Different routine laboratory tests in assessment of COVID-19: A casecontrol study
Imene Adouani, Tassaadit Bendaoud, Hadjer Belaaliat, Wahiba Teniou, Faiza Keriou, Farida Djabi
1Faculty of Medicine, University Ferhat Abbas Setif-1, Setif, Algeria
2Laboratory of Biotechnology and Genomics in Medical Sciences, Setif, Algeria
3Central Laboratory, Setif Hospital, Setif, Algeria
4Unit of Pneumology, Setif Hospital, Setif, Algeria
ABSTRACT Objective: To identify helpful laboratory paprameters for the diagnosis and prognosis of COVID-19. Methods: An observational retrospective study was conducted to analyze the biological profile of COVID-19 patients hospitalized in the Unit of Pulmonology at Setif hospital between January and December 2021. Patients were divided into two groups: the infection group and the control group with patients admitted for other pathologies. The infected group was further divided according to the course of the disease into non-severe and severe subgroups. Clinical and laboratory parameters and outcomes of admitted patients were collected. Results: The infection group included 293 patients, of whom 237 were in the non-severe subgroup and 56 in the severe subgroup. The control group included 88 patients. The results showed higher white blood cells, neutrophils, blood glucose, urea, creatinine, transaminases, triglycerides, C-reactive protein, lactate dehydrogenase, and lower levels of lymphocyte, monocyte and platelet counts, serum sodium concentration, and albumin. According to ROC curves, urea, alanine aminotransferase, C-reactive protein, and albumin were effective diagnosis indices on admission while neutrophil, lymphocyte, monocyte, glycemia, aspartate aminotransferase, and lactate dehydrogenase were effective during follow-up.Conclusions: Some biological parameters such as neutrophil, lymphocyte, monocyte, glycemia, aspartate aminotransferase, and lactate dehydrogenase are useful for the diagnosis of COVID-19.
KEYWORDS: Algeria; Alteration; Biological parameters; COVID-19; Pneumology
1. Introduction
COVID-19 out-brake in Algeria began in March 2020. A considerable number of infected subjects remain asymptomatic, while symptomatic cases have different symptoms ranging from minimal nonspecific symptoms to acute respiratory distress syndrome with various complications and high mortality[1-6]. It might be a challenge to diagnose and manage the infection and its complications. Laboratory parameters might be important to confirm the diagnosis, determine its severity, monitor the treatment, and reduce mortality[7,8]. There is a lack of data analyzing the different routine biological parameters in an Algerian population up to now, our study aims to screen these laboratory parameters related to COVID-19.
2. Patients and methods
2.1. Study setting
A case-control study was conducted on patients recruited from the Unit of Pneumology of the University Hospital of Setif from January to December 2021.
2.2. Ethical statement
This study was conducted following the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Ferhat Abbas Setif-1 on November 2021 (SC11-2021). The informed patient consent was waived by obtaining approval from the Ethics and Scientific Committees of the University of Ferhat Abbas Setif-1 due to the retrospective nature.
2.3. Inclusion and exclusion criteria
All patients admitted to the Unit of Pneumology from January to December 2021 were included. Patients with incomplete medical and/or biological records were excluded. The included patients were stratified into the infection group of patients diagnosed with COVID-19 using clinical symptoms and/or chest computed tomography (chest-CT) at admission and the control group with patients hospitalized for other pathologies. The infection group was further classified into non-severe and severe subgroups based on the outcomes (discharge from the hospital, transfer to other units, or death).
2.4. Primary outcomes
Data on demographic characteristics, underlying comorbidities, clinical characteristics, and para-clinical findings, including radiological and laboratory tests, were collected from paper medical records. Hospitalization time, hospital discharge time, and time from disease onset to hospitalization were also noted.
2.5. Statistical analysis
Epitools?online calculator has been used to estimate the sample size needed for our case-control study (P=0.04, power=80%, confidence level=95%, assumed odds ratio=5). A minimum of 82 patients were needed in each group.
IBM?SPSS 26.0 was used for data analysis. Categorical variables were presented as absolute numbers and percentages, while continuous variables were presented as mean±SD or median (Q1, Q3). Student-t test or Mann-Whitney U test were used to compare continuous variables. Chi-square or Fisher’s exact tests were used to compare categorical variables. Odds ratios (ORs) were used to explore the association between different laboratory test abnormalities and the severity of infection. Receiver Operating Characteristic (ROC) curves were used to test the effectiveness of the studied parameters for COVID-19 diagnosis and prognosis.
3. Results
A total of 434 patients were admitted to the pneumology unit from January to December 2021. Incomplete medical and/or biological records led to the exclusion of 53 cases. The infection group comprised 293 COVID-19 patients while the control group included 88 non-COVID-19 patients (Figure 1). Among COVID-19 patients, there were 56 (19.1%) severe patients while the non-severe group included 237 (80.9%) patients. A total of 40 (13.6%) patients died and 16 (5.4%) were transferred to other units such as Intensive Care Unit, Internal Medicine Department, Infectious Diseases Department, and 56 non-severe patients were discharged from the hospital.
