN Mengzhu,ZHANG Yujie,SONG Yafang,GUO Jing,ZHAO Tingting,WANG Yuhang,PEI Lixia,SUN Jianhua
SUN Mengzhu,ZHANG Yujie,SONG Yafang,GUO Jing,ZHAO Tingting,WANG Yuhang,Department of Acupuncture and Rehabilitation,the Affiliated Hospital of Nanjing University of Chinese Meidicne,Nanjing 210023,China
PEI Lixia,SUN Jianhua,Department of Acupuncture and Rehabilitation,Jiangsu Province Hospital of Chinese Medicine,Nanjing 210029,China
Abstract OBJECTIVE: To investigate whether electroacupuncture(EA) at bilateral Tianshu (ST25) and Zusanli (ST36)acupoints could alleviate stress-induced irritable bowel syndrome (IBS) and evaluate its effect on gut microbiota and corticotropin-releasing factor (CRF).METHODS: Thirty C57BL/6 mice were randomly divided into the normal,water avoidance stress (WAS),and WAS+EA groups (10 mice per group).An experimental model of IBS was established by exposing the animals to WAS.The mice were treated with EA at the bilateral Tianshu (ST25) and Zusanli (ST36) acupoints.The abdominal withdrawal reflex test was conducted to evaluate visceral sensitivity in IBS.Gut microbiota was analyzed using 16S rRNA sequencing and analysis.Theexpression of CRF was determined using immuneofluorescence and quantitative real-time polymerase chain reaction.RESULTS: EA alleviated visceral hypersensitivity in a mouse model of WAS-induced IBS.It modulated the dysbiosis of gut microbiota induced by WAS.Moreover,it suppressed the WAS-induced overexpression of CRF in colon tissues.CONCLUSION: The findings of this study suggest that EA alleviated WAS-induced IBS via mechanisms possibly involving the modulation of the dysbiosis of gut microbiota and suppression of CRF expression.
Keywords: electroacupuncture;irritable bowel syndrome;gastrointestinal microbiome;corticotropin-releasing hormone
1.INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder (FGID) characterized by abdominal pain and alteration of bowel habits,with a prevalence of 10%-20%.1Although IBS is not lifethreatening,it significantly reduces the life quality and productivity of the affected patients and increases the health-care use.2
The pathophysiology of IBS remains to be completely elucidated;however,considerable attention has been focused on the relationship between IBS and the gut–brain axis in recent years.The gut–brain axis is a term used to describe the interactions between the GI tract and the central,peripheral,and autonomic nervous systems.Gut–brain interactions are thought to play a vital role in the pathophysiology of functional GI disorders such as IBS.3Corticotropin-releasing factor (CRF),as the main mediator of gut–brain interactions,plays an important role in triggering IBS.4There is ample evidence suggesting that CRF expression is associated with changes in visceral sensory function.A previous study showed that administration of CRF could enhance visceral sensitivity in the animals.5Meanwhile,another study showed that the response of visceral hypersensitivity caused by stress was blocked by the injection of a CRF antagonist.6
A correlation between gut microbiota and IBS has been demonstrated.Under normal conditions,gut microbiota can metabolize compounds,stimulate the immune system,and produce micronutrients and neuromodulators to maintain the physiological functions of the host ecosystem.7However,differences in diversity,richness,and composition of gut microbiota have been identified between patients with IBS and healthy controls.8Dysbiosis of gut microbiota has been associated with IBS symptoms.Transplantation of the gut microbiota from animals or patients with IBS to normal individuals was found to lead to the development of IBS symptoms.9,10Probiotic interventions may alleviate the symptoms of IBS,further indicating a role of gut microbiota in the pathophysiology of IBS.11,12The gut–brain axis has expanded to include the gut microbiota,leading to the microbiota–gut–brain axis.The microbiota–gut–brain axis is crucial in maintaining homeostasis and controlling the motor,sensory,autonomic,and secretory functions of the GI tract.13Furthermore,more recently,IBS has been described as a disorder of the microbiota-gut-brain axis.14
Nowadays,electroacupuncture (EA) is widely used for treating various diseases.There is ample evidence that EA has a definite effect in alleviating IBS.15-17However,the detailed mechanism underlining the effect of EA in this context remains to be fully elucidated.Hence,in this study,we aimed to investigate whether EAcould alleviate water avoidance stress (WAS)-induced IBS and observe its effects on the microbiota–gut–brain axis.
