Formation of composite hydrogel of carboxymethyl konjac glucomannan/gelatin for sustained release of EGCG
Lin Wang*, Ning zhou, Shengxuan Zheng, Jie Pang,*
a Department of Engineering Mechanics, Tsinghua University, Beijing 100084, China
b Institute of Superlubricity Technology, Research Institute of Tsinghua University in Shenzhen, Shenzhen 518057, China
c College of Food Science, Fujian Agriculture and Forestry University, Fuzhou 350002, China
Keywords:
Hydrogel
Carboxymethyl konjac glucomannan
Gelatin
Tannic acid
Biodegradation
Sustained release
A B S T R A C T
Development of functional bioinspired hydrogels that have good releases control character is necessary for the application of these materials in biomedical engineering. Herein, we report a composite hydrogel prepared from several biocompatible carboxymethyl konjac glucomannan (CKGM)/gelatin (G)/tannic acid (TA) functional nano-hydroxyapatite (TA@n-HA), which has good biodegradability and pH sensitivity. The mechanism of interaction between hydrogels was con firmed by Fourier transform infrared spectroscopy, X-ray diffraction,Scanning electron microscopy and Thermogravimetric analysis. The physico-chemical properties of CKGM/G hydrogels have been significantly improved through the incorporation of TA@n-HA within the matrix. Studies in the sustained release of epigallocatechin gallate (EGCG) demonstrated that the TA@n-HA/CKGM/G hydrogels exhibit not only better pH sensitive properties, but also enhanced biocompatibility and encapsulation in comparison to the matrix devoid of TA@n-HA. Consequently, TA@n-HA/CKGM/G hydrogels using EGCG as a drug release model show the potential for drug delivery.
1. Introduction
Hydrogels with desirable characteristics have been supposed to be potential materials for bioactive molecules delivery [1-3]. Due to the good water holding capacity and biocompatibility, hydrogel has been widely used for delivering functional compositions [4].However, some crucial challenges including the biodegradable,biocompatibility, pH sensitive properties and eco-friendly of hydrogels remain to be solved [5,6]. Compared with synthetic materials,natural polysaccharides tend to be environment friendly [7,8],biodegradable [9,10], and biocompatible [11,12]. As for natural macromolecules, such as chitosan [13,14], carboxylated chitosan [15],konjac glucomannan (KGM) [16,17], sodium alginate (SA) [18],carrageenan [19], and cellulose [20,21], they are easily formed hydrogels by a facile method in environmentally aqueous solution .
Among the polymers widely used in hydrogel applications,KGM is a naturally occurring polysaccharide obtained by theAmorphophallustubers [22]. It is composed of glucose and mannose units viaβ-1,4 glycosidic bonds linkages in a molar ratio around 1:1.6, and with the acetyl groups attach to the saccharide units and scatter randomly along the molecule [16,23]. In addition, approximately 1 per 19 sugar residues is common at the C-6 position [24]. Natural KGM is a kind of insoluble dietary fiber with high molecular weight and high viscosity [25]. Studies have shown thatβ-mannanase catalyzes the pyrolysis of KGM to produce mannose, which reduces the molecular weight of KGM. The low molecular weight KGM can quickly reach the colon and aid fermentation to produce more beneficial short-chain fatty acids, making it a potential carrier. [26,27]. Due to its good biocompatibility [28], biodegradability [29], easily modified ability [30],and gel-forming properties [31], KGM has become an inevitable source for both research and application purposes in the fields of biomedical and food industries [32]. However, various limitations including poor pH sensitivity behavior and strong water absorption are still associated with these promising materials [33].
To address these drawbacks, some studies have been performed via modifying KGM. Carboxymethylation is a common strategy to modify the charge property of polysaccharides [34]. Carboxymethylated KGM (CKGM) has been used by some authors as anionic polysaccharides to construct pH-sensitive delivery systems [35]and to prepare sponge dressings with good biocompatibility [36].
Nevertheless, compared with carboxymethylated polysaccharides,the application of CKGM is still quite limited. Gelatin (G) has unique gelling properties and excellent mechanical properties, making it an ideal material for preparing hydrogels [37], such as biocompatible nanofiber films [38]. Besides, due to a large number of functional side groups, gelatin readily undergoes chemical cross-linking reaction with CKGM via electrostatic interaction between amino groups and carboxyl groups. However, the main limitation of gelatin is its rapid dissolution in aqueous environments leading to fast drug release [39].In order to overcome this problem, hydrogels that are reinforced by nano filler to slow down the release of the loaded bioactive molecules have recently been considered [40,41].
Nano hydroxyapatite (n-HA) as a main inorganic component of natural bone mineral [42,43], which has excellent biocompatibility [44],can be used in human skeletal tissue [45]. While, since it forms active connection with the tissue due to its strong surface area and agglomerate [46], its development and application are limited.Surface modification of n-HA can overcome the agglomeration problem caused by the high surface activity of nanoparticles [47,48].Tannic acid (TA) featured with colorless and low cost is recognized as a better alternative plant phenolic for the design of biocompatible functional surface engineering [49]. Accordingly, the n-HA was modified by the oxidative polymerization of TA and then the functionalized TA n-HA was used as reinforcing nanofiller in the CKGM/G hydrogels. Moreover, it finds applications as a carrier for drug delivery [50], in repairing enamel [51], as a filler in bone repair or replacement [52]. Epigallocatechin gallate (EGCG) is a natural polyphenolic compound with antioxidant, anticancer and antibacterial activities. Effective encapsulation of EGCG enables it to be widely used in biomedical field, especially in the treatment of atherosclerosis and cardiac hypertrophy [53-56]. EGCG possesses many hydroxyl groups in its molecular structure, which can form hydrogen bond interactions with KGM [57]. EGCG has been added into polysaccharides to produce hydrogel [58]. Therefore, we choose it as the bioactive ingredient for the preparation of gel.
