MIL-53-based homochiral metal-organic framework as a stationary phase for open-tubular capillary electrochromatography
Xiaodong Sun,Bing Niu,Qi Zhang,Qin Chen,**
aSchool of Medicine,Shanghai University,Shanghai,200444,China
bSchool of Life Sciences,Shanghai University,Shanghai,200444,China
cSchool of Pharmacy,Jiangsu University,Zhenjiang,Jiangsu,212013,China
ABSTRACT
Homochiral metal-organic frameworks(MOFs)have attracted considerable attention in many fields of research,such as chiral catalysis and chiral chromatography.However,only few homochiral MOFs can be effectively used in capillary electrochromatography(CEC)and their performances are far from adequate.In this study,we successfully synthesized achiral nanocrystalline MIL-53.A facile post-synthetic modification strategy was then implemented to functionalize the product,yielding a homochiral MOF:L-His-NH-MIL-53.This MOF was then employed as a chiral coating in open-tubular CEC mode(OT-CEC),and,as such,it exhibited high enantioselectivities for several racemic drugs.The homochiral MOF and the fabricated capillary coating were systematically characterized using transmission electron microscopy,scanning electron microscopy(with energy-dispersive X-ray spectrometry),Fourier-transform infrared spectroscopy,X-ray diffractometry,thermogravimetric analysis,circular dichroism spectroscopy,Brunauer-Emmett-Teller surface area measurements,and X-ray photoelectron spectroscopy.This study is expected to provide a new strategy for the design and establishment of MOF-based chiral OT-CEC systems.
Keywords:
Homochiral MOF
L-His-NH-MIL-53
Chiral stationary phase
Capillary electrochromatography
Enantioseparation
Peer review under responsibility of Xi'an Jiaotong University.
1.Introduction
Chirality,a ubiquitous phenomenon in nature,describes the situation whereby an object(mostly a molecule)is nonsuperimposable with its mirror image.The increasing use of enantiopure products in the pharmaceutical,food,and agricultural industries has made the development of efficient enantioseparation methods a highly interesting research area[1-3].Among the various separation approaches,capillary electrochromatography(CEC)has proven to be highly effective for chiral analysis,because it incorporates the advantages of the electrophoretic(high separation efficiency)and chromatographic(high selectivity)techniques[4-7].
Based on the process employed to fabricate the capillaries,the CEC techniques can be divided into three main categories:the packed column CEC,monolithic column CEC,and open-tubular modes(OT-CEC)[8].Among these modes,the OT-CEC mode exhibits many advantageous characteristics,such as relative ease of preparation,high column efficiency,no eddy diffusion effects,and rapid analysis[9].Nevertheless,OT-CEC is characterized by some deficiencies,including a low phase ratio when nonporous particles are used as the stationary phases.To address the shortcomings of OT-CEC,the application of various functional materials,such as ionic liquids,nanomaterials,and porous organic frameworks,has been proven to be highly effective,and it has thus been the focus of considerable attention of researchers[10-17].
Metal-organic frameworks(MOFs),a class of porous materials that is constructed by joining organic ligands and metalcontaining units(inorganic secondary building units),have drawn substantial attention in the separation science community as an emerging class of microporous materials.High porosity,large surface area,variable chemical functionalities,and well-defined pore size make MOFs promising materials for molecular separations[18,19].Previous studies have indicated that MOFs with microporous supramolecular architectures are potential candidates for use as chromatographic stationary phases.Indeed,impressive separation performance has been achieved using several MOFs as stationary phases in OT-CEC[20,21].Yu et al.[22]successfully utilized zeolitic imidazolate framework(ZIF)-90 as an OT-CEC coating to separate several isomers as well as neutral and basic compounds.Sun et al.[23]introduced an achiral MOF,HKUST-1,into a capillary to serve as a stationary phase;evidence indicated that with the thus-prepared system,improved enantioresolutions and enantioselectivities could be achieved as compared with the bare-capillary system.
In this study,we successfully synthesized achiral nanocrystalline MIL-53.A facile post-synthetic modification strategy was developed to functionalize the MOF product,yielding a chiral MOF L-His-NH-MIL-53.It was then employed to serve as a chiral coating,which was used in the OT-CEC mode and,as such,exhibited high enantioselectivities for several racemic drugs.A series of characterization techniques,scanning electron microscopy(SEM),energydispersive X-ray spectrometry(EDS),transmission electron microscopy(TEM),Fourier-transform infrared(FT-IR)spectroscopy,X-ray diffractometry(XRD),thermogravimetric analysis(TGA),circular dichroism(CD)spectroscopy,Brunauer-Emmett-Teller(BET)surface area measurements,and X-ray photoelectron spectroscopy(XPS),were employed to analyze the morphology and properties of the homochiral MOF and capillary coating.Different synthetic strategies for MIL-53 as well as CEC experimental conditions(e.g.,buffer pH and applied voltage),which are known to influence the efficacy of chiral separations,were evaluated and optimized.This study not only reports a novel homochiral MOF-based opentubular capillary column but also demonstrates that the enantioselectivity of weak chiral recognition reagents(e.g.,L-histidine(LHis))can be significantly enhanced when they are integrated with particular MOFs.