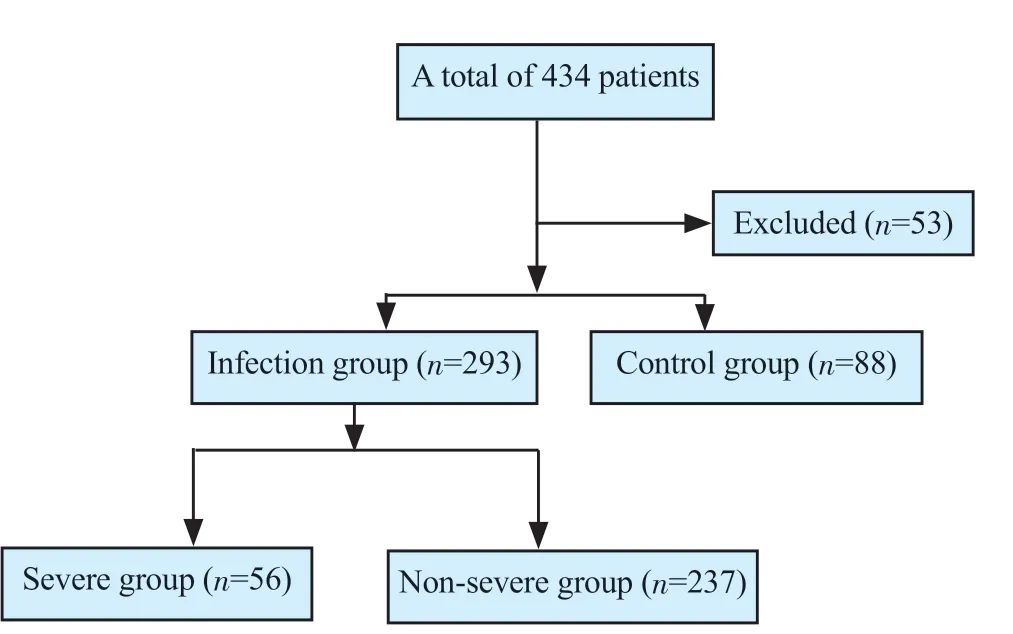
Figure 1. The study flowchart.
3.1. Socio-economic and demographic characteristics
The age ranged from 16 to 103 years. The median age (Q1, Q3) in the infection group, control group, non-severe, and severe groups were 66 (53, 74), 55 (36, 70), 64 (52, 73), and 72 (60, 78) years. The sex ratio in the infection group, control, non-severe and severe groups were 2.02, 3.00, 1.82, and 3.31 with male predominance. Most patients lived in Setif, were married, employed, and had a medium socioeconomic level.
Only age and socioeconomic level showed significant differences among all groups. Infected patients and severe cases were older. Furthermore, there were more patients with good socioeconomic levels in the infection group (Tables 1&2).
3.2. Comorbidities
In the infection group, comorbidities were observed in 76.8% of patients, and these were represented predominantly by arterial hypertension (41.3%), benign prostatic hyperplasia (36.2%), diabetes (31.7%), respiratory diseases (25.3%), heart disease (9.2%), dysthyroidism (5.8%), and anterior COVID-19 (2.7%). Comorbidities were present in 74.3% of non-severe and 87.5% of the severe subgroups. Among the control cases, 62.2% had comorbidities including respiratory diseases (33%), arterial hypertension (19.3%), diabetes (13.9%), and anterior COVID-19 (13.6%) (Tables 1&2).
Patients with comorbidities (OR: 1.98, 95% CI: 1.19-3.30, P=0.008) and medication history (OR: 1.80, 95% CI: 1.06-3.04, P=0.027) were more in infection group than the control group, particularly among severe cases (P=0.035 and P=0.01, respectively) (Tables 1&2). There were more subjects without unhealthy habits (OR: 2.21, 95% CI: 1.35-3.60, P<0.05) in the infection group than the control group. More patients in the control group [35 (11.9%)] were active smokers compared to the infection patients [29 (33.0%)]. Furthermore, more patients who were in contact with suspected or confirmed COVID-19 subjects were in the infection group (OR: 11.63, 95% CI: 4.61-29.41, P<0.05), but the difference was not significant between the severe and non-severe groups.