2.MATERIALS AND METHODS
2.1.Animals and group allocation
Male C57BL/6 mice [(20 ± 2) g] aged 8 weeks were purchased from Shanghai SLAC Laboratory Animal Co.Ltd.and housed under specific-pathogen-free conditions with temperature [(23 ± 1) °C] and light/dark cycle(12/12 h) regulation.Food and water were provided ad libitum.All experimental procedures were performed per the National Institutes of Health guidelines for the care and use of laboratory animals and approved (No.2018 DW-02-03) by the Animal Ethics Committee of Affiliated Hospital of Nanjing University of Traditional Chinese Medicine (TCM).The mice were randomly divided into normal,WAS (subjected to WAS),and WAS+EA (subjected to WAS and EA) groups (10 mice per group).
2.2.Model establishment
The IBS model was established by exposing the mice to WAS as described previously.18Briefly,the mice were placed on a round platform in the center of a plexiglass pool filled with water up to 1 cm below the platform height at room temperature for 1 h daily on 10 consecutive days.
2.3.EA treatment
The mice were fixed with a special mouse rack.Acupuncture needles (diameter: 0.19 mm) were inserted into the bilateral Tianshu (ST25) and Zusanli (ST36)acupoints at a depth of 3 mm.The needles were connected to an EA stimulator (HANS-200A,Beijing,China).The parameters for EA were set as follows:current intensity,0.5 mA;dense-sparse wave frequency,2/15 Hz;and retention time,15 min.The treatment was conducted once daily for 7 consecutive days.
2.4.Assessment of the visceral sensitivity in IBS
A distension balloon catheter with a diameter of 2 mm was placed in the descending colon of lightly anesthetized mice.Colorectal distention (CRD) was performed after the mice woke up and adapted for 15 min.The animals were exposed to each of the following distention pressure level for 20 s followed by a resting period of 4 min: 15,30,45,and 60 mm Hg.The process was repeated thrice for each pressure level.Further,abdominal withdrawal reflex (AWR) scores were calculated as previously described.19
2.5.Sample collection
The mice were sacrificed by cervical dislocation.Colon tissue was obtained from the descending colon;3-5 g of fecal matter was collected and then immediately frozen in liquid nitrogen.All samples were maintained at a temperature of-80 ℃ for preservation.
2.6.Hematoxylin and eosin (HE) staining
HE staining was performed for a histological analysis of the colon tissue samples.The colon tissues were fixed in 10% formalin for 24 h and embedded in paraffin.Sections with a thickness of 4 μm were stained with hematoxylin and eosin for the histological assessment.Each specimen was observed at a magnification of ×200.
2.7.Immunofluorescence analysis
Immunofluorescence analysis was performed to determine the expression of CRF.All colonic sections were blocked in 5% bovine serum albumin,followed by incubation with rabbit anti-CRF antibody (1:50;ab198798,Abcam,Cambridge,UK) at 4°C overnight.The sections were then washed in phosphate-buffered saline (PBS) thrice and incubated at room temperature with goat anti-rabbit IgG H&L (FITC;1:500;ab6717,Abcam,Cambridge,UK).Diaminobenzidine was used as the chromogen.4',6-diamidino-2-phenylindole (DAPI)was added and incubation was performed for 5 min at room temperature.The samples were then viewed under a fluorescence microscope (magnification,×200;Olympus-BH2,Tokyo,Japan).