2.Experimental
2.1.Chemicals
Aluminum(III)chloride hexahydrate(AlCl3?6H2O,99%)and 2-aminoterephthalic acid(98%)were purchased from Heowns Co.,Ltd.(Tianjin,China).3-Aminopropyltriethoxysilane(APTES,99%)and glutaraldehyde(50% water,V/V)were sourced from Aladdin Biochemical Technology Co.,Ltd.(Shanghai,China).1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride(EDC,98%)and N-hydroxysuccinimide sulfonic acid salt(NHSS,99%)were provided by Macklin Biochemical Co.,Ltd.(Shanghai,China).L-His and six model racemic drugs(phenylalanine,tryptophan,amlodipine,metoprolol,bisoprolol,and esmolol),at purity higher than 99%,were provided by the Jiangsu Institute for Food and Drug Control(Nanjing,China).Phosphoric acid(H3PO4)and sodium hydroxide(NaOH,analytical grade)were supplied by Nanjing Chemical Reagent Co.,Ltd.(Nanjing,China).Potassium permanganate(KMnO4,99.5%)was purchased from Shanghai Chemical Reagent Co.,Ltd.(Shanghai,China).Other organic solvents(analytical grade)were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Nanjing,China).Bare capillaries(75 μm i.d.× 365 μm o.d.)were purchased from Yongnian Ruifeng Chromatography Devices Co.,Ltd.(Handan,China).
2.2.Instrumentation
The morphology characterization of the prepared MOF nanocrystals and MOF functionalized capillary(MOF@capillary)was performed on TEM(JEM-1400plus,JEOL,Tokyo,Japan)and SEM(Quanta FEI,FEF 250,Hillsboro,OR,USA).The surface elemental composition of the MOF coatings was measured by EDS.The FT-IR spectra of the nanocrystals were recorded with the FTIR-8400S(Shimadzu,Kyoto,Japan).XRD patterns were obtained using a BRUKER D8 advance(Bruker,Karlsruhe,Germany).The BET surface area and total pore volume of the MOF coatings were determined using a surface area analyzer(Beckman Coulter SA3100,Pasadena,CA,USA).The thermostability of the MOF nanocrystals was determined by TGA performed using the TGA4000(Perkin Elmer,Waltham,MA,USA).The elemental composition of MOFs was evaluated using XPS(AXIS,Shimadzu,Tokyo,Japan),and MOFs’optical activities were determined using CD spectroscopy(Jasco-815,Jasco,Tokyo,Japan).
2.3.CEC experiments
CL1030 CE system equipped with a UV-photometric detector was utilized to perform all CEC experiments(Beijing Huayang Liming Instrumental Co.,Ltd.,Beijing,China).The capillary columns used were 50-cm long(41.5 cm effective length).The concentration of the racemic drugs was 0.5 mg/mL(solvent:50% methanol,V/V).All running buffers were degassed by being subjected to sonication for 5 min and then filtered through a 0.45-μm membrane filter prior to use.Between the analyses,the capillary column was rinsed with NaOH 0.01 M for 60 s,H2O for 100 s,and running buffer to obtain a stable baseline.Thiourea was used as a neutral marker.
2.4.Preparation of L-His-NH-MIL-53
2.4.1.Synthesis of NH2-MIL-53
NH2-MIL-53 MOFs were synthesized through a solvothermal synthetic approach[24].Briefly,AlCl3?6H2O(0.76 g,3.1 mmol)and 2-aminoterephthalic acid(0.56 g,3.1 mmol)were accurately weighed and dissolved in 30 mL of dimethylformamide(DMF,unless stated otherwise).After a 30-min sonication,the obtained solution was transferred into a Teflon-lined steel autoclave(total volume:100 mL),which was heated for 24 h at 150°C.The brown solid thus produced was collected via centrifugation at 3,000 r/min and activated by DMF for 24 h to remove the trapped ligands(replacement between DMF and ligands).The product was then flushed with methanol for three consecutive times to remove the DMF inside pores.The obtained yellow powder was dried under vacuum for 12 h at 120°C.
2.4.2.Post-modification of NH2-MIL-53 with L-His
The grafting of L-His onto NH2-MIL-53 was assisted by the coupling agents,EDC,and NHSS.In a typical reaction,120 mg of MOF nanocrystals,0.155 g of L-His(1 mmol),0.192 g of EDC(1 mmol),and 0.043 g of NHSS(0.2 mmol)were accurately weighed and dissolved in 20 mL of phosphate buffer(100 mM,pH 5.0);the resulting solution was then stirred at 25°C for 5 days.Subsequently,the L-His-functionalized MOF nanocrystals were collected via centrifugation at 3,000 r/min and flushed with H2O three consecutive times.Finally,the obtained yellow solid product was dried under vacuum at ambient temperature for 24 h.