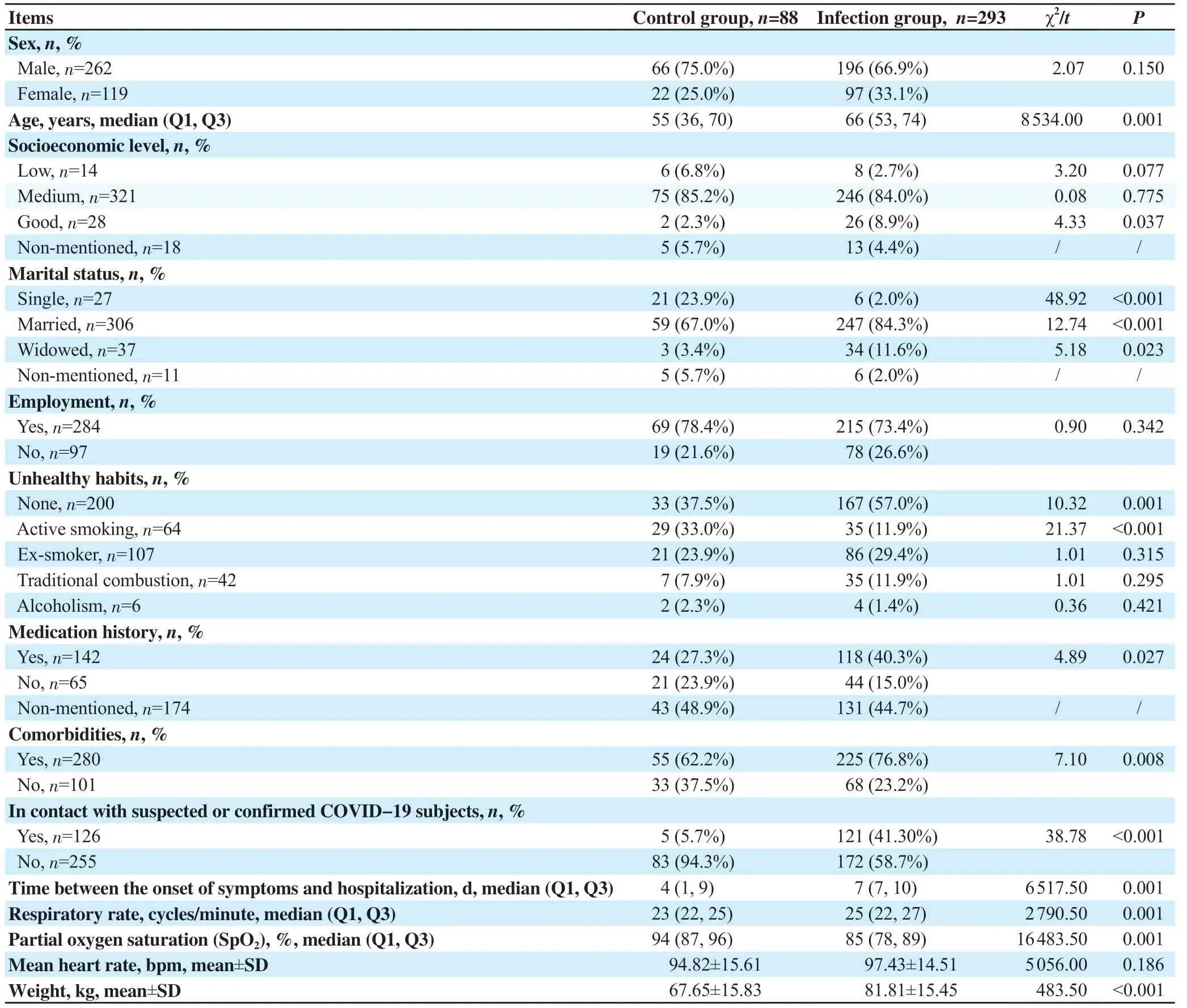
Table 1. Socio-economic and demographic characteristics and physical signs of control group and infection group (n=381).

Table 2. Socio-economic and demographic characteristics and physical signs of severe group and non-severe group (n=293).
3.3. COVID-19 clinical signs and symptoms
The time between the onset of symptoms and hospitalization was [7 (7, 10)] d in the infection group. In infection group, main symptoms were dyspnoea [268 (91.5%)], asthenia [264 (90.1%)], fever [193 (65.9%)], dry cough [176 (60.1%)], anorexia [170 (58.0%)], chest pain [96 (32.8%)], productive cough [93 (31.7%)], profuse sweating [83 (28.3%)], diarrhoea [69 (23.5%)], nausea [69 (23.5%)], anosmia [64 (21.8%)], ageusia [59 (20.1%)], weight loss [56 (19.1%)], vomiting [46 (15.7%)], throat irritation [31 (10.6%)], burning of the urine [26 (8.9%)], abdominal pain [22 (7.5%)] and dysuria [19 (6.5%)]. The main symptoms in the control group included pneumothorax, haemoptysis, asthma, chronic obstructive pulmonary disease, tuberculosis, and specific treatment intolerance, tumoral process, etc.
Polypnoea was more frequent in the infection group compared with the control group (P<0.001) and particularly pronounced in severe cases compared with the non-severe group (P=0.027). Patients with normal lung auscultation were more in the infection group (OR: 2.567, 95% CI: 1.314-5.013, P<0.05) (Tables 1&2).
On admission, the median respiratory rate, partial oxygen saturation (SpO2), the mean heart rate, and weight were 25 vs. 23 cycles/minute, 85% vs. 94%, (97.43±14.51) vs. (94.82±15.61) bpm, (81.81±15.45) vs. (67.65±15.83) kg in the infection and control groups, respectively. Table 2 demonstrates that SpO2was significantly lower in infected patients and in severe cases with higher respiratory rates. Figure 2A shows that SpO2on admission was effective in COVID-19 diagnosis with a sensitivity of 91.6%.
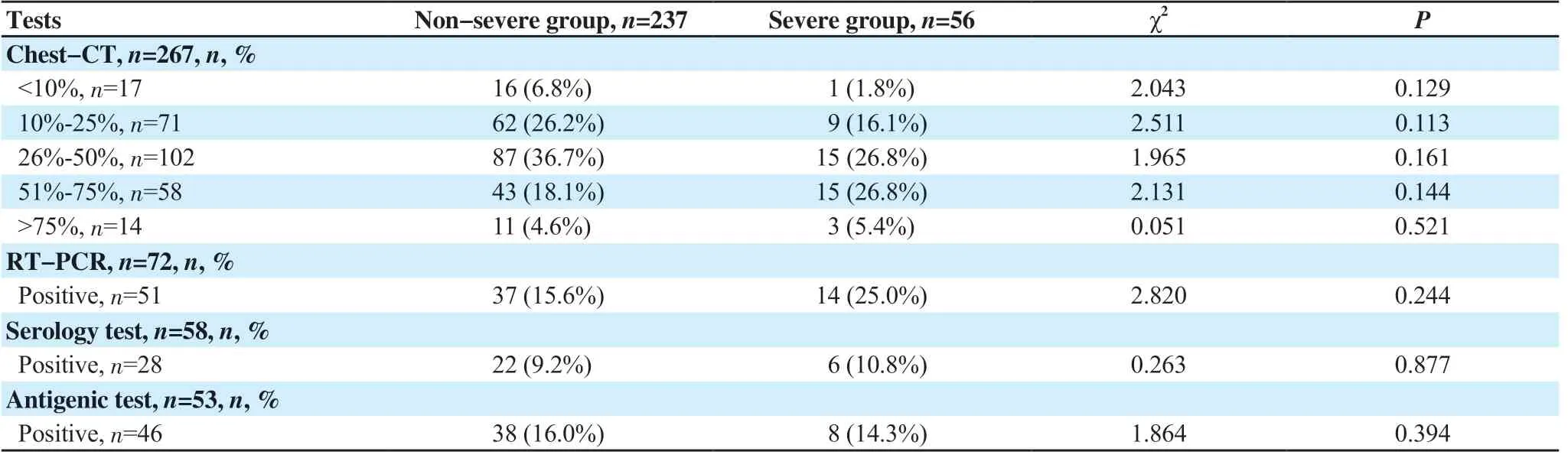
Table 3. Chest CT and laboratory test result .