Mussel chemistry is an emerging technology [59]. Due to its foot protein containing the catechol group of dihydroxyphenylalanine(DOPA) and the amino group of lysine, mussels can interact with the substrate through non-covalent and covalent chemical action to achieve adhesion [60]. Inspired by the mussel-inspired adhesive mechanism, we propose a CKGM/G hydrogel with simultaneous enhancement of sustainable release properties by incorporating TA-coated n-HA (TA@n-HA) motifs into the CKGM-G dynamic networks. Among them, electrostatic interaction and hydrogen bonding interactions were further studied. The properties and the crosslinking mechanism of the hydrogel were investigated by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction patterns(XRD), scanning electron microscopy (SEM) and Thermo-gravimetric analysis (TGA). To our knowledge, this is the first report on the TA@n-HA/CKGM/G hydrogels. We hypothesized that electrostatic interaction and hydrogen bonding interactions were the features of hydrogel, which can enhance sustainable release properties (Fig. 1).The aim of our study was to produce a unique and efficient hydrogel to be used in biomedical applications, and this work can also provide useful information for extending the application and offer a new perspective for the design and development of hydrogels.
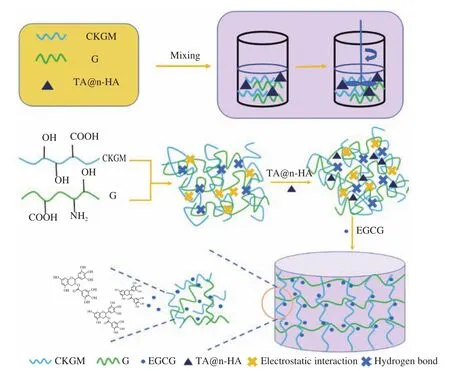
Fig. 1 Scheme showing the preparation and releasing mechanism of TA@n-HA hydrogels.
2. Experiment
2.1 Materials
KGM (purity ≥ 95%) was purchased from Shaotong San Ai Konjac Development Co. (Yunnan, China). EGCG (purity 95%) was purchased from Sigma Chemical Reagent Co., Ltd. Gelatin, n-HA,tannic acid (purity 98%), tris (hydroxymethyl)-aminomethane,sodium acetate, monochloroacetic acid (MCA) and silver nitrate were purchased from Sigma-Aldrich (St. Louis, MO,USA). Cellulase E0240 was purchased from Shanghai Bio Life Science and Technology Co. Ltd, China (Mw = 5.2 × 103g/mol,enzymatic activity ≥ 15 U/mg). Glycerin and absolute ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. All the chemicals used as received without further purification.
2.2 Preparation of CKGM
CKGM was prepared by the method of Xiao et al. [61]with minor modifications. KGM powder (10 g) was immersed in 30 mL of 50% by weight methanol aqueous solution. After 40 min,the swollen KGM was suspended in 50 mL of methanol, and a 30%(m/m) sodium hydroxide aqueous solution was gradually added while stirring at 50 °C. After 30 min, 0.6 g of monochloroacetic acid was added to 25 mL of 70% (V/V) ethanol and added to the previous step.At this time, the mixture was neutralized with hydrochloric acid.The product was washed successively with 70%, 80% and 100% by weight methanol to remove impurities and filtered off. The residue was air-dried and dried in vacuum at 50 °C.
2.3 Preparation of TA@n-HA
The TA@n-HA suspension was prepared by a one-pot water-based process based on a previously reported method with minor modifications [62], In details, Tris buffer solution (1 mol/L)was added dropwise to 200 g of n-HA suspension (containing approximately 6 g n-HA) until the pH reached 8.0. Subsequently, 3 g of TA was added, the suspension was magnetically stirred for 8 h at 25 °C, and the collected TA@n-HA suspension was sonicated (300 W,20 min) and stored at 4 °C before usage.
2.4 Preparation of TA@n-HA/CKGM/G hydrogel
The TA@n-HA powder (1.0, 2.0, and 3.0 g) was dissolved into 100 mL of the distilled water (70 °C) to obtain 1 g TA@n-HA, 2 g TA@n-HA as well as 3 g TA@n-HA, respectively. And then 0.5 g gelatin was dissolved into the TA@n-HA solutions (70 °C). After the gelatin was completely dissolved, the solution was prepared by dissolving 1.0 g of CKGM powder into the mixture (45 °C) in a water bath under magnetic stirring at 400 r/min for 1.5 h, and finally obtain TA@n-HA/CKGM/G hydrogel.
For the preparation of EGCG, CKGM, CKGM/G, TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel was loaded. EGCG was dissolved in water to a concentration of 1.0 mg/mL, 2.0 g hydrogel was equilibrated in 200 mL of 1.0 mg/mL EGCG solution for 12 h at 25 °C to load it into CKGM, G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel.
The sample codes for TA@n-HA/CKGM/G hydrogel(TA@n-HA-x) are marked by the weight ratio of the TA@n-HA.For instance, 1g TA@n-HA/CKGM/G hydrogel that is designated as TA@n-HA-1, and the detailed compositions of TA@n-HA/CKGM/G hydrogels are summarized in Table 1. For neat gels (CKGM, CKGM/G),they were prepared by the same procedures without incorporation of TA@n-HA.