2.5.Preparation of L-His-NH-MIL-53@capillary column
In general,five steps were involved in this procedure:1)to fully expose the Si-OH group,a 50-cm uncoated bare capillary was continuously flushed with NaOH 1.0 M for 1 h,then with H2O for 30 min,and finally with HCl 1.0 M for 30 min;it was then rinsed with methanol for 30 min.The residual solvent was removed by flushing the capillary with N2gas.The capillary was then dried at 100°C in a vacuum oven overnight.A 50% APTES solution(prepared in methanol)was then used to rinse the interior of the capillary column for 30 min.Later,both sides of the capillary were sealed with rubber septa and reacted(silanization reaction between APTES and capillary inner wall occurred)in a water bath maintained at 55°C for 12 h.2)A 2% glutaraldehyde(V/V,pH 11)solution was used to wash the APTES capillary at ambient temperature for 2 h.3)After removing residual glutaraldehyde with ultrapure water,KMnO4(0.1 M)was continuously injected into the aldehyde-functionalized capillary for 1 h at 40°C to achieve its oxidation.Subsequently,to obtain the carboxyl-functionalized capillary,the residual KMnO4was removed by flushing the capillary with H2O for 3 h.4)The preparation of L-His-NH-MIL-53@capillary was performed according to the synthesis reaction(refer to Section 2.4).Notably,in the present case,a carboxyl-functionalized capillary was utilized instead of the Teflon-lined steel autoclave.AlCl3?6H2O(0.127 g,0.53 mM)and 2-aminoterephthalic acid(0.093 g,0.53 mM)were accurately weighed and dissolved in 5 mL of DMF with H2O(from 0% to 100%).The 50% DMF(in H2O,V/V)solution just prepared was continuously flushed into the column for 10 min,and the column was subsequently sealed with rubber septa and heated to a temperature of 150°C for 24 h.The obtained capillary was flushed with DMF and activated for 24 h with both sides sealed with rubber septa to remove the trapped ligands(replacement between DMF and ligands).Ultimately,the NH2-MIL-53-modified capillary was flushed with methanol so that the DMF located inside the MOF pores would be replaced by the said methanol.The obtained column was named NH2-MIL-53@capillary column.5)L-His(1 mmol),EDC(1 mmol),and NHSS(0.2 mmol)were dissolved in phosphate buffer(100 mM,5 mL,pH 5.0),and the obtained solution was employed to rinse under an applied external pressure of 0.05 MPa with the NH2-MIL-53@capillary column at ambient temperature for 5 h(sealed with rubber and reacted for 15 min every hour).After flushing the capillary with H2O to remove the residual reactants,the L-His-NH-MIL-53@capillary was prepared.A schematic diagram describing the preparation process is provided in Fig.1.