2.8.Quantitative real-time polymerase chain reaction(qRT-PCR)
qRT-PCR was performed to determine the mRNA expression of CRF.RNA was extracted from the colon tissue by using Trizol reagent (JIALAN,Beijing,China).The RNA was converted to complementary DNA by using the High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific,Waltham,MA,USA).qRT-PCR was performed using SYBR? Premix Ex Taq? (Bio Rad,Hercules,CA,USA) on a Light Cycler 480 system(A650t-1,Dalian,China);three runs were used.The primers used were as follows: CRF: forward,5′-GGGATATCGCCAAACACCCA-3′;CRF: reverse,5′-TGGATGGCTGGTATGAGCTG-3′;and GAPDH:forward,5′-GCCTTCCGTGTTCCTACC-3′;GAPDH:reverse,5′-GCCTGCTTCACCACCTTC-3′.
2.9.16S rRNA sequencing and analysis
Microbial DNA from stool samples was extracted,and then 16S rRNA at the V3-V4 hypervariable region was amplified.Sequencing libraries were generated using the Illumina TruSeq DNA PCR-Free Library Preparation Kit(Illumina Inc.,San Diego,CA,USA) following the manufacturer’s recommendations,and index codes were added.Sequencing was performed using the Illumina Hiseq platform (Illumina Inc.,San Diego,CA,USA).The sequences were analyzed using the QIIME software package,and in-house Perl scripts were used to analyze α-(within sample) and β-(between sample) diversity.
2.10.Statistical analysis
Data were analyzed using SPSS software (IBM Corp.,Released 2012.IBM SPSS Statistics for Windows,Version 21.0.,Armonk,NY,USA) and GraphPad Prism(Version 5.0,La Jolla,CA,USA).All data are presented as mean ± standard deviation (± s) values.For parametric data,statistical comparisons among groups were performed using one-way analysis of variance(ANOVA) followed by least significant difference tests.For non-parametric data,the Kruskal-Wallis test and one-way ANOVA were used.P< 0.05 was considered statistically significant.
3.RESULTS
3.1.EA alleviated visceral hypersensitivity in the WAS group
At stimulation pressures of 15,30,45,and 60 mm Hg,AWR scores in the WAS group were significantly higher than the corresponding scores in the normal group (P=0.000,P=0.000,P=0.003,andP=0.014,respectively).Further,the corresponding AWR scores in the WAS+EA group were lower than those in the WAS group;however,the intergroup difference at the stimulation pressure of 60 mm Hg was not significant (P=0.010,P=0.029,P=0.029,andP=0.249,respectively;Table 1).
Table 1 AWR scores at different stimulation pressures in normal,WAS and WAS+EA groups ( ± s)
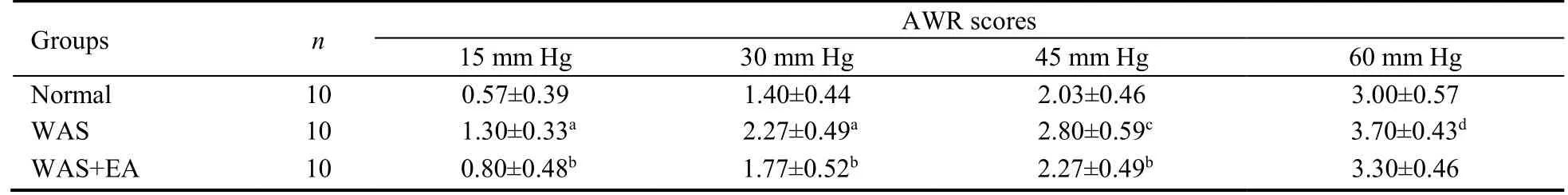
Table 1 AWR scores at different stimulation pressures in normal,WAS and WAS+EA groups ( ± s)
Notes: Normal group: without treatment;WAS group: subjected to WAS for 1 h daily on 10 consecutive days;WAS+EA group: subjected to WAS for 1 h daily on 10 consecutive days and EA at bilateral Tianshu (ST25) and Zusanli (ST36) for 7 consecutive days.WAS: water avoidance stress;EA: electroacupuncture.aP < 0.001,cP < 0.01,dP < 0.05 versus normal group;bP < 0.05 versus WAS group.