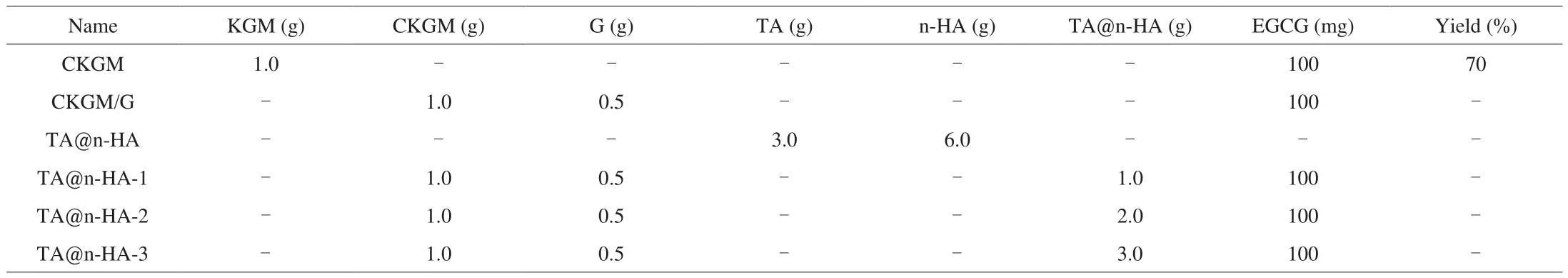
Table 1Composition of initial reaction mixtures used for the preparation of hydrogels.
2.5 Rheological analysis
All rheological measurements were carried out using a rotational rheometer (Rheometrics, NJ, USA). The parallel-plate mode (PP-50,50 mm, and 2 mm gap) was carried out. Steady shear flow behavior and strain sweep of CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2,and TA@n-HA-3 hydrogel. Samples were measured in triplicate (The status of TA@n-HA-x samples is sol).
2.6 FT-IR
The FT-IR spectra of TA, n-HA, TA@n-HA, CKGM, CKGM/G,TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel were recorded to search for interactions between TA@n-HA, CKGM and G (Bruker Vertex 70, Madison, USA), and the scanning wavelengths were in the range of 4 000 and 400 cm-1.
2.7 XRD
X-ray diffraction pattern is an effective method to reflect the crystal and amorphous structure of hydrogels [63]. The X-ray diffraction patterns were obtained on freeze-fried of TA, n-HA, TA@n-HA,CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel by an X-ray diffractometer (XRD, Bruker Inc., Germany)equipped with Ni- filtered Cu Kα radiation, and the data were recorded from angles of 2–60° at a scan rate of 10 min-1.
2.8 TGA
The thermal stability of the hydrogel was tested using TA instruments (TGAQ500, Newcastle, DE, USA). The temperature parameters were set from 25 °C to 600 °C at a rate of 10 °C/min under nitrogen atmosphere.
2.9 SEM
The structures of the n-HA, TA@n-HA, CKGM, CKGM/G, TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel was examined by a scanning electron microscope (SEM, Hitachi, SU8010, Tokyo, Japan).The hydrogel was coated with a gold-plated layer.
2.10 Biodegradation
The biodegradation experiment was carried out according to the method we used before [64]. The enzymatic degradation of the TA@n-HA hydrogels were determined in a flask containing a 30 mL buffer of the generated Cellulase E0240.
2.11 EGCG loading determination
The entrapment efficiencies (EEs) of the CKGM, G, TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel for EGCG was determined by the following equation:
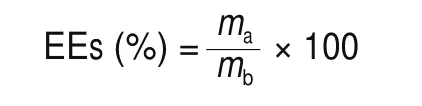
wheremaandmbare the actual and theoretical EGCG contents of the CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel, respectively. TA@n-HA hydrogel samples are made in parallel three times.
2.12 In vitro release study
EGCG was used as a model drug to investigate the sustained release property [65].In vitroexperiments were conducted to evaluate the effects of different media on the sustained release behavior of CKGM CKGM/G TA@N-HA-1 TA@N-HA-2 and TA@N-HA-3 hydrogels in simulated gastric fluid (SGF, pH 1.2) simulated intestinal fluid (SIF, pH 6.8) and simulated colorectal fluid (SCF, pH 7.4) [66].
Loaded with 100 mg EGCG of CKGM, CKGM/G, TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel was immersed in 50 mL each of SGF, SIF and SCF at 37 °C under constant shaking at 60 r/min. These 10 mL EGCG loaded with CKGM, CKGM/G,TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 solutions were transferred to a dialysis bag. Then, 5 mL of medium was quantified using UV-Vis spectrophotometer (UV-2600, Shimadzu, Japan) at 272 nm, and withdrawn volume with 5 mL fresh medium buffer at 37 °C.