3.Results and discussion
3.1.Characterization of L-His-NH-MIL-53
The morphologies of NH2-MIL-53 and L-His-NH-MIL-53 were determined using TEM and SEM(Fig.2A).In fact,after modification of NH2-MIL-53 with L-His,achieved through a typical EDC/NHSS coupling reaction,no apparent change was observed in the crystal morphology of the MOF.The XRD patterns of NH2-MIL-53 and LHis-NH-MIL-53(Fig.2C)were consistent with published literature data[24],indicating the successful grafting of L-His in the cavities of NH2-MIL-53.The success of this modification was further confirmed by FT-IR spectroscopy(Fig.2B)and TGA data(Fig.2D).In the FT-IR spectra,the presence of a strong absorption at 1670 cm-1is indicative of the formation of amide bonds between the carboxylic groups of L-His and the amino groups of NH2-MIL-53.The appearance of the TGA curves indicates that a certain amount of LHis was incorporated into the NH2-MIL-53(approximately 13.8%,m/m).The CD spectra of NH2-MIL-53 and L-His-NH-MIL-53 nanocrystals(Fig.2E)exhibit significant differences in the 205-220 nm wavelength range,indicating that modification with L-His caused the optical activity of the MOF to change with respect to that in the case of achiral NH2-MIL-53.Notably,to determine the porosity of the prepared MOFs,N2adsorption-desorption isotherms were obtained using the BET technique.Results indicated that the pore diameter values mostly fell in the 13-18-nm range,and the average pore diameter was 14.7 nm in the case of NH2-MIL-53 and 12.8 nm in the case of L-His-MIL-53(Fig.2F).Moreover,XPS images of the MOFs(Fig.S1)showed an increment in relative proportions of C and N elements in the products,while a decreased relative proportions of O and Al was also observed.This is another evidence of the successful modification of L-His on the MOFs.
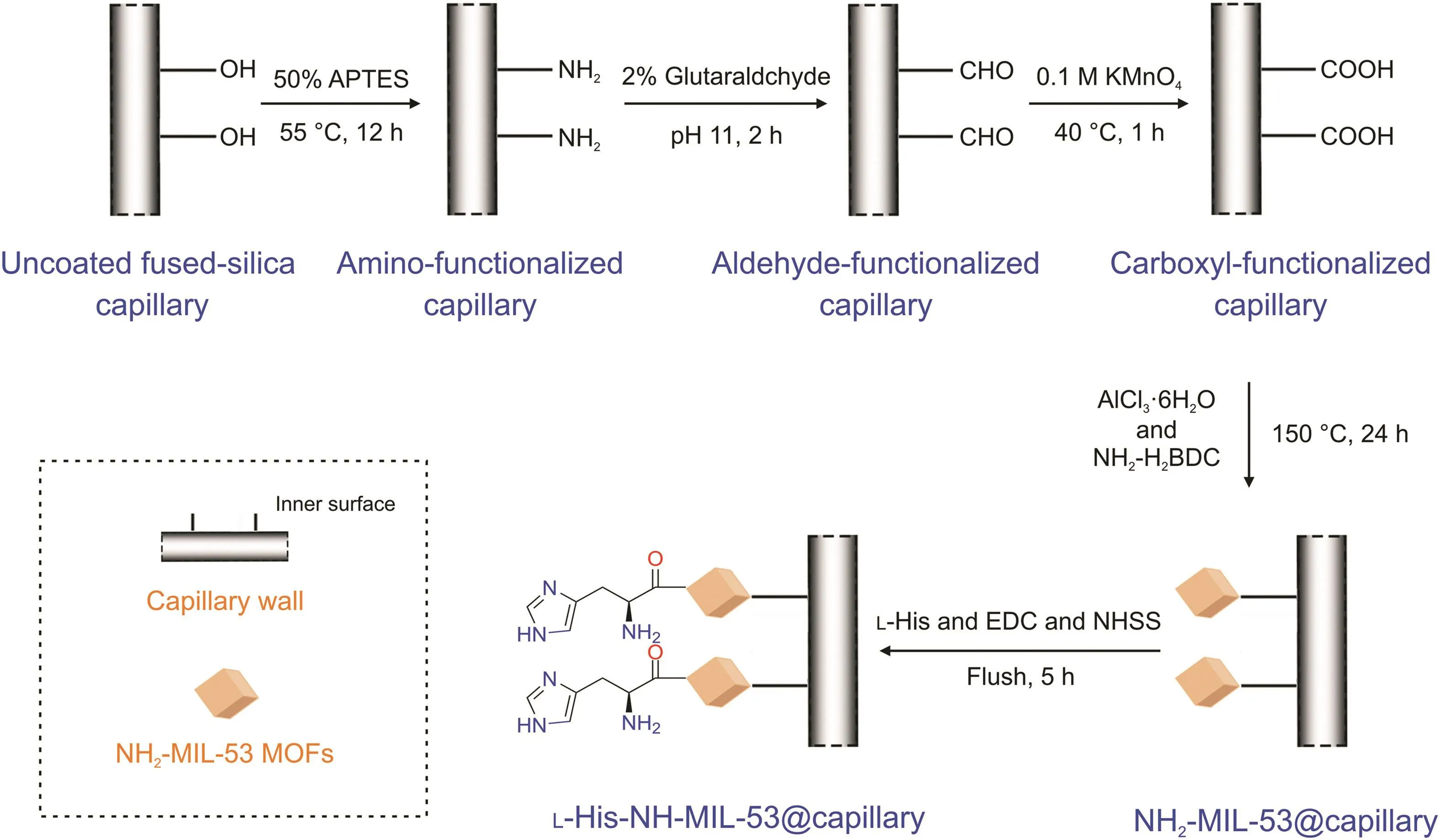
Fig.1.Process for the preparation of the L-histidine(L-His)-NH-MIL-53@capillary column.APTES:3-aminopropyltriethoxysilane;NH2-H2BDC:2-aminoterephthalic acid;EDC:1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride;NHSS:N-hydroxysuccinimide sulfonic acid salt;MOF:metal-organic framework.
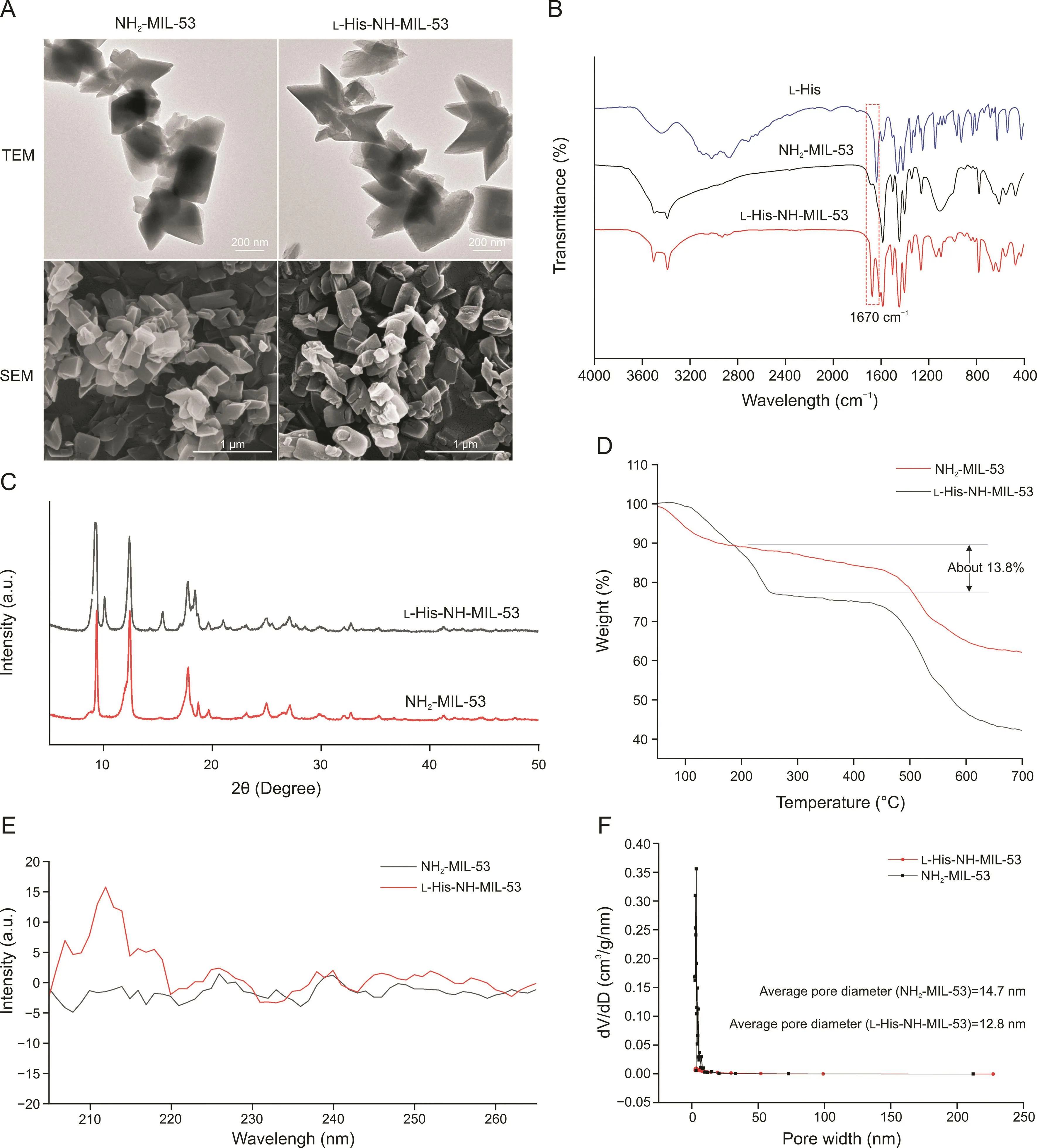
Fig.2.Characterization of NH2-MIL-53 and L-His-NH-MIL-53.(A)Scanning electron microscopy(SEM)and transmission electron microscopy(TEM)images.(B)Fourier-transform infrared spectra.(C)X-ray diffractometry patterns.(D)Thermogravimetric analysis curves.(E)Circular dichroism spectra.(F)N2adsorption-desorption isotherms.
