Hepatocellular carcinoma and immunotherapy:Beyond immune checkpoint inhibitors
TO THE EDITOR
We read with great interest the review by Mattos
[1]on the immune landscape of hepatocellular carcinoma(HCC),which covered the immune aspects and markers of HCC as well as the immunotherapeutic modalities used in this malignancy.Considering the immunogenicity of HCC,it comes as no surprise that clinical and basic research has been directed to dive deeper into the immune-biological and therapeutic upside of HCC,especially with the rise of immunotherapy in oncology.
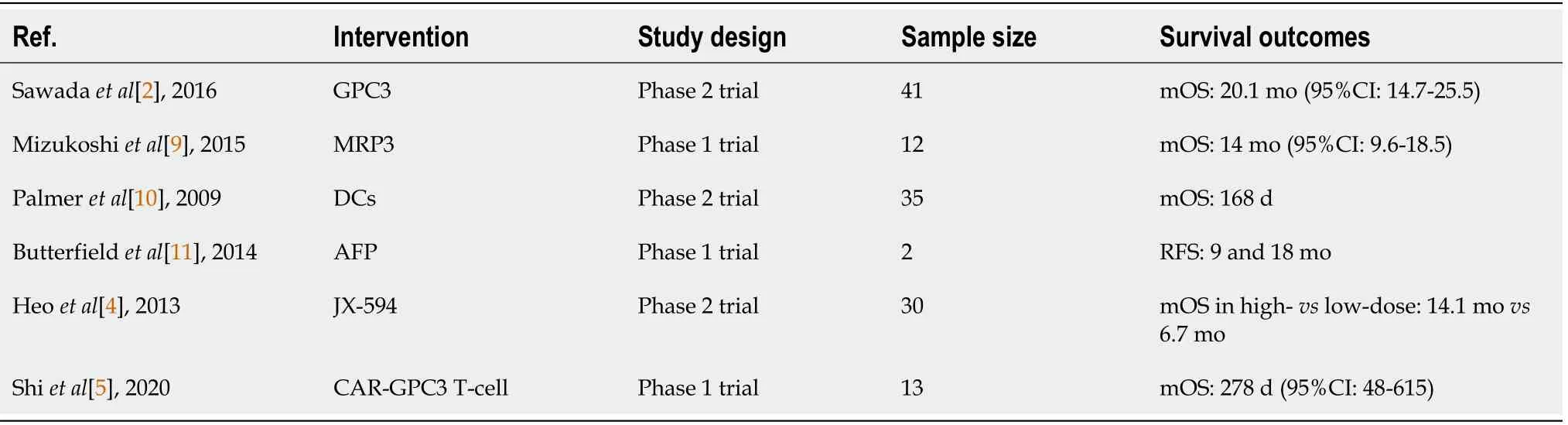
While the authors thoroughly discussed the therapeutic use of immune checkpoint inhibitors(ICIs),such as anti-programmed cell death protein 1 and its ligand(nivolumab,pembrolizumab,and atezolizumab)and anti-cytotoxic T-lymphocyte-associated protein 4(ipilimumab),we would like to highlight the role of other promising immunotherapeutic modalities in HCC.The first being tumor-associated antigen vaccines,including the oncofetal antigen glypican-3(GPC3)vaccine,which was investigated in adjuvant settings in HCC patients in a phase 2 trial and resulted in a median overall survival(mOS)of 20.1 mo[2].Another potential vaccine antigen is the multidrug resistance-associated protein 3(MRP3),a member of the adenosine triphosphate-binding cassette transporters highly expressed in HCC tissue[3].MRP3-derived peptide vaccines resulted in a mOS of 19 mo in a phase 1 trial of 12 HCC patients.Oncolytic virotherapy is another immune modality that has been widely investigated in solid malignancies.Heo
[4]conducted a phase 2 trial assessing the efficacy and safety of high- and lowdose JX-594,an oncolytic poxvirus,in HCC patients[4].The investigators reported a significantly longer mOS with high-dose compared to low-dose JX-594(14.1 mo
6.7 mo;
= 0.02).Lastly,adoptive cellular therapy,which is a promising option that is being used more in hematological and solid cancers,has been investigated in HCC,specifically through genetically modified T cells expressing chimeric antigen receptors for GPC3 in a phase 1 trial on 13 patients,which resulted in a mOS of 278 d[5].Table 1 includes the characteristics of the clinical trials on non-ICI immunotherapeutic options for HCC patients.
I am truly grateful to you for your hospitality, which was so magnificent that I could not imagine that you would be offended by my taking such a little thing as a rose
We would also like to emphasize the importance of identifying biomarkers predictive of the immunotherapy response in HCC.To date,limited evidence exists on this topic,yet some preclinical and clinical data point to potential targets.For instance,emerging evidence suggests that activated Wnt/betacatenin signaling can predict primary immunotherapy resistance in HCC[6].There is also growing interest in the microbiome’s predictive value to ICI response in other cancers.For HCC,this is especially relevant since chronic liver disease alters the microbiome components[7].Established ICI predictive biomarkers in other malignancies,such as microsatellite instability and high tumor mutational burden,are of limited use in HCC due to their rarity[6,8].