3. Results and discussion
3.1 Rheological characterization
The steady rheological properties of CKGM, CKGM/G, TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel was shown in Fig. 2a. The formation and rupture of hydrogels are easily affected by shear force,which is a typical behavior of polysaccharide solution. High shear forces can change the inter-chain and intra-chain interactions of hydrogels,so hydrogels have shear thinning properties, indicating that hydrogels are a typical non-Newtonian pseudoplastic fluid. Gong et al. [67]reported the similar behavior of polysaccharide, which are due to solutes with higher molecular weight and high polymer structure, and shear-thinning behavior provides advantageous characteristics [68].Notably, the initial viscosity of all hydrogels at low shear rates increases with increasing shear rates, which may be due to the strong interaction between hydrogel particles that makes the hydrogels tend to flow in the opposite direction [69]. As shown in Fig. 2a,over the shear rate range from 0.001 s-1to 1 s-1, TA@n-HA-3 hydrogel showed a higher viscosity than another hydrogel, TA@n-HA can produce mussel chemical reaction in hydrogel system to increase viscosity. The viscosity of TA@n-HA-3 hydrogel reaches 59.2 Pa·s, while the viscosity of CKGM/G hydrogel is only 17.44 Pa·s, and always maintained a sol state. The viscosity curve exhibited an upward trend. It suggested that the hydrogen bonding interactions of the hydrogel is enhanced with the addition of TA@n-HA.This observation indicates that TA@n-HA could increase the formation of hydrogen bonding. In a similar study on n-HA, theβ-sheet ratio of secondary structure of gelatin in HAPO film decreased from 40.69% to 34.20% with n-HAP, which were HAP10 and HAP50 films respectively, indicating that the structure of the fiber was denser due to the hydrogen bond between gelatin and n-HAP [70].
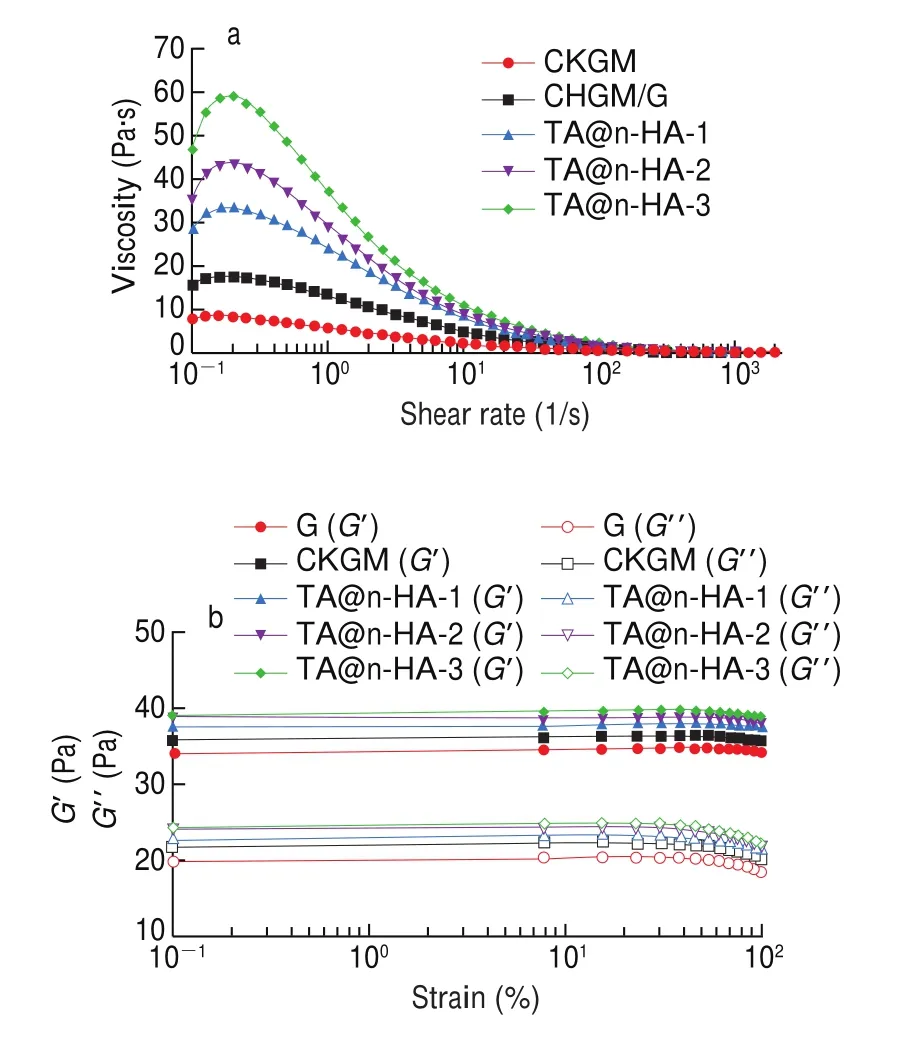
Fig. 2 Steady rheological properties (a) and strain sweeps (b) of CKGM,CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel.
To study the viscoelastic properties of TA@n-HA hydrogels, the linear viscoelastic region (LVR) was performed for hydrogels though dynamic strain sweep [71]. As shown in Fig. 2b, the value of the storage modulus (G’) was higher than that of loss modulus (G’’), and the viscoelasticity of TA@n-HA-3 was higher than CKGM, CKGM/G,TA@n-HA-1 and TA@n-HA-2 hydrogel, the more TA@n-HA,the greater the viscoelasticity of the hydrogel. These results indicated that the interaction between CKGM/G and the entanglement of intermolecular chains were enhanced with the increasing of TA@n-HA.Pure CKGM hydrogel showed the lowestG’, which may be attributed to the fact that it has maintained a sol state, and there is no solid network structure inside the sol. However, the value ofG’of hydrogels increased with TA@n-HA proportion, suggesting the formation of a more stable networks at high TA@n-HA concentration.This phenomenon may be ascribed to TA@n-HA in hydrogels interacting with CKGM/G molecules by forming hydrogen bonds and forming a more compact network, which is moreflexible. Moreover,the value ofG’ andG’’ remained constant (0.1% to 10.0%), while the other region is non-linear, with the values ofG’ andG’’ changing(> 10%). Furthermore, when the strain is less than 10%, the morphology of the hydrogel remains unchanged. When the strain exceeded 10%, the molecular chains in the hydrogel were destroyed and began to show a downward trend.