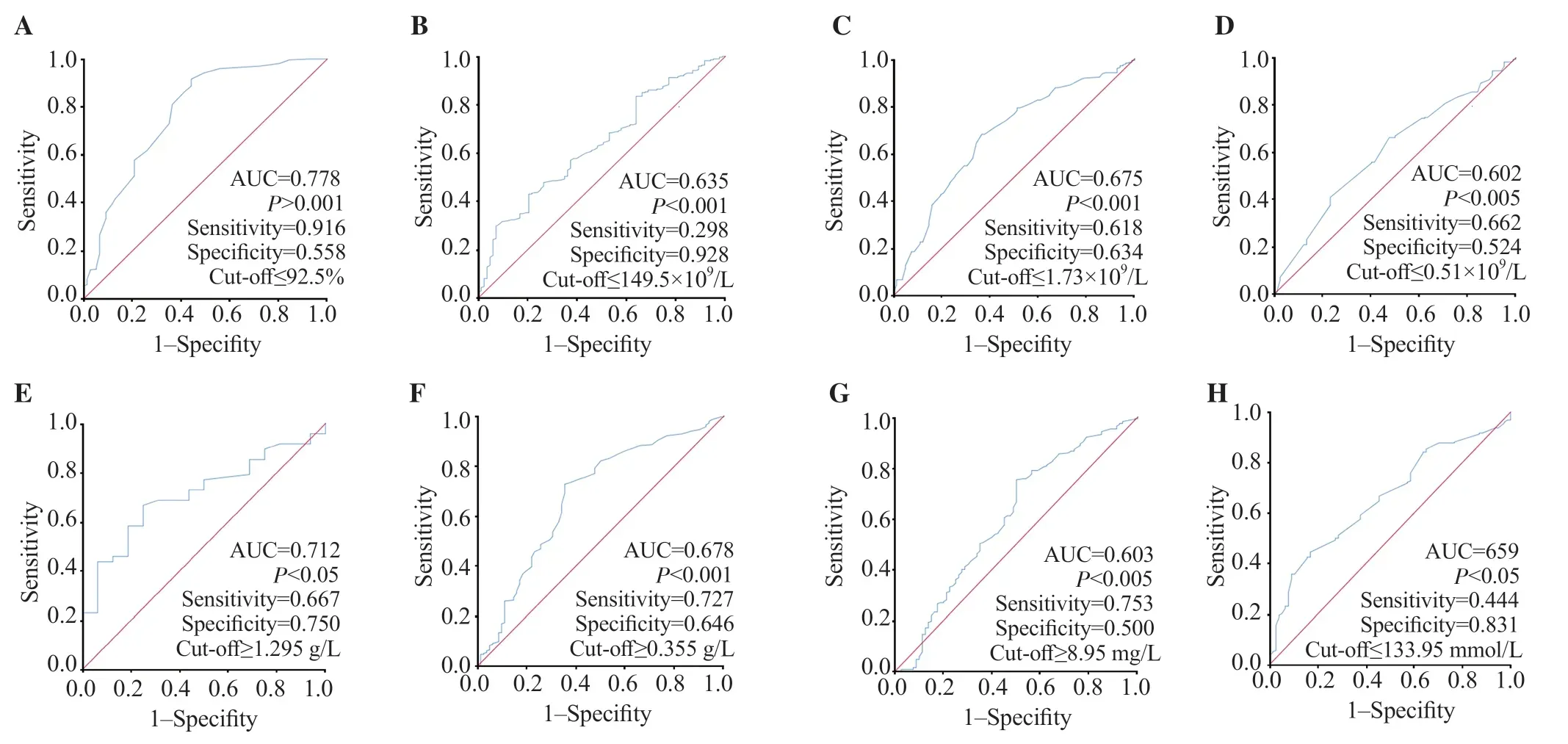
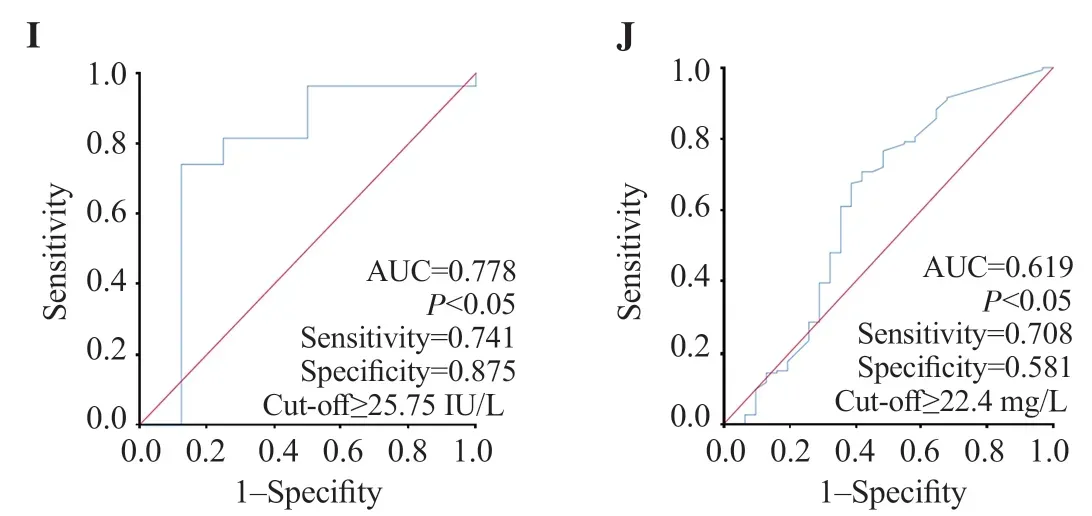
Figure 2. ROC curves of different routine biological markers for the prediction of COVID-19 infection at admission. A: SpO2; B: platelet count; C: lymphocyte count; D: monocyte count; E: blood glucose; F: urea; G: creatinine; H: Na+; I: ALT; J: CRP.