3.2.EA affected the richness and evenness of gut microbiota
We assessed the gut microbiota by using 16S rRNA gene sequencing analyses.An average of 90114 reads was obtained per sample,and 642 discrete bacterial taxa(OTUs) were identified at ≥ 97% sequence identity.We observed microbial community richness in different groups by using α-diversity analyses based on the Chao1,ACE,Simpson,and Shannon indices.The Chao1 and ACE indices were significantly higher in the WAS group than in the normal group,while the Simpson and Shannon indices were not significantly different between the two groups (Chao1 index,P=0.0042;ACE index,P=0.0045;Simpson index,P=0.5361;Shannon index,P=0.9077;Figure 1).The Chao1 and Simpson indices were higher in the WAS+EA group than in the WAS group,implying that the richness and evenness of species communities increased significantly in the WAS+EA group (Chao1 index,P=0.0042;Simpson index,P=0.0022;Figures 1A and C).However,the ACE and Shannon indices were not significantly different between the WAS and WAS+EA groups (ACE index,P=0.6573;Shannon index,P=0.0799;Figures 1B,1D).
3.3.EA altered the structure of gut microbiota
We further measured the extent of the similarity of gut microbial communities among the normal,WAS,and WAS+EA groups by assessing β-diversity on the basis of principal co-ordinate analysis (PCoA) and non-metric multidimensional scaling (NMDS).The PCoA plots showed that the gut microbiota composition clustering of the WAS group was distinctly separated from that of the normal group while the gut microbiota composition clustering of the WAS+EA group was distinctly separated from those of the other two groups,suggesting that the gut microbial community was altered by WAS and EA treatment (Figures 2A,2B).PCoA of the unweighted UniFrac distance showed that the two principal coordinates accounted for 24.01% and 13.29%of total variations,respectively (Figure 2A).PCoA of the weighted UniFrac distance showed that the two principal coordinates accounted for 59.97% and 17.31% of total variations,respectively (Figure 2B).Moreover,the NMDS ordination results indicated that the microbialcommunities in the three groups were significantly different (stress=0.072;Figure 2C).We also found differences in the structures of the microbial communities among the three groups.Differential abundance analysis at the phylum level revealed that the Firmicutes/ Bacteroidetes ratio was higher in the WAS group than in the normal group,and the ratio decreased after EA treatment(Figure 2D).Differential abundance analysis at the genus level revealed that the relative abundance of Lactobacillus was lower in the WAS group than in the normal group,and the relative abundance of Lactoba-cillus significantly increased after EA treatment (Figure 2E).
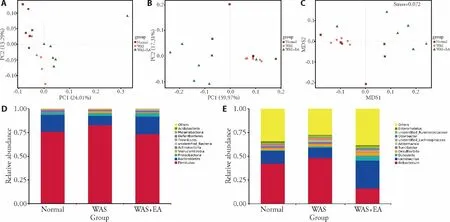
Figure 2 Structure of the gut microbiota
3.4.EA modulated the composition of gut microbiota
To identify the specific bacterial taxa associated with EA,we compared the composition of gut microbiota using MetaStat analysis and linear discriminant analysis effect size (LEfSe).MetaStat analysis at the genus level showed that Ileibacterium,Dorea,Candidatus Saccharimonas,Dubosiella,Lactobacillus were significantly enriched in the WAS+EA group compared to the WAS group.However,the relative abundances of Ileibacterium and Dorea were lower while the relative abundances of Candidatus Saccharimonas,Dubosiella and Lactobacillus were higher in the WAS+EA group(Figure 3A).MetaStat analysis at the order level showed that Lactobacillales and Erysipelotrichales were significantly enriched in the WAS+EA group compared to the WAS group.However,the relative abundance of Lactobacillales was higher while that of Erysipelotrichales was lower in the WAS+EA group(Figure 3B).LEfSe comparisons identified 47 taxa (2 phyla,5 class,7 order,11 families,13 genera and 9 species) that were differentially abundant among the three groups.Significant enrichments in class Bacilli,order Lactobacillales,Bifidobacteriales and Campylobacterales,families Lactobacillaceae,Peptostreptococcaceae,Bifidobacteriaceae,Peptococcaceae and Helicobacteraceae,genera Lactobacillus,Dubosiella,Candidatus,Bifidobacterium,Anaerotruncus and Helicobacter,and species Clostridiales bacterium,Candidatus Arthromitus and Clostridium were identified in the WAS+EA group (Figure 3C).Interestingly,the linear discriminant analysis (LDA) score of Lactobacillus was the highest,which was consistent with the above conclusions.The cladogram also showed a significantly different level of bacteria among the three groups.Moreover,the cladogram indicated that Lactobacillus and Bifidobacteriales played important roles in the WAS+EA group (Figure 3D).
