Nanosecond pulsed electric field interrupts the glycogen metabolism in hepatocellular carcinoma by modifying the osteopontin pathway
Shn Hu , Yn Zhu , Yu Chn , Pu Chng , Gui-Rong Wng , Li-Qun Wng , , Xin-Mi Chn
a Department of Obstetrics, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310052, China
b Department of Respiratory, Shulan Hospital, Hangzhou 310 0 04, China
c Department of Pediatric Surgery, Guangdong Women and Children Hospital, Guangzhou 511400, China
d Department of Gynecology, the Second Affiliated Hospital, Zhejiang University, Hangzhou 310052, China
e Department of Surgery, the State University of New York, Upstate Medical University, Syracuse, New York, USA
f School of Pharmacy, Shandong University of Chinese Medicine, Jinan 250355, China
TotheEditor:
Hepatocellular carcinoma (HCC) is one of the most common lethal cancers and is the leading cause of cancer mortality world- wide [1]. In recent years, developments in locoregional thera- pies have provided new options for the treatment of HCC, which are recommended by the international guidelines including the Barcelona Clinic Liver Cancer (BCLC) and the American Joint Com- mittee on Cancer (AJCC) [2] . For locoregional therapies, radiofre- quency ablation (RFA) is the most commonly used method which is based on thermal ablation. Although RFA is a minimal inva- sive treatment, the damage of adjacent normal tissues caused by the thermal effect occasionally occurs during treatment. Research has also revealed that the heat might promote tumor dissemina- tion [3] . Thus, there is urgent need to find new non-thermal ab- lation method for HCC treatment. Unlike RFA, the temperature rise caused by nanosecond pulsed electric field (nsPEF) does not exceed 3 °C [4] , which greatly reduces thermal damage to surrounding tis- sues. In addition, nsPEF has great capability to initiate antitumor immune reaction [5] . The current study aimed to investigate the therapeutic effect of nsPEF and the possible molecular mechanism on glycogen metabolism of nsPEF in HCC.
The HCC cell line MHCC97H was purchased from Shanghai Cell Bank (Shanghai, China) and cultured in DMEM supplemented with 10% fetal bovine serum and 1% antibiotics (penicillin 100 U/mL and streptomycin 100 mg/mL) at 37 °C in 5% CO 2 . BALB/c nude mice (20 ± 2 g, 6-8 weeks) were purchased from Shanghai Model Or- ganisms Center (Shanghai, China) and housed in a standard specific pathogen free environment. The nsPEF instrument was supplied by Ready Bio-technology Ltd. (Hangzhou, China).
The HCC-bearing animal model was created according to a pre- vious study [6] . First, 1 × 106freshly collected HCC cells were sus- pended into 0.2 mL phosphate buffer saline. The mice were in- jected subcutaneously with HCC cells. The tumors were visible af- ter 4 weeks’ injection. Then the primary tumors were excised from the skin, cut into 1-mm3pieces and implanted subcutaneously into mice. After four weeks’ implantation, the length and width of the secondary tumor were measured with calipers. Tumor vol- ume was calculated with the following formula: tumor volume (mm3) = length (mm) × width (mm) × depth (mm) × 1/2.
The HCC-bearing animals were assigned randomly into 3 groups: nsPEF-treated group (n= 6), control group without treat- ment (n= 6) and surgery group with radical resection (n= 6).
The follow-up period for the nsPEF-treated group was 3 months. However, the tumors in the control group grew faster. When the tumor reached the maximum size of 1.2 cm allowed by the animal experiment protocol, the animals were euthanized to ensure animal welfare.
The nsPEF generator with duration of 100 ns was set up [4] . Electric fields released to the HCC tumor with a needle punc- ture electrode with a previously optimized parameter (100 ns, 40 Kv/cm, 500 pulses) ( Fig. 1 ).
Fig. 1. Photograph of the nsPEF treatment system. The nsPEF treatment system can deliver the real time image-guided minimum invasive ablation with the monitor which visualizes the ablation procedure. The oscilloscope records the pulse shape and treatment parameters. nsPEF: nanosecond pulsed electric field.