3.2.Characterization of the L-His-NH-MIL-53 stationary phase
SEM images clearly show the presence of homogeneous layers on the inner surface of the NH2-MIL-53@capillary and L-His-NH-MIL-53@capillary columns(Fig.3A).The surface element composition of the two MOF-coated columns was determined by SEM-EDS.Results from this analysis further demonstrated that the NH2-MIL-53 and LHis-NH-MIL-53 MOFs were successfully fixed onto the inner surface of the capillaries(Fig.3B).BET analysis results(Fig.3C)indicate that the surface area of L-His-NH-MIL-53@capillary was 4.905 m2/g,which was lower than that of the NH2-MIL-53@capillary(48.723 m2/g),suggesting that L-His ended up filling the MOF pores.However,a value of 4.905 m2/g for the surface area of the coated capillary was still significantly higher than that of the bare capillary(<1 m2/g).This difference is critical for improving the phase ratio in OT-CEC separations.The stability of the prepared MOFs was verified over a 1-month storage period under refrigerated conditions(-4°C)and by conducting repeatability tests(Figs.S2 and S3).
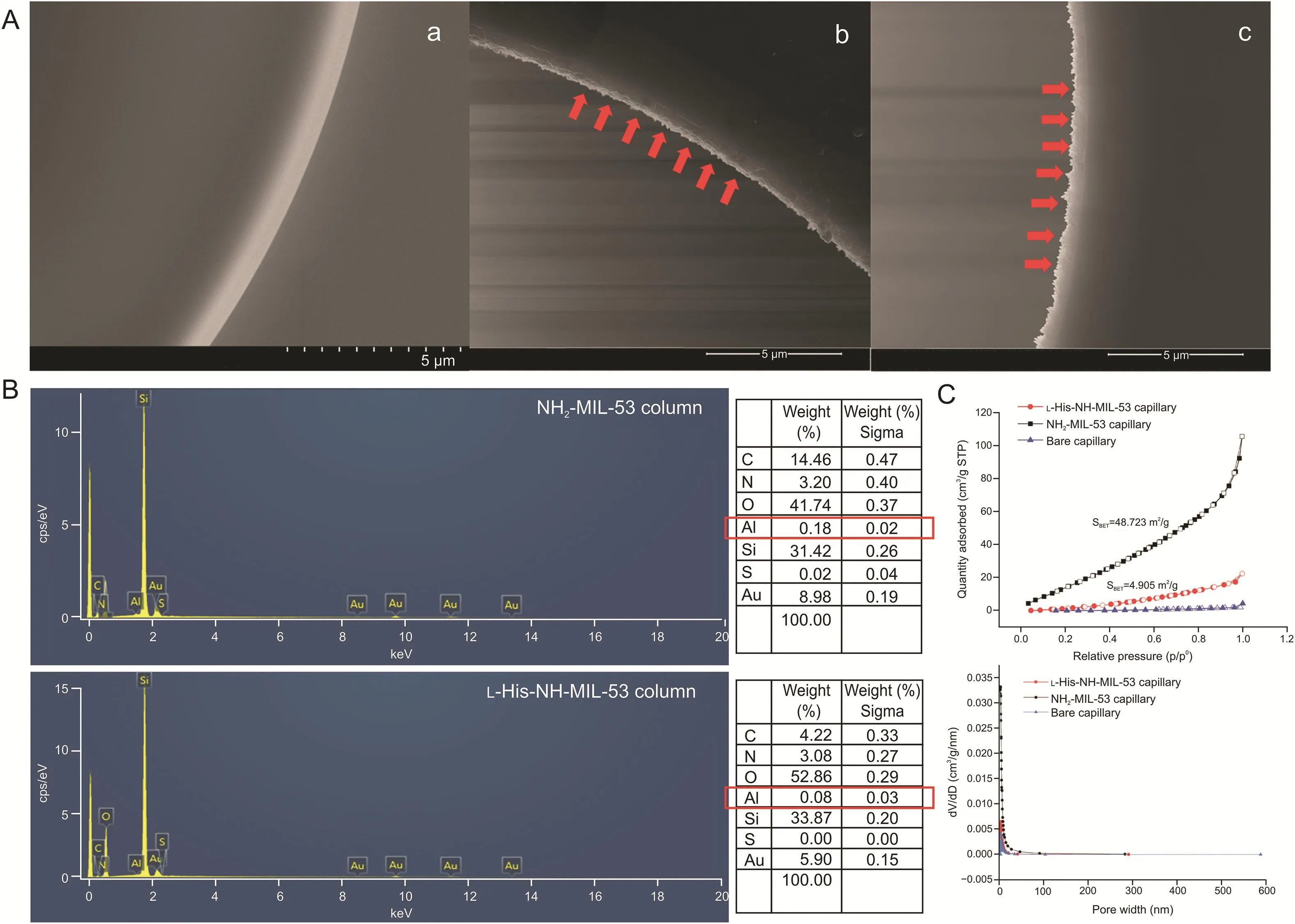
Fig.3.Characterization of different capillary columns.(A)SEM images for(a)bare-capillary column,(b)NH2-MIL-53@capillary column,and(c)L-His-NH-MIL-53@capillary column.(B)SEM-energy-dispersive X-ray spectrometry results.(C)N2adsorption-desorption isotherms and pore size distributions.