3.4. Chest CT and laboratory tests
Chest-CT was performed on 267 (91.1%) COVID-19 patients with 151 (51.5%) typical and 91 (31.1%) compatible radiological pictures. Whilst, 25 (8.5%) showed non-evocative chest-CT of COVID-19. As for the extent of lung damage, the infection at minim (< 10%), mild (10%-25%), moderate (26%-50%), severe (51%-75%) and critical (>75%) levels were found in 17 (5.8%), 71 (24.2%), 102 (34.8%), 58 (19.8%) and 14 (4.8%) of cases, respectively. The lung damage extent was not mentioned in 5 files. Table 3 doesn’t show any differences between severe and non-severe patients. Chest-CT was prescribed for only 24 non-COVID-19 patients and none of them was in favour of COVID-19.
Real-time polymerase chain reaction (RT-PCR) was prescribed in 72 (24.6%) infected patients, of which 51 (17.4%) came back positive. Whereas, serology and antigenic test were in favour of COVID-19 in 28 (9.6%) and 46 (15.7%), respectively. Table 3 dosen’t show any differences in these tests between severe and nonsevere cases.
3.5. Hemo-biochemical markers
Tables 4&5 show higher neutrophils and white blood cells (WBC), but lower lymphocyte, and platelet counts among infected patients, particularly in the severe subgroup, during admission and/or follow-up (all P<0.05). While higher international normalized ratio (INR) and D-dimer values and lower prothrombin ratio (PR) were observed only in the severe subgroup and lower monocyte count in the COVID-19 group (all P<0.05). However, there were no differences in fibrinogen (which was exclusively prescribed for COVID-19 patients during follow-up) [(5.43±1.59) and (5.41±0.87) g/L, P=0.983] or hemoglobin (Hb) levels in all cases in the infection, control, non-severe and severe groups were (13.66±1.79), (14.02±1.20), (13.72±1.71), and (13.39±2.13) mg/dL on admission, P=0.124 and P=0.242, respectively) and (13.07±2.13), (13.48±1.87), (13.50±1.83), and (13.41±2.04) mg/dL during follow-up, P=0.246 and P=0.837, respectively). Figures 2B-D, and 3A-D show that WBC, neutrophil, lymphocyte, monocyte, and platelet counts were effective in COVID-19 diagnosis at admission and/or during followup.
Infected patients had higher blood glucose levels at admission and follow-up than control cases (P<0.05), but there was no significant difference between severe and non-severe groups. During followup, COVID-19 patients had higher triglyceride (TG) levels than the control group (P<0.05) (Tables 4&5). Figures 2E, 3E, and 3H show glucose and TG were effective in diagnosis.
Tables 4&5 demonstrate that urea was higher in COVID-19 patients at admission and/or during follow-up and creatinine was higher in severe patients (P<0.05). COVID-19 patients had lower Na+on admission while severe cases had higher K+during followup (P<0.05). Figures 2F, 2G and 3F show that renal markers were interesting in COVID-19 diagnosis.
Infected cases had higher transaminases [alanine aminotransferase (ALT), aspartate aminotransferase (AST)], and lower albumin at admission and/or during follow-up (P<0.05) (Tables 4&5). However, transaminases and albumin did not impact the severity of the disease. As shown in Figures 2I anf 3G-I, transaminases and albumin were effective in COVID-19 diagnosis at admission and/or during followup.
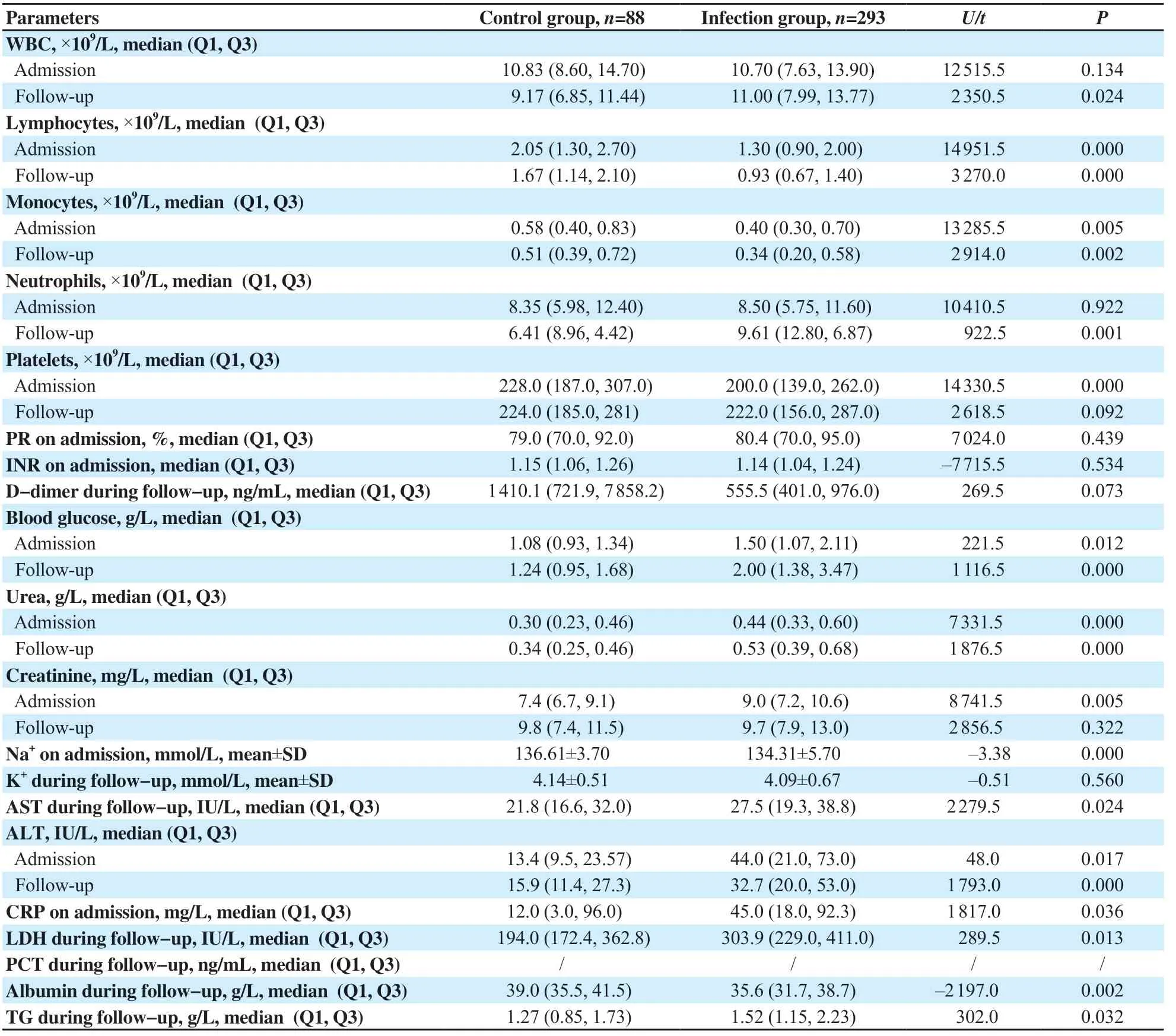
Table 4. Biochemical parameters of control group and infection group (n=381).
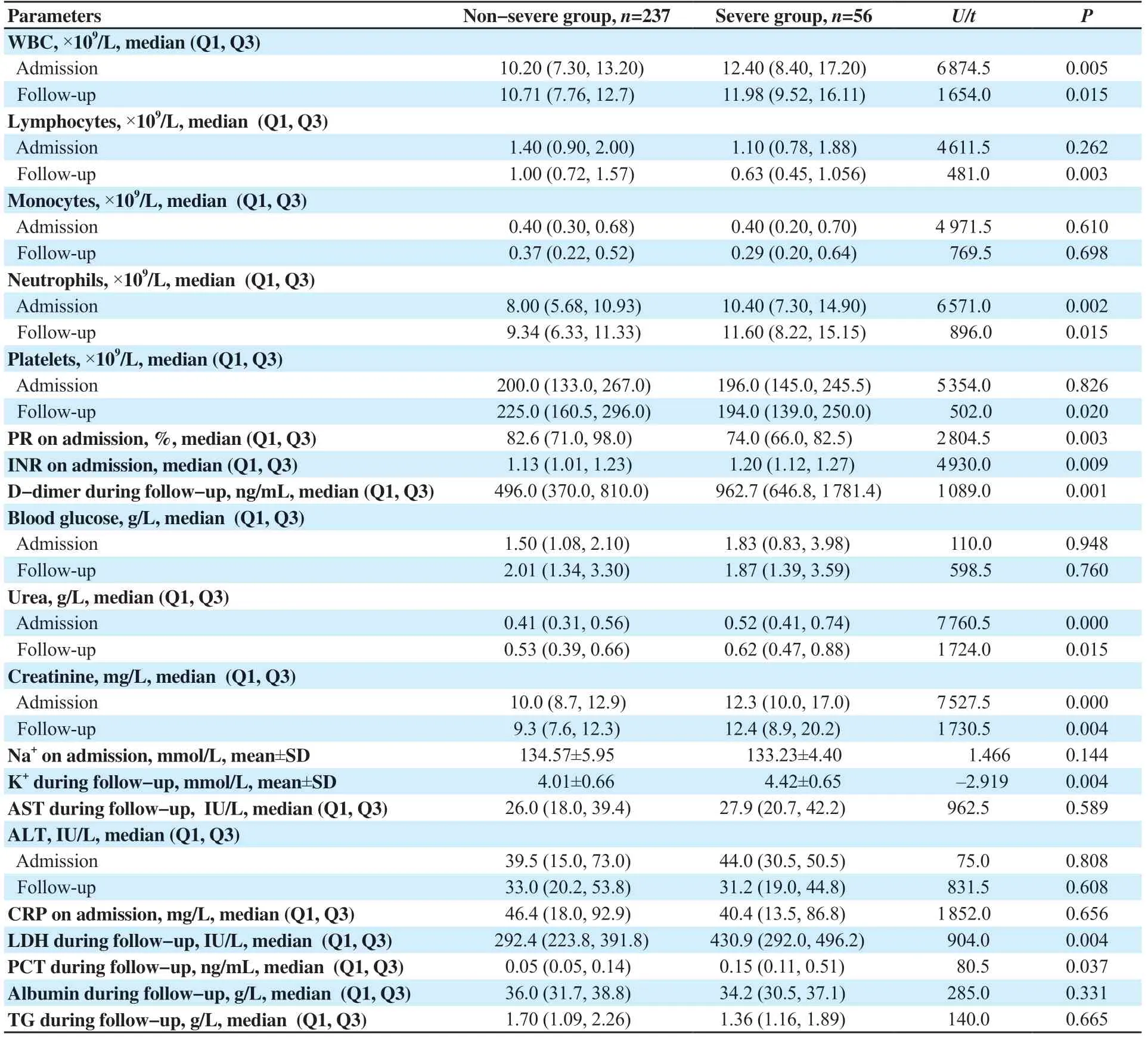
Table 5. Biochemical parameters of severe group and non-severe group (n=293).