3.2 FTIR spectra analysis
The FTIR spectra of CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2,and TA@n-HA-3 hydrogel was given in Fig. 3, which were observed to study the functional groups and interactions between CKGM, G, and TA@n-HA. n-HA exhibited absorption peaks at 1 089.7 cm-1and 961.4 cm-1that were attributed to the stretching vibrations of asymmetrical P-O stretching and symmetrical P-O stretching [72],respectively. After n-HA was modified by the oxidative polymerization of TA, the characteristic absorption peaks of n-HA were well reserved, and a new band appeared at 1 182.3 cm-1in the TA@n-HA. These results indicated the successful coating of TA on the n-HA surface (Fig. 3a).
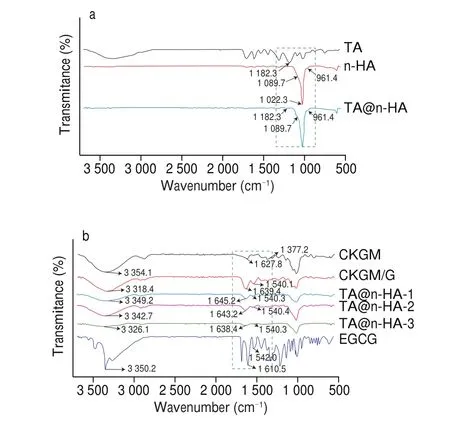
Fig. 3 FTIR spectra of CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel at wavenumbers between 500 and 4 000 cm-1.
For CKGM, the broad peak at 3 354 cm-1was attributed to both inter- and intra-molecular hydrogen bonding interactions among OH groups in CKGM [73]. Because of the hydrogen bonding and the number of hydroxyl groups of each components are different, a peak shift occurred at around 3 300 cm-1, and a peak has appeared at 1 377 cm-1, which clearly confirms the presence of carboxymethyl groups on the CKGM [73](Fig. 3b). Around 3 318 cm-1, a wide band can be observed in the spectrum of G, referring to the combined peaks of OH and NH stretching vibration [74]. The absorption peak at 1 639 cm-1was attributed to molecular hydrogen bond, which was consistent with our previous studies [75,76]. New peak has appeared at 1 540 cm-1in the hydrogel, which clearly con firms the presence of EGCG in the hydrogel. Compared to the FT-IR spectrum of CKGM and CKGM/G, the location of some characteristic peaks shifted after TA@n-HA was added, which suggested that hydrogen bond was the main interaction force between CKGM/G matrix and TA@n-HA. The absorption peaks around at 1 640 cm-1were significantly increased for CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel comparing to CKGM. This phenomenon was attributed to the formation of more intramolecular hydrogen bonds. These results indicated the electrostatic interactions between carboxyl groups(—COO—) on CKGM and the amino groups (—NH3+) on gelatin. In addition, the change in the intensity of FT-IR peaks may be attributed to good compatibility.
3.3 XRD analysis
The XRD patterns of the CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2,and TA@n-HA-3 hydrogel was presented in the Fig. 4. A broad peak at 2θ= 21.0° was observed in the CKGM, indicating that CKGM was an amorphous material, which was consistent with previous research [64].The X-ray patterns of CKGM/G was similar to the CKGM, indicating the good homogeneity of CKGM and G. After the combination of CKGM and G, the peak value becomes lower, this may be ascribed to the synergistic interactions promoting the movement of segments chain segment thus impacting the crystallinity [77,78].

Fig. 4 X-ray diffraction patterns of CKGM, CKGM/G, TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel.
Compared with CKGM and CKGM/G hydrogel, the intensity of diffraction peaks of TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel at 2θ= 25.83° and 31.81° were enhanced, indicating that the crystallinity of CKGM and G networks increased during the adding of TA@n-HA. TA@n-HA-3 hydrogel showed two diffraction peaks at around 2θ= 26.23° and 32.30°. These observed peaks can be ascribed to the crystal regions of TA@n-HA. For the TA@n-HA hydrogel,2θ= 26.18° and 32.17° of TA@n-HA-2 hydrogel did not change significantly, and TA@n-HA-2 hydrogel and TA@n-HA-3 hydrogel were both slightly shifted, corresponding to the characteristic crystalline structure of TA@n-HA. The above results have shown that TA@n-HA is well integrated with the CKGM/G matrix, and no new diffraction peaks appear, indicating that no other new substances have been generated, further indicating that the intermolecular interactions between several substances have not changed. It may be caused by the electrostatic interaction between the amino group and the carboxyl group and the hydrogen bond between TA@n-HA and the CKGM/G matrix. Other reason can be the large amount of TA@n-HA promoted the hydrogen bonding interaction between CKGM, G and TA@n-HA, resulting in a similar crystal structure. As depicted in Fig. 4,TA@n-HA hydrogels appeared similar diffractogram profiles. The peaks associated with the crystal planes of TA@n-HA were observed for TA@n-HA hydrogels, proving the incorporation of TA@n-HA within the hydrogels. These results supported the results of FT-IR.