FOOTNOTES
Abushukair HA drafted the manuscript and conceptualized the concepts;Saeed A conceptualized the core concepts and critically revised the draft.
Anwaar Saeed reports research grants from AstraZeneca,Bristol Myers Squibb,Merck,Exelixis,KAHR Medical,and Incyte,and advisory board fees from AstraZeneca,Bristol Myers Squibb,Merck,Exelixis,and Pfizer.The other author has no conflicts of interest to declare.
Hassan Mohammed Abushukair 0000-0002-0068-5201;Anwaar Saeed 0000-0001-8024-9401.
Then the Prince seized the girl s hand and cried out, This is the Princess Hyacinthia! You re right again, said the Magician in amazement37; but I ve still another task for you to do
From the very beginning, the girl s family objected strongly to her dating this guy, saying that it had got to do with family background and that the girl would have to suffer for the rest of her life if she were to be with him
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
United States
Fan JR
Filipodia
Fan JR
1 Mattos ?Z,Debes JD,Boonstra A,Vogel A,Mattos AA.Immune aspects of hepatocellular carcinoma: From immune markers for early detection to immunotherapy.
2021;13: 1132-1143[PMID: 34616518 DOI: 10.4251/wjgo.v13.i9.1132]
2 Sawada Y,Yoshikawa T,Ofuji K,Yoshimura M,Tsuchiya N,Takahashi M,Nobuoka D,Gotohda N,Takahashi S,Kato Y,Konishi M,Kinoshita T,Ikeda M,Nakachi K,Yamazaki N,Mizuno S,Takayama T,Yamao K,Uesaka K,Furuse J,Endo I,Nakatsura T.Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients.
2016;5: e1129483[PMID: 27467945 DOI: 10.1080/2162402X.2015.1129483]
3 Mizukoshi E,Honda M,Arai K,Yamashita T,Nakamoto Y,Kaneko S.Expression of multidrug resistance-associated protein 3 and cytotoxic T cell responses in patients with hepatocellular carcinoma.
2008;49: 946-954[PMID: 18619700 DOI: 10.1016/j.jhep.2008.05.012]
4 Heo J,Reid T,Ruo L,Breitbach CJ,Rose S,Bloomston M,Cho M,Lim HY,Chung HC,Kim CW,Burke J,Lencioni R,Hickman T,Moon A,Lee YS,Kim MK,Daneshmand M,Dubois K,Longpre L,Ngo M,Rooney C,Bell JC,Rhee BG,Patt R,Hwang TH,Kirn DH.Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer.
2013;19: 329-336[PMID: 23396206 DOI: 10.1038/nm.3089]
5 Shi D,Shi Y,Kaseb AO,Qi X,Zhang Y,Chi J,Lu Q,Gao H,Jiang H,Wang H,Yuan D,Ma H,Li Z,Zhai B.Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials.
2020;26: 3979-3989[PMID: 32371538 DOI: 10.1158/1078-0432.CCR-19-3259]
6 Pinter M,Scheiner B,Peck-Radosavljevic M.Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups.
2021;70: 204-214[PMID: 32747413 DOI: 10.1136/gutjnl-2020-321702]
7 Keenan BP,Fong L,Kelley RK.Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation,fibrosis,and the immune response.
2019;7: 267[PMID: 31627733 DOI: 10.1186/s40425-019-0749-z]
8 Kole C,Charalampakis N,Tsakatikas S,Vailas M,Moris D,Gkotsis E,Kykalos S,Karamouzis MV,Schizas D.Immunotherapy for Hepatocellular Carcinoma: A 2021 Update.
2020;12[PMID: 33020428 DOI: 10.3390/cancers12102859]
9 Mizukoshi E,Nakagawa H,Kitahara M,Yamashita T,Arai K,Sunagozaka H,Iida N,Fushimi K,Kaneko S.Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma.
2015;369: 242-249[PMID: 26325606 DOI: 10.1016/j.canlet.2015.08.020]
10 Palmer DH,Midgley RS,Mirza N,Torr EE,Ahmed F,Steele JC,Steven NM,Kerr DJ,Young LS,Adams DH.A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma.
2009;49: 124-132[PMID: 18980227 DOI: 10.1002/hep.22626]
11 Butterfield LH,Economou JS,Gamblin TC,Geller DA.Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients.
2014;12: 86[PMID: 24708667 DOI: 10.1186/1479-5876-12-86]
 World Journal of Gastrointestinal Oncology2022年6期
World Journal of Gastrointestinal Oncology2022年6期
- World Journal of Gastrointestinal Oncology的其它文章
- Insight on BRAFV600E mutated colorectal cancer immune microenvironment
- Biofeedback therapy combined with Baduanjin on quality of life and gastrointestinal hormone level in patients with colorectal cancer
- Does chronic kidney disease affect the complications and prognosis of patients after primary colorectal cancer surgery?
- Characterizing the patient experience during neoadjuvant therapy for pancreatic ductal adenocarcinoma:A qualitative study
- Clinicopathological differences,risk factors and prognostic scores for western patients with intestinal and diffuse-type gastric cancer
- Contemporary,national patterns of surgery after preoperative therapy for stage II/III rectal adenocarcinoma