3.5.EA suppressed the overexpression of CRF in colon tissues
The relative mRNA expression of CRF in the WAS group (2.21 ± 0.10) was significantly higher than that in the normal group (1.00 ± 0.22) (P=0.000).The relative mRNA expression of CRF in the WAS+EA group (1.52± 0.09) was significantly lower than that in the WAS group (P=0.000) (Figures 4A).Similar trends were found in the immunofluorescence analysis results: CRF expression in the WAS group (2.08 ± 0.26) was significantly higher than that in the normal group (1.00 ±0.30;P=0.002).On the other hand,the CRF expression in the WAS+EA group (1.52 ± 0.17) was significantly lower than that in the WAS group (P=0.03) (Figures 4B,4C).
4.DISCUSSION
In this study,we established a mouse model of WASinduced IBS and evaluated the therapeutic effects of EA on the IBS in WAS-treated mice.We found WAS increased the visceral sensitivity of mice without obvious colonic histopathological changes (Supplement Figure 1),while EA at Tianshu (ST25) and Zusanli (ST36) alleviated the WAS-induced visceral hypersensitivity of IBS.In TCM,IBS is classified as abdominal pain,diarrhea,or constipation on the basis of its main clinical manifestations.Spleen and stomach dysfunction are important pathogeneses of IBS.EA,as a special type of acupuncture,as well as an electrical stimulation,is widely used for FGID,including IBS.Acupoint selection is vital in EA treatment.According to the classical theory of acupoints,Tianshu (ST25) is the “Front-Mu point” of the large intestine,and Zusanli (ST36) is the “l(fā)ower He-Sea point” of the stomach.They are both located in the stomach meridian,which can regulate the functions of the spleen and stomach thus improve enteric function.In this study,we found that EA at Tianshu (ST25) and Zusanli (ST36) significantly alleviated the visceral hypersensitivity in animal models of IBS,which was consistent with the findings of our previous study.16Appropriate parameter setting also plays a crucial role in EA treatment.In