3.3.Optimization of OT-CEC experimental conditions
The influence that the following parameters had on the tryptophan separation performance afforded by the L-His-NH-MIL-53@capillary column was evaluated as follows.
1)Solvent composition was conducted in the preparation process of NH2-MIL-53.In this study,three solvents of different volumetric compositions(i.e.,100%DMF,50%DMF/50% H2O,and 100% H2O)were used to prepare theMOFs.As can be evidenced from the data reported in Fig.4B,the MOF precursors(2-aminoterephthalic acid and AlCl3?6H2O)exhibited different dispersibility in the three solvents.However,no obvious morphological differences were observed for the final MOF products prepared form the three obtained solutions(Fig.4B).Nonetheless,optimal separation was obtained when 100% H2O was used as the solvent, indicating that solvent composition during MOF synthesis might still affect the architecture and porosity of the final products,and thus,influence the extent of enantiorecognition in CEC.
2)Buffer pH:The influence of the pH of the running buffer on the efficacy of enantioseparations was evaluated in the 7.3-7.9range.pH7.5 was found to be the most favorable value for the L-His-NHMIL-53@capillary column.The tryptophan enantiomers(isoelectric point:5.89)were negatively charged in the 7.3-7.9 pH range,while the L-His residues on the MOF coating were protonated(the pKa value of the amino group of L-His is 9.2).Therefore,it is reasonable to speculate that the electrostatic interactions between the enantiomers and L-His residues may be a critical factor contributing to enantiorecognition.
3)Applied voltage:In CEC separations,a moderate increase in applied voltage affords the analysis time to be shortened,as long as the separation efficiency is not adversely affected by the excessive Joule heat.Herein,applied voltages ranging from12.5 to 17.5 kV were tested,and the most efficient separations were obtained at 15.0 kV.
Typical data demonstrating the influence of the factors discussed in this section on CEC separation efficiency are reported in Figs.4 and S4.
3.4.Separation performance of the L-His-NH-MIL-53@capillary column
Under the optimized OT-CEC conditions, the enantioseparationperformance of L-His-NH-MIL-53@capillary column was furthertested with several chiral compounds. As can be evidenced from thedata reported in Fig. 5, the separation between the tryptophan enantiomers was conducted, and satisfactory values for the Rs (resolutions) and α (selectivity) parameters were obtained. Partialseparations were also achieved for the other racemic drugs. Notably, asa small-molecule chiral