In the infection group, C-reactive protein (CRP) was higher at admission (P<0.05), but no difference was found between severe and non-severe cases, both at admission and during follow-up (P=0.656 and 0.177, respectively). In contrast, lactate dehydrogenase (LDH) levels increased during follow-up. Furthermore, severely infected patients had higher LDH and procalcitonin (PCT) levels (P<0.05). According to Figures 2J and 3J, only CRP and LDH were effective in diagnosis of COVID-19.
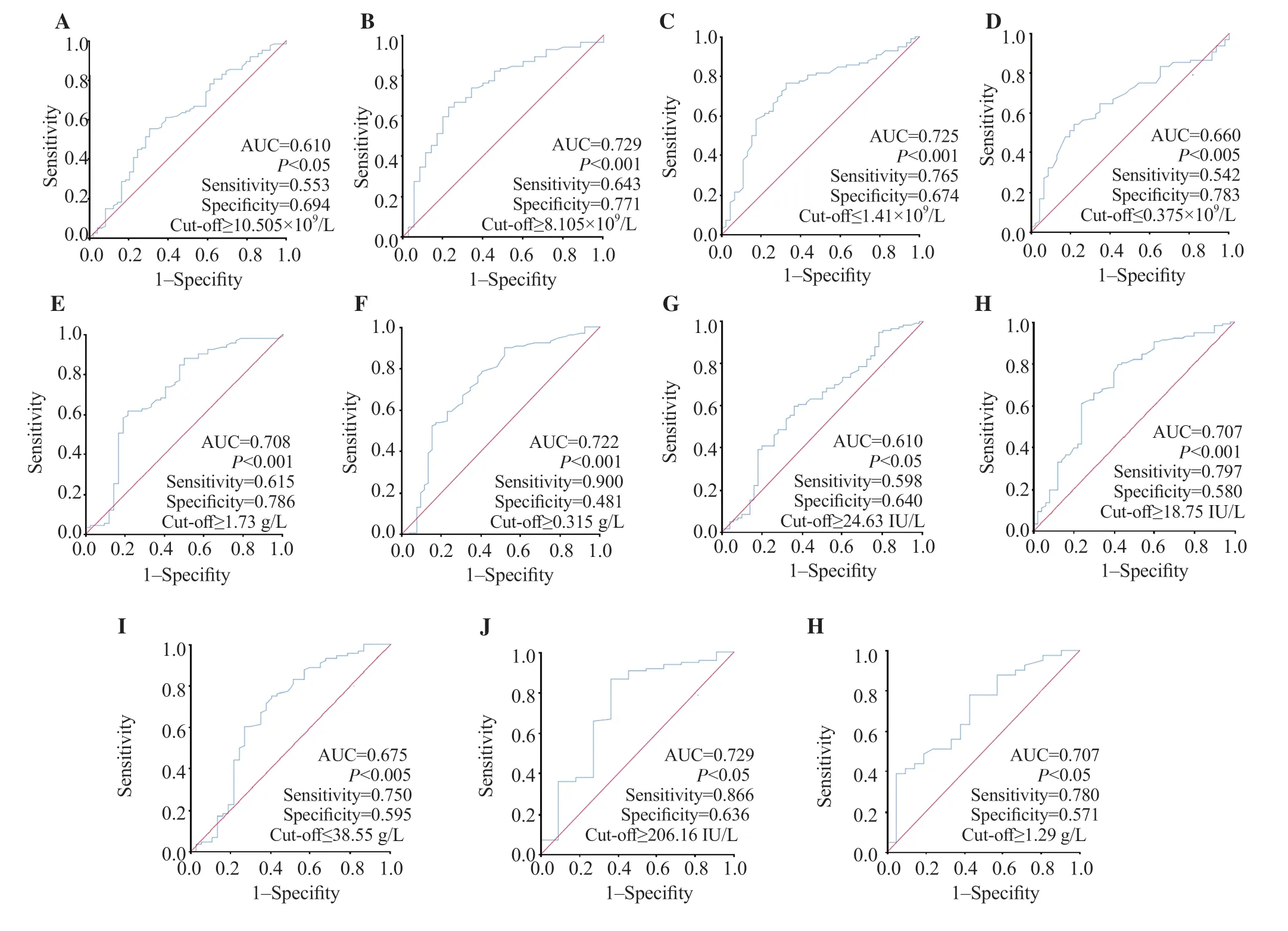
Figure 3. ROC curves of different routine biological markers for the prediction of COVID-19 infection during follow-up. A: WBC; B: neutrophils; C: lymphocyte count; D: monocyte count; E: blood glucose; F: urea; G: AST; H: ALT; I: albumin; J: LDH; H: triglyceride.
4. Discussion
The risk of severe COVID-19 is higher in males and increases with age[6,9,10]. Older patients with associated comorbidities have a poor prognosis and a high risk[13]. Overall, the signs of COVID-19 included but not limited to fever[3,6,11,12], cough[3,6,11,12], fatigue[3,11,12], muscle soreness[11], chest tightness[11,12], anosmia[3], ageusia[3], myalgia[3,12], shortness of breath[3,12], expectoration[3,12] and digestive signs including diarrhea[3] and nausea/vomiting[3]. Most of these symptoms were also noticed in our study. The variety of symptoms could be explained by the fact that SARS-CoV-2 targets angiotensin-converting enzyme-2 receptors which are expressed in different tissues[1,6].