this study,EA parameters were set as follows: current intensity,0.5 mA;dense-sparse wave;frequency,2/15 Hz;and retention time,15 min.Current intensity is directly associated with the safety of stimulation.A low current intensity (0.5 mA) has been demonstrated to be safe for animals in our previous study.20A dense-sparse wave consists of a pulse sequence with an alternating low-and high-frequency output.Evidence shows that stimulation at low and high frequencies alternately induces a much more potent analgesic effect than that induced by a constant frequency stimulation.21A retention time of 15 min is commonly used in animal studies of EA for IBS.15,20
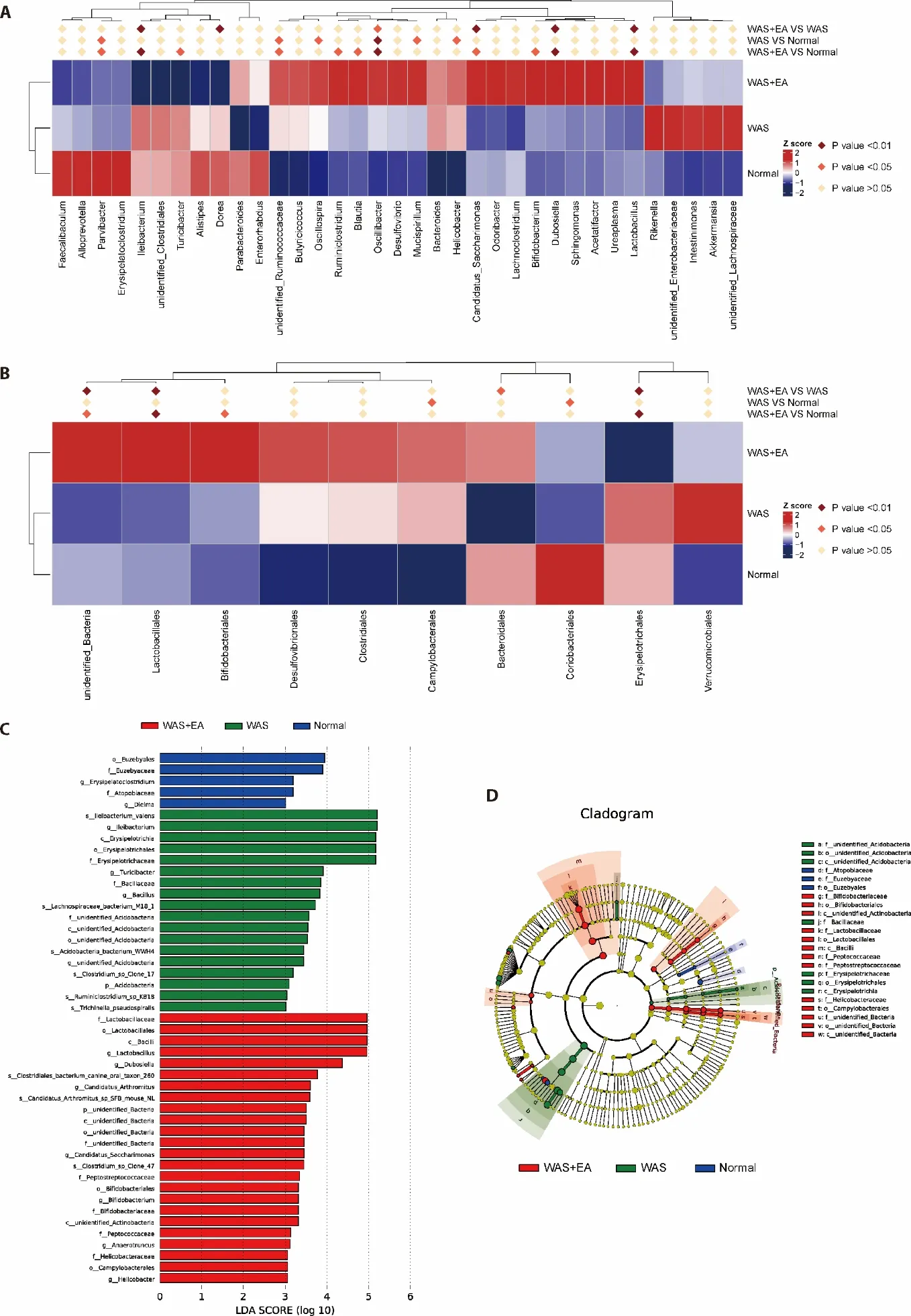
Figure 3 Composition of the gut microbiota
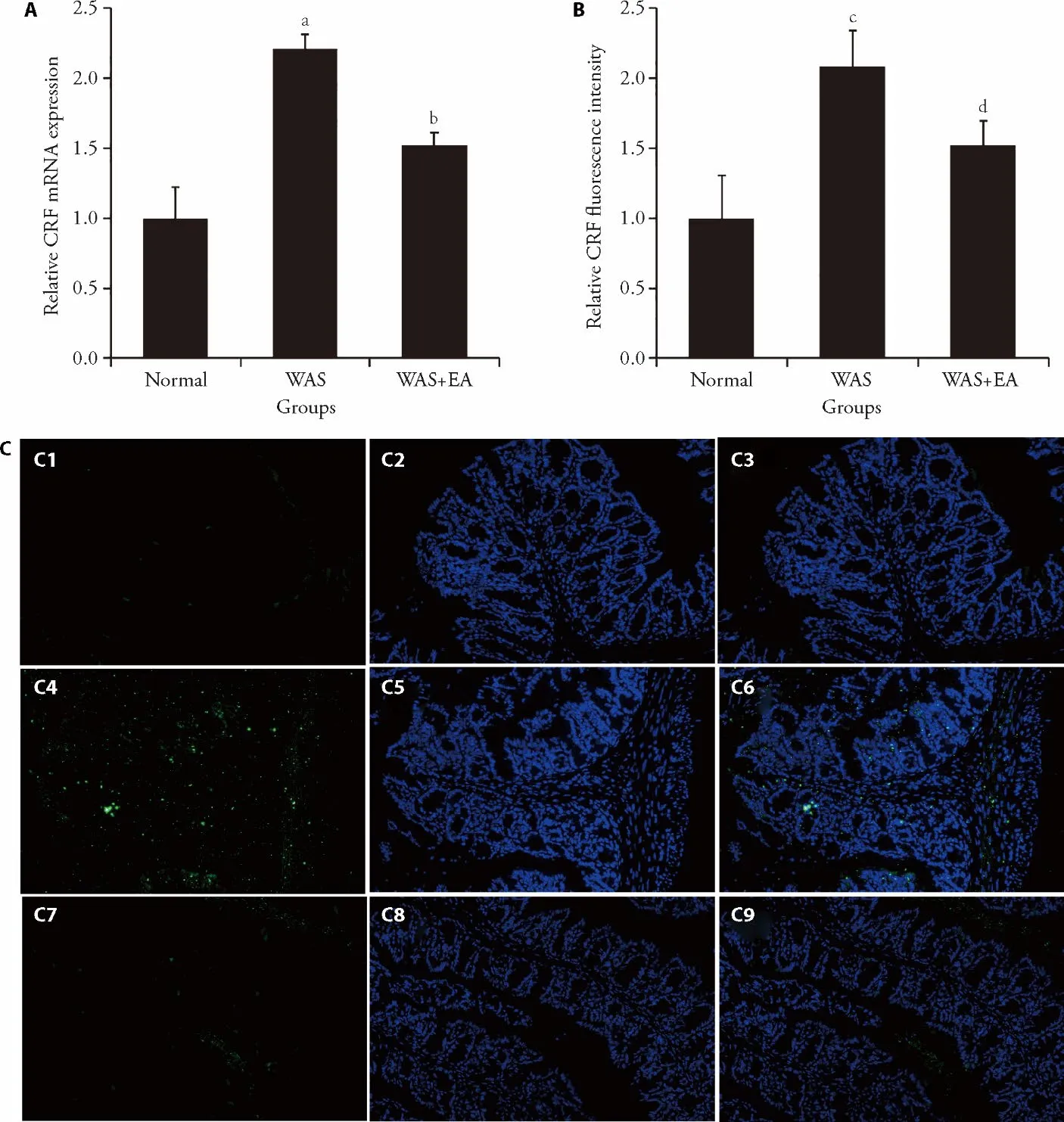
Figure 4 Effect of EA on the expression of CRF in colon tissues (magnification,× 200)
The mechanisms underlying the effects of EA on visceral hypersensitivity remain obscure.Since dysfunction of the microbiota–gut–brain axis has been regarded to play an important role in the pathogenesis of IBS,14we first observed gut microbiota using 16S rRNA gene sequencing analyses.We found differences in the αdiversity (chao1 and ace indices) and β-diversity (PCoA and NMDS) of the microbial communities between the WAS and normal groups,suggesting that dysbiosis of the gut microbiota was prevalent in WAS-induced IBS mice.Richness and diversity are considered essential for maintaining microbiota function.22Most studies,23,24but not all,18showed that the gut microbiota of IBS subjects had lower richness and diversity than the gut microbiota of healthy controls.Our study showed that the αdiversity and β-diversity were significantly higher in the WAS+EA group compared to the WAS group,implying that EA increased the richness and diversity of gut microbiota.We also found that the Firmicutes/Bacteroidetes ratio was higher at the phylum level in the WAS group.In healthy controls,the largest group of taxonomic phyla