recognition reagent, L-His usually cannotafford favorable enantioseparation capability when acting as solechiral selector. In this study, no separation of any enantiomeric pair was observed when L-His was utilized as a chiral additive,either in the bare capillary or in the unmodified NH2-MIL-53@capillary(see the typical electropherograms in Figs.S5 and S6).However,when L-His was incorporated into NH2-MIL-53,the L-His-functionalized homochiral MOF@capillary exhibited considerable enantioselectivity for the model drugs(tryptophan)as shown in Fig.5.These results indicate that the enantioseparation capability of weak chiral recognition reagents could be significantly enhanced following their integration in specific MOFs.
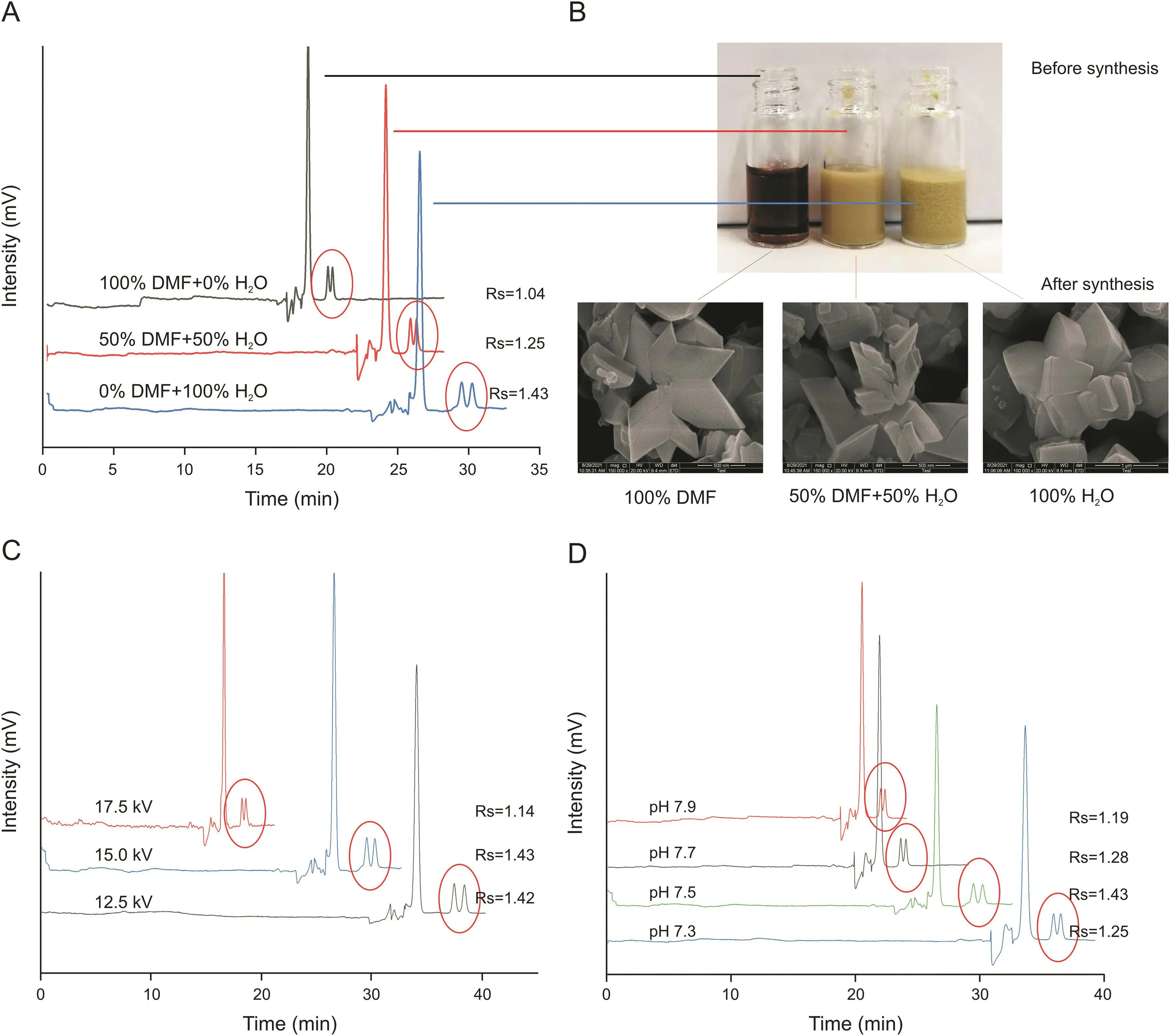
Fig.4.Effect of different conditions on the efficiency of the enantioseparation of tryptophan using the L-His-NH-MIL-53@capillary.(A and B)Different synthetic strategies.(C)Different applied voltages.(D)Different pH values.Other conditions:20-mM phosphate buffer.DMF:N,N-dimethylformamide;Rs:resolutions.
3.5.Separation mechanisms
MOFs are known to possess large specific surface areas and tunable micropores/channels that can accommodate many types of molecules,such as enantiomers. In this study, the porous network of NH2-MIL-53 was observed to be able to provide sufficient sites for LHis-enantiomer interactions,and thus,play a vital role in improving the enantiorecognition capability of L-His[24].In fact,when the racemic drug enters the pores of L-His-NH-MIL-53,the interaction between different enantiomers and L-His may be enhanced.Additionally,since the organic ligand 2-aminoterephthalic acid possesses abundant benzene rings,the L-His-NH-MIL-53 stationary phases can afford extra aromaticity and hydrophobicity for the enantiomers.The π-π interactions and hydrophobic effect between the organic ligands in L-His-NH-MIL-53 and the aromatic analytes are speculated to provide additional contributions to the favorable chiral recognition[25].Fig.6 shows the schematic illustration of the speculated mechanism.The molecular modeling structure of L-His-NH-MIL-53 is reported in Fig.S7.
3.6.A comparison of the present work with the results of other OTCEC studies
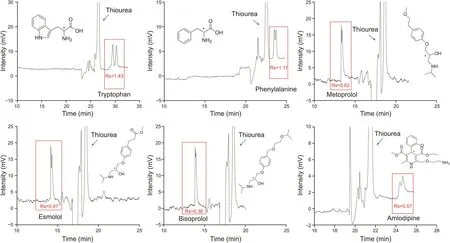
Fig.5.Performance of open-tubular capillary electrochromatography-based enantioseparation of six model drugs conducted usingL-His-NH-MIL-53@capillary.Conditions:phosphate buffer 20 mM at pH 7.5;applied voltage:15 kV.
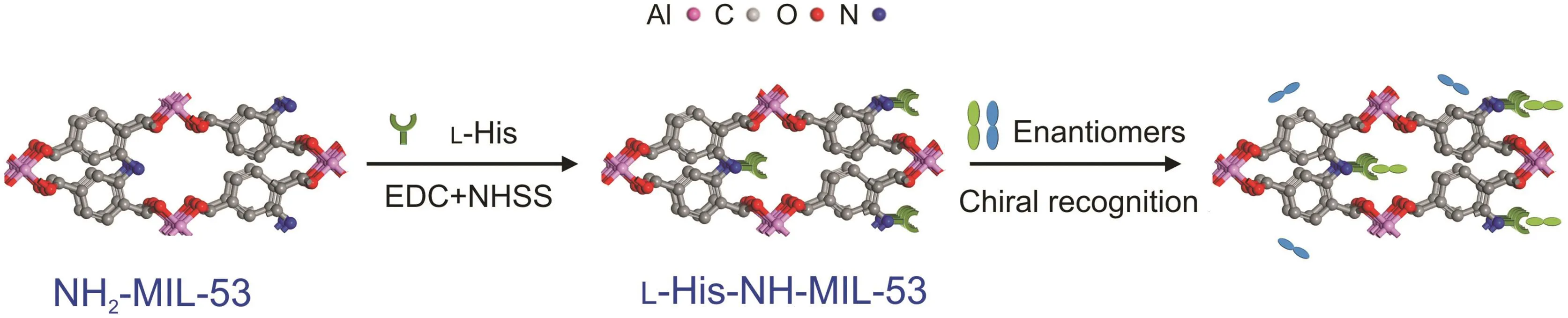
Fig.6.Schematic illustration of the mechanism of chiral recognition by L-His-NH-MIL-53 coating.