The confirmation of the diagnosis of COVID-19 relies on specific symptoms and characteristic radiological and biological signs[3]. Most infected patients show chest-CT lesions[1]. Chest-CT was used to confirm the diagnosis in almost all our patients. According to our Unit of Pneumology, the pulmonary damage extend was classified into <10%, 10%-25%, 25%-50%, 50%-75% and ≥75%[3,13].
Despite its limited sensitivity (63%), RT-PCR with nasal swab has been used to confirm COVID-19 in some studies[3,6,14]. And serological tests are usually used to assess SARS-CoV-2 infection. In our study, RT-PCR and serological tests have been poorly examined.During the progression of COVID-19, some biological parameters are changed abnormally[3,14,15]. As previously reported, anemia and thrombocytopenia appear rare in COVID-19 patients[3]. In contrast, Lippi et al.[14] reported a low concentration of Hb. Whereas, neutrophilia, lymphopenia, and leukocytosis have been frequently observed in COVID-19[3,13,14]. In addition, leukocytosis[7,12,16], neutrophilia[16], lymphopenia[7,12,16] and lower monocyte[7,12], eosinophil[7,12], basophil[12], and platelet[7,14] count with lower Hb values[7] have been reported in severe cases.
Results showed that D-dimer levels increase among COVID-19 patients, particularly in those with unfavorable progression which makes it a relevant predictive factor for in-hospital mortality[1,3,5,14,15,17,18]. In our study, the mean of D-dimer concentrations didn’t differ between severe and non-severe groups at admission, but it was higher in the severe group during hospitalization. Lippi et al.[14] and Kukla et al.[19] observed an increase in prothrombin time (PT) while Pla?ais et al.[3] noticed a decrease in PT, which is similar to our results. In contrast, Kodavoor et al.[20] found that PT and INR were normal in most patients. High concentrations of fibrinogen were remarked by Eljilany et al.[1] but Grobler et al.[15] demonstrated its depletion in COVID-19 patients. Hyperglycemia has been reported in COVID-19 patients[3,14]. Our results also showed an increased glycemia, particularly in severe subjects. Moreover, elevated serum urea and/or creatinine have been observed in infected patients and they may be associated with a pejorative prognosis[3,7,14,21]. Ok et al.[16] remarked that severe cases had higher urea while Poggiali et al.[22] found that creatinine increased. Similarly, urea and creatinine levels were higher in COVID-19 patients, especially in the severe group of our study.
Many studies have evaluated the incidence of hepatic abnormalities in SARS-CoV-2 infected patients and showed that transaminase elevations are very common[3,4,6,7,14,19,20,22-24]. In addition, elevation of total bilirubin[20] and hypoalbuminemia[3,7,14,19,22] with normal alkaline phosphatase and γ-glutamyl transpeptidase levels[6,20] have been reported. These abnormalities increased during hospitalization, which is associated with the severity of the infection. In contrast, Wagner et al.[23] reported no established link between high total bilirubin, hypoalbuminemia, and the need for intensive care. The sensitivities and specificities of abnormal AST and ALT to predict mortality were 90.6%, 84.4%, 67.0%, and 89.3% respectively[20]. In our study, higher transaminase levels and lower albuminemia with normal total bilirubin, alkaline phosphatase, and γ-glutamyl transpeptidase were observed in the COVID-19 group. ROC curves showed that AST and ALT could be helpful for the diagnosis of COVID-19 with lower sensitivity and specificity than those recorded in the previous study.
According to previously reported studies, SARS-CoV-2 viral infection induces host defense mechanisms resulting in an inflammation characterized by high levels of inflammatory markers including CRP, PCT, LDH, and ferritin which might be very useful in predicting mild and severe cases[1,7,11,13,14,16,17,22,25,26]. Procalcitonin doesn’t increase during viral infection, whilst its gradual increase probably reflects bacterial co-infection in COVID-19 patients[14]. Our findings suggested that CRP levels were higher in the COVID-19 group and PCT was more associated with the outcome. Studies proved that PCT, CRP, and LDH are effective in the diagnosis of COVID-19[11,17,22,26]. In our study, CRP and LDH thresholds were lower than those observed in the previously mentioned studies with different effectiveness.
In conclusion, our results indicated that WBC, neutrophils, lymphocytes, monocytes, platelets, PR, INR and D-dimers, blood glucose, triglycerides, urea, creatinine, Na+, transaminases, albumin, CRP, PCT, and LDH are useful parameters for diagnosis of COVID-19.
Conflict of interest statement
The authors report no conflict of interest.
Funding
This study received no extramural funding.
Authors’ contributions
I.A.: concept, design, definition of intellectual content, literature search, clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, manuscript review; T.B.: literature search, clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, manuscript review; H.B.: literature search, clinical studies, data acquisition, data analysis, manuscript preparation; W.T.: literature search, clinical studies, data acquisition, manuscript preparation; F.D. and F.K.: guarantor.
 Journal of Acute Disease2022年4期
Journal of Acute Disease2022年4期
- Journal of Acute Disease的其它文章
- Challenges of COVID-19 prevention and control: A narrative review
- Delayed post-hypoxic leukoencephalopathy following barbiturate overdose: A case report
- Severe progression of autoimmune hepatitis in a young COVID-19 adult patient: A case report
- Associated risk factors for post-COVID-19 mucormycosis at a tertiary care centre: A cross-sectional study
- Hematological indices as predictors of mortality in dengue shock syndrome: A retrospective study
- Effect of oral premedication of midazolam, ketamine, and dexmedetomidine on pediatric sedation and ease of parental separation in anesthesia induction for elective surgery: A randomized clinical trial