are Firmicutes and Bacteroidetes.25The Firmicutes/Bacteroidetesratio is regarded as an indicator of bacterial population changes,and this ratio has been reported to be high in IBS.26However,theratio decreased after EA in this study,which was consistent with the findings of other EA studies.27Furthermore,the relative genus-level abundance of Lactobacillus was lower in the WAS group compared to that in the normal group.Lactobacillus is commonly considered a beneficial bacterial genus that confers distinct health benefits to the host.28It is widely used for treating IBS and has been demonstrated to effectively alleviate visceral hypersensitivity.12,29In this study,EA treatment significantly increased the relative abundance of Lactobacillus in animal models of IBS,suggesting that EA alleviated the visceral hypersensitivity of IBS by regulating Lactobacillus.EA has been also confirmed to increase Lactobacillus to treat gut microbiota dysbiosisrelated diseases such as diabetes and ulcerative colitis in other studies.30,31Moreover,MetaStat analysis showed that EA treatment significantly increased Ileibacterium,Dorea,Candidatus Saccharimonas,Dubosiella andLactobacillusabun-dances at the genus level and significantly increased Lactobacillalesand Erysipelotrichales abundances at the order level.LEfSe indicated thatLactobacillusand Bifidobacteriales played important roles in the therapeutic effects of EA on IBS.Notably,Bifidobacteriales is also considered a probiotic bacterium for treating IBS.These results suggested that EA alleviated IBS potentially by modulating the gut microbiota in WAS mice.
We then observed the expression of CRF to determine the effects of EA on the brain–gut axis.Dysfunction of the brain–gut axis is associated with the presence of functional GI disorder symptoms.32CRF is regarded as the main mediator of the brain–gut axis and an important factor in the development of IBS.We found that the expression of CRF in the colon tissues of mice was significantly higher in the WAS group compared to that in the normal group,but it decreased after EA.These results suggested that EA alleviated IBS potentially by modulating the expression of CRF.