A comparison of coating preparation methods,merits,analytes for the L-His-NH-MIL-53@capillary and other coating materials in OT-CEC (e.g., gold nanoparticles,graphene oxide, Fe3O4nanoparticles,and silica nanoparticles)is summarized in Table 1[26-29].Notably,the chiral functional groups of the previously investigated materials are mostly based on traditional chiral selectors,which are characterized by strong enantioselectivities(e.g.,cyclodextrins and enzymes).By comparison,a much weaker chiral recognition reagent(L-His)was selected for this study.This would better reflect the ability of MOFs to enhance the enantioselectivity of a certain chiral selector.Other advantages of the LHis-NH-MIL-53@capillary column are its high porosity and the ability to provide multiple enantiorecognition forces,such as electrostatic interactions,π-π interactions,and hydrophobic effects[26-29].The results of the present study were also compared with those obtained in other MOF-based studies,and their(UiO-66-NH2,γ-CD-MOF(Cu-SD),ZIF-90,HKUST-1,JLU-Liu23 and NH2-MIL-53)respective merits are listed in Table S1[23,30-33].
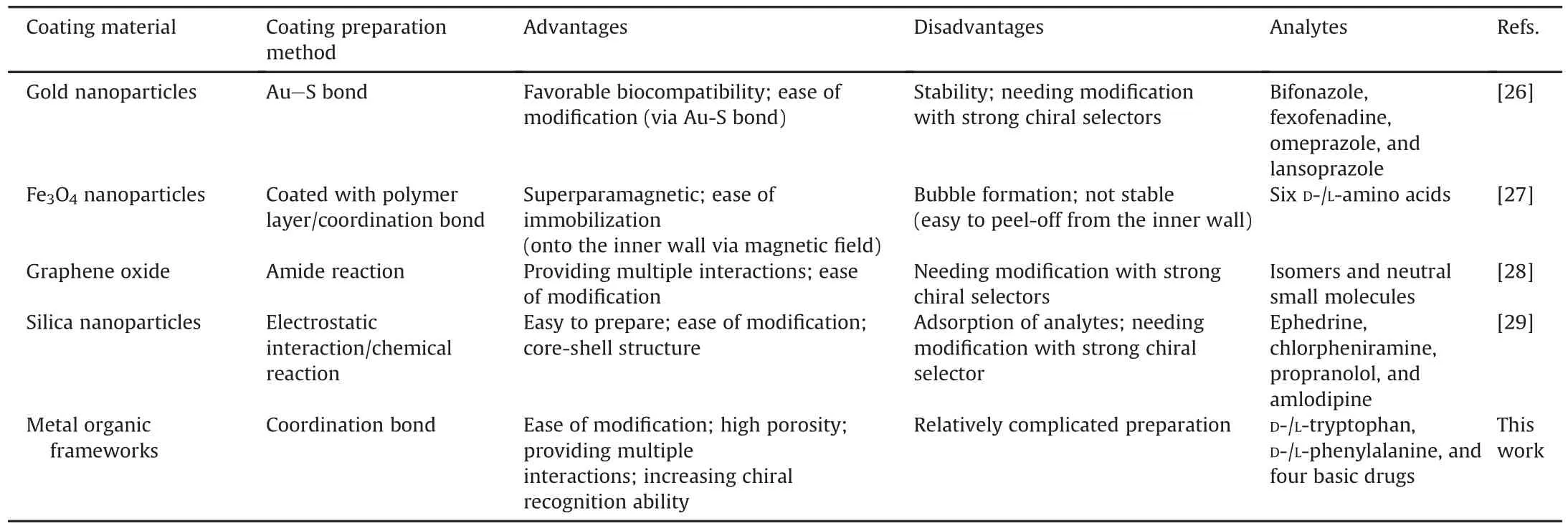
Table 1A comparison of the present open-tubular capillary electrochromatography mode methods with some other works.
4.Conclusion
In this study,we described the preparation of a homochiral MOF,L-His-NH-MIL-53.This MOF was employed to construct an OT-CEC system,which exhibited an encouraging chiral separation performance for several model drugs.Various characterization approaches,such as TEM,SEM(with EDS),FT-IR,XRD,TGA,CD,BET,and XPS,were employed to verify the formation of L-His-NH-MIL-53 andL-His-NH-MIL-53@capillary.The porous network of the prepared homochiral MOFs is deemed to play a vital role in improving the enantiorecognition capability of L-His,because it can provide sufficient sites for L-His-enantiomer interactions.The hydrophobic effect and π-π interactions are speculated to additionally contribute to the favorable chiral recognition.This study is expected to afford a new strategy for the design and establishment of MOF-based chiral OT-CEC systems.Moreover,it demonstrates the potential of improving the enantioselectivities of weak chiral recognition reagents by fully utilizing the inherent characteristics of porous organic frameworks.
CRediT author statement
Xiaodong Sun:Methodology,Writing-Original draft preparation;Bing Niu:Investigation,Writing-Original draft preparation;Qi Zhang:Conceptualization,Methodology,Writing-Reviewing and Editing;Qin Chen:Conceptualization,Investigation.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgment
This study was funded by the National Natural Science Foundation of China(Grant No.:82003705).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.12.004.
 Journal of Pharmaceutical Analysis2022年3期
Journal of Pharmaceutical Analysis2022年3期
- Journal of Pharmaceutical Analysis的其它文章
- Recent advances in quantum dots-based biosensors for antibiotics detection
- Qualitative and quantitative evaluation of Flos Puerariae by using chemical fingerprint in combination with chemometrics method
- Multiple rapid-responsive probes for hypochlorite detection based on dioxetane luminophore derivatives
- Nitrogen-doped carbon@TiO2double-shelled hollow spheres as an electrochemical sensor for simultaneous determination of dopamine and paracetamol in human serum and saliva
- Compatibility and stability studies involving polymers used in fused deposition modeling 3D printing of medicines
- A preparation strategy for protein-oriented immobilized silica magnetic beads with Spy chemistry for ligand fishing