3.4 The thermal stability (TGA& DTG) analysis
To investigate the influence of TA@n-HA on thermal stability of the hydrogels, the thermal characteristic of TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel was analyzed and compared according to their TGA and DTG (Derivative thermogravimetry)curves, and the results are displayed in Fig. 5. The results of TGA and DTG curves showed multistep decomposition, the hydrogel underwent two degradation stages, and the specific information of TGA of hydrogels has been listed in Table 2. All hydrogels have a slight weight loss near 100 °C, corresponding to the vaporization of the adsorbed moisture [6,79]. The weight loss of hydrogel mainly appeared at 150–600 °C with approximately 31%-63%, which was ascribed to the disaggregation and fracture of C—O and C—C bonds of the saccharide ring [80](Fig. 5a). For CKGM, thermal degradation between 150–600 °C with 63.52% weight loss can be assigned to the decomposition of the main backbone of CKGM. For TG curves, the initial decomposition temperature of these hydrogels was similar and close to 200 °C, suggesting the introductions of TA@n-HA had not significantly influence on initial decomposition temperature of the hydrogels.
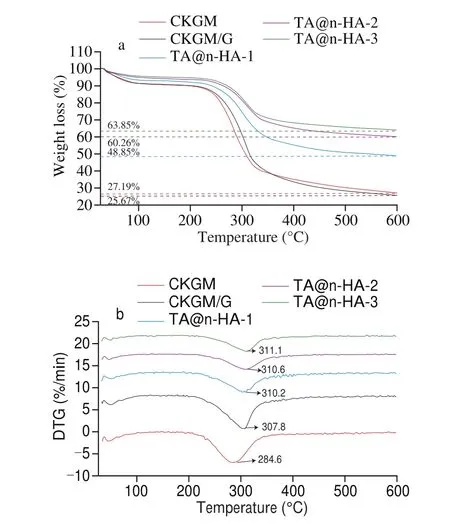
Fig. 5 TG thermograms (a) and DTG (b) of CKGM, CKGM/G, TA@n-HA-1,TA@n-HA-2, and TA@n-HA-3 hydrogel.
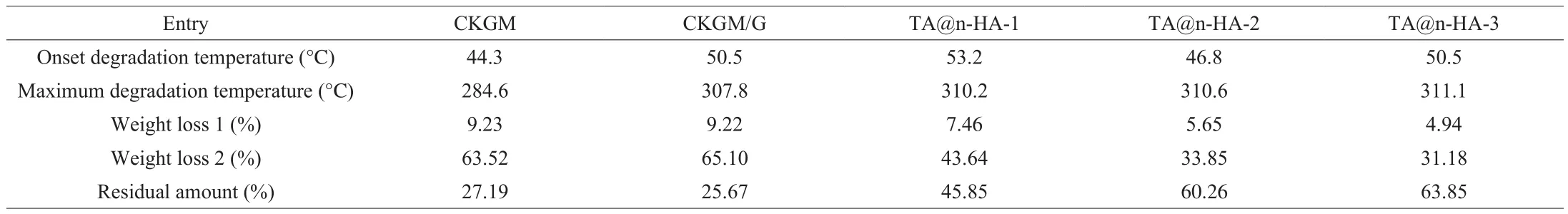
Table 2Quantitative TGA of TA@n-HA hydrogels.
Besides, The DTG curves (Fig. 5b) showed the relationship between temperature and weight losing rate which presented a temperature peak at 284.6, 307.8, 310.2, 310.6 and 311.1 °C for CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel, respectively. Notably, the maximum degradation temperature of TA@n-HA hydrogels increased from 310.2 °C to 311.1 °C as the increase of TA@n-HA ratio, and was much higher than that of CKGM (284.6 °C) or CKGM/G (307.8 °C) (Fig. 5b and Table 2),suggesting that the combination of CKGM, G and TA@n-HA via electrostatic interactions and hydrogen bonds were helpful for the enhancement of thermal stability. TA@n-HA hydrogels showed an enhanced thermal stability at higher temperature (> 300 °C)followed by a decrease of weight loss after heating to 500 °C, which was attributed to the resistant characteristic of TA@n-HA, results are similar to those of Dai et al. [6]and Haa fiz et al. [81].
3.5 SEM analysis
To obtain the information regarding microstructure, the hydrogel was hlyophilized for morphology observation using SEM [82]. The surface morphology of n-HA before and after coating treatment were monitored using SEM (Figs. 6a-b). The unmodified n-HA showed a smooth surface. TA@n-HA exhibited a rough surface, therefore,the TA successfully attached to the n-HA surface, findings similar to those of Kang et al. [83], this behavior was in accordance with the results of FT-IR and XRD.
It was observed that all hydrogels showed an interconnected porous structure, which is similar to our previous reports [75,84].Moreover, the interconnection between pore structure could be due to the network formed by the electrostatic interaction between amino and carboxyl, and the hydrogen bonds between TA@n-HA and CKGM/G matrix, which supports our hypothesis.
Compared with CKGM and CKGM/G hydrogel (Figs. 6c-d), the TA@n-HA hydrogels have more condensed structure and the pore size decreases with the increasing TA@n-HA content (Figs. 6e-g).Moreover, the TA@n-HA-3 hydrogel exhibit more stable structure and compact morphology (Fig. 6g). This attributed to the increased hydrogen bonding and accelerated gelation process between CKGM,G and TA@n-HA. This result confirmed that the compact internal structure of the TA@n-HA hydrogel provides good conditions for the encapsulation of active compounds.