In conclusion,we demonstrated that EA alleviated stressinduced IBS and modulated the dysbiosis of gut microbiota and the expression of CRF.Thus,the mechanisms underlying the effects of EA on IBS may be associated with the regulation of the microbiota–gut–brain axis.Dysbiosis of the microbiota–gut–brain axis has been suggested to be correlated with the occurrence and development of IBS,14and some studies reported that it could be regulated by acupuncture.15,16However,our study was limited to observing changes at the gut level.Further studies are needed to better explore the relationship between the microbiota–gut–brain axis and the effects of EA on IBS.
 Journal of Traditional Chinese Medicine2022年5期
Journal of Traditional Chinese Medicine2022年5期
- Journal of Traditional Chinese Medicine的其它文章
- Shugan Jieyu capsule (舒肝解郁膠囊) improve sleep and emotional disorder in coronavirus disease 2019 convalescence patients: a randomized,double-blind,placebo-controlled trial
- Application value of Qisexingtai hand diagnostic method in diagnosis of coronary artery disease
- An infrared thermographic analysis of the sensitization acupoints of women with primary dysmenorrhea
- Preliminary single-arm study of brain effects during transcutaneous auricular vagus nerve stimulation treatment of recurrent depression by resting-state functional magnetic resonance imaging
- Effect of treatment with Fufang Huangqi decoction (復(fù)方黃杞湯劑)on dose reductions and discontinuation of pyridostigmine bromide tablets,prednisone,and tacrolimus in patients with type I or II myasthenia gravis
- Wenshen Jianpi recipe (溫腎健脾方) induced immune reconstruction and redistribution of natural killer cell subsets in immunological nonresponders of human immunodeficiency virus/acquired immune deficiency syndrome: a randomized controlled trial