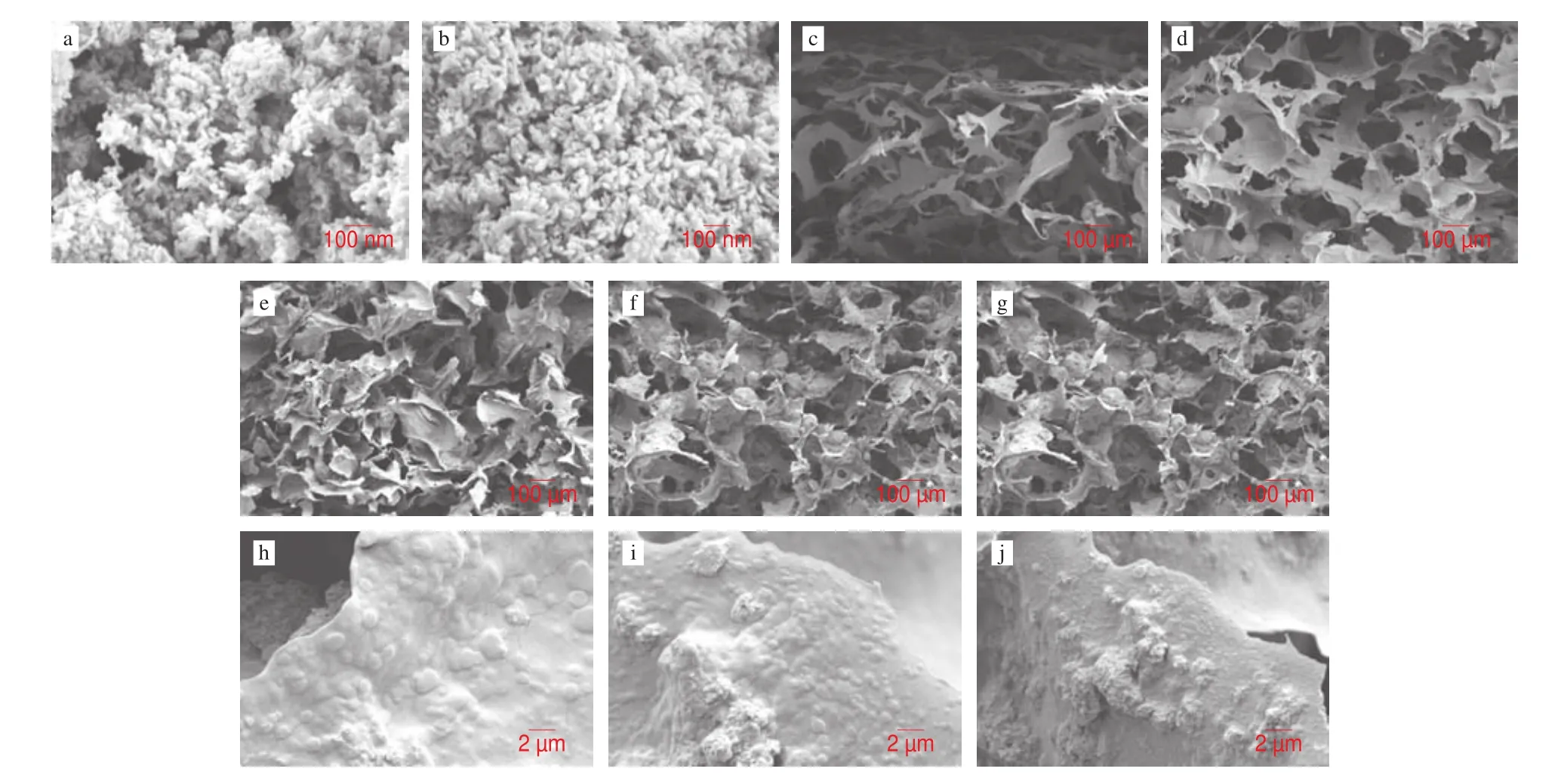
Fig. 6 The surface and cross-section microstructures of n-HA (a), TA@n-HA (b), CKGM (c), CKGM/G (d), TA@n-HA-1 (e,h), TA@n-HA-2 (f,i) and TA@n-HA-3 (g,j).
3.6 Biodegradation study
Biodegradability is an important factor in developing hydrogels for applications in delivery system [79]. The biodegradability of the TA@n-HA hydrogels were investigated in phosphate buffer containing Cellulase E0240. As shown in Fig. 7a, after 12 h of incubation, the degradation of CKGM was the fastest with a remaining weight of about 30.15%. For TA@n-HA-1, TA@n-HA-2 and TA@n-HA-3 sample, the weight remaining was 42.63%,46.45% and 52.94%, respectively. The results suggested that KGM-based hydrogel can be degraded by Cellulase E0240. Moreover,the diffusion and degradation rate of the enzyme in TA@n-HA hydrogel were improved [85]. Therefore, the degradation rate of hydrogels can be tailored by modifying the compositions of hydrogels that was controlled by altering the content of TA@n-HA [78].Hydrogel degradation and release of EGCG is shown in Fig. 7e. In different pH environments, TA@n-HA hydrogel degrades and EGCG is continuously released.
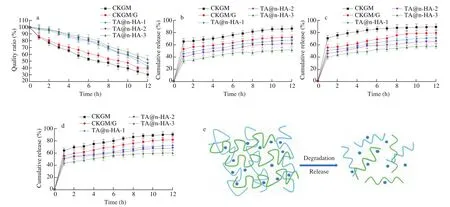
Fig. 7 The biodegradation study (a) and releasing properties of EGCG from CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel(b: SGF; c: SIF; d: SCF), hydrogel degradation and release of EGCG (e).
3.7 EGCG encapsulation efficiencies and release behaviors
EGCG have limited release ability which confines their application [79]. To explore the EGCG controlled release behavior of the CKGM, CKGM/G, TA@n-HA-1, TA@n-HA-2, and TA@n-HA-3 hydrogel, the EESwas studied to obtain the EGCG content of TA@n-HA hydrogel. The EEs of TA@n-HA hydrogels were carried out their UV spectra (UV-2600, Shimadzu, Japan) at 272 nm and were(17.34 ± 3.15)%, (20.24 ± 3.67)%, (28.36 ± 3.07)%, (33.43 ± 2.61)%,and (38.74 ± 2.45)%, respectively. TA@n-HA-3 hydrogel higher EEs, which may be attributed to electrostatic interaction and hydrogen bonding interactions. It increased the pore structure of TA@n-HA-3 hydrogel and provided more free space for EGCG storage.
The release of EGCG in several hydrogels in three media SGF,SIF and SCF was present in Figs. 7b-d. As demonstrated at SGF, a burst release occurs in about 1 h for all the hydrogels and sustained release can be observed thereafter, because of the large concentration gradient between solution and simulated gastric fluid [86].
As shown in Fig. 7c, the cumulative release rates of EGCG were as 88.72% and 79.35% for hydrogels without TA@n-HA at SIF condition, respectively, the reason is that when EGCG is loaded, some EGCG will be loaded on the surface of the hydrogel and the internal porous structure. The EGCG on this part of the surface will enter the gastrointestinal simulation fluid at the initial stage of controlled release, causing burst release. In contrast, more EGCG can be released at SCF, indicating the pH sensitive release of the TA@n-HA hydrogels. This situation arises from the presence of amino groups of gelatins having polyelectrolyte properties in the structure of hydrogel.TA@n-HA hydrogels showed a quick release at the beginning and more than 45.85% of EGCG was released in the first 1 h [87-89],which was attributed to the EGCG on the surface of the hydrogels readily diffuses into the release medium. Notably, the release speed slowed down for TA@n-HA-3 hydrogel, this is due to electrostatic interactions and hydrogen bond interactions, which make the internal structure of the hydrogel more compact.
It is also clear that at weakly alkaline condition, a burst release of EGCG from CKGM/G hydrogel can be observed and over 82.03% EGCG is released within 12 h. After the introduction of TA@n-HA,the initial burst release is depressed significantly, and the release rate decreases with the increase of TA@n-HA content. The addition of TA@n-HA makes the structure of the hydrogel more stable, the interaction between internal molecules is enhanced, and the filling effect is achieved. For TA@n-HA-3 hydrogel, the accumulative release percent increases gradually and reaches 60.46% after 12 h,which was much lower than CKGM, showing good controlled release performance. n-HA has the advantages of controllable structure, large drug loading, and high encapsulation efficiency [50,90,91].
Loading EGCG after surface modification can solve the problem of drug burst release. The molecular chain of TA@n-HA hydrogels contain amino groups and residual ionizable carboxyl. As the change of pH, the groups ionized, resulting in the dissociation of electrostatic interaction and hydrogen bonds between the macromolecular chains,showing pH-responsive abilities.
4. Conclusion
In this work, we have successfully developed a novel TA@n-HA hydrogels, which can be easily prepared from polysaccharides and proteins. The good interactions and network structure characteristics of the composite gel molecules were revealed by FT-IR, XRD, TGA and SEM. While the trend ofG’ increased following the increase of TA@n-HA ratio. The TA@n-HA improved the thermal stability and entrapment efficiency of the hydrogels. Thein vitrorelease profile of TA@n-HA hydrogels showed a pH-responsive and sustained release property. After released to SCF for 12 h, the maximum release amount of CKGM could even reach 90.35%. Moreover, TA@n-HA hydrogels showed good biodegradability. Based on these results,TA@n-HA hydrogels can as a potential delivery carrier, has a great prospect for the site-specific small molecule delivery in the intestine.
conflict of interest
No conflict of interest exists in the submission of this manuscript.The work described was original research that has not been published previously, and not under consideration for publication elsewhere.
Acknowledgment
This research was financially supported by the National Natural Science Foundation of China (Grant No. 31772045), the program on Fujian Agriculture and Forestry University of doctoral students going abroad (Grant No. 324-112110089) and scientific research foundation graduate school of Fujian Agriculture and Forestry University (Grant No. 324-1122yb064).
- 食品科學(xué)與人類健康(英文)的其它文章
- Yogurt-derived Lactobacillus plantarum Q16 alleviated high-fat diet-induced non-alcoholic fatty liver disease in mice
- The levels of osteopontin in human milk of Chinese mothers and its associations with maternal body composition
- Lactobacillus fermentum as a new inhibitor to control advanced glycation end-product formation during vinegar fermentation
- Screening and identification of purine degrading Lactobacillus fermentum 9-4 from Chinese fermented rice- flour noodles
- Characteristic and effect analysis of protein and peptide in Cantonese cured meat processing
- Antioxidant effect of Lactobacillus fermentum HFY02-fermented soy milk on D-galactose-induced aging mouse model