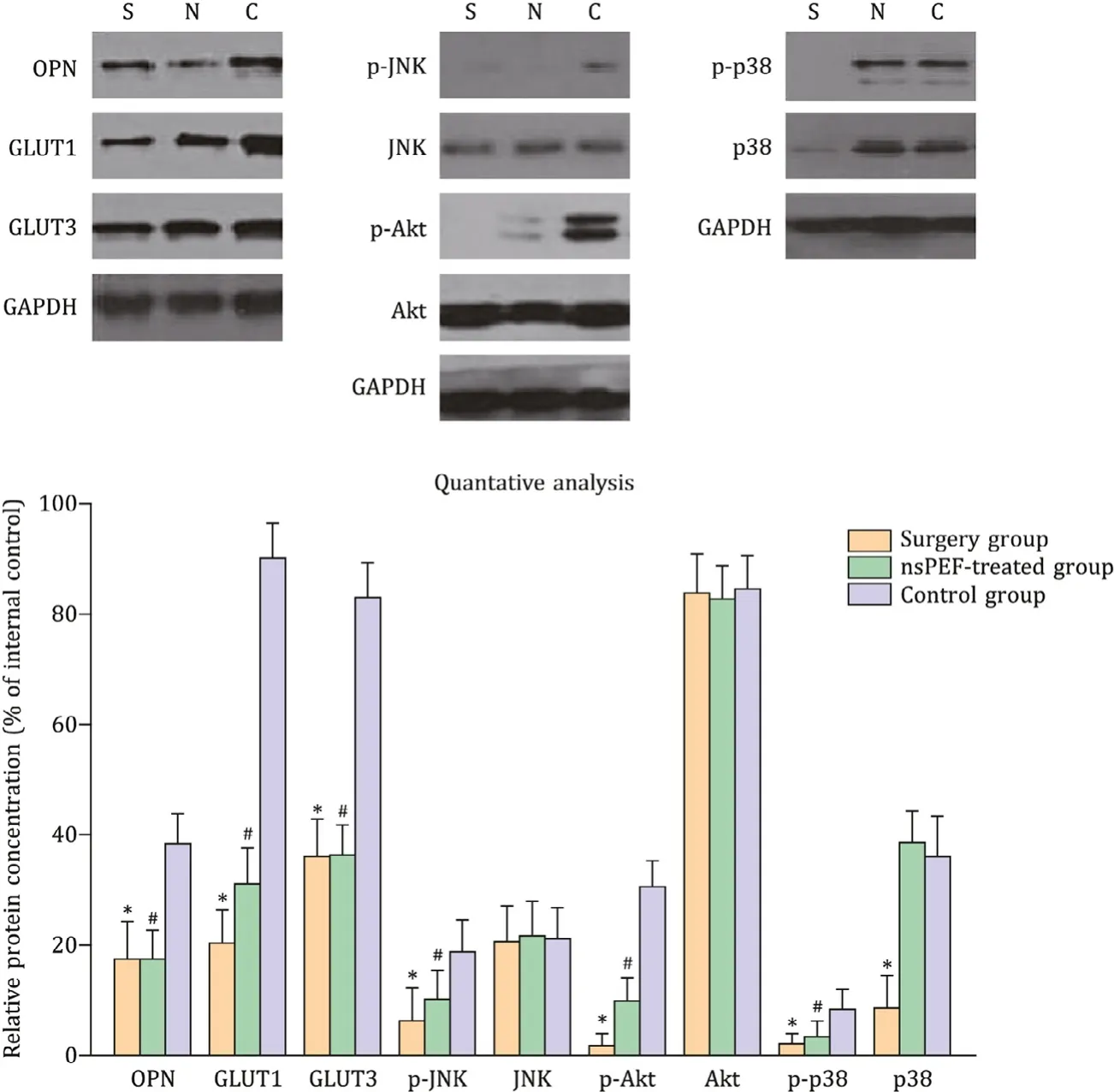
Fig. 2. The key proteins expression after exposure to the nsPEF. *: P < 0.05, the surgery group vs. the control group; #: P < 0.05, the nsPEF-treated group vs. the control group. The protein expression of OPN, GLUT1, GLUT3 and the phosphorylated levels of JNK, Akt (protein kinase B) and p38 when HCC tumors were treated with nsPEF and surgery. S: surgery group; N: nsPEF-treated group; C: control group; OPN: osteopontin; GLUT: glucose transporter; JNK: c-Jun N-terminal kinases; Akt: alpha kinase threonine; HCC: hepatocellular carcinoma; nsPEF: nanosecond pulsed electric field.
Tumor samples were collected and lysed with sonication. The proteins were quantified. Equal amounts of proteins were loaded and separated with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene fluo- ride membrane and blocked with milk and 0.05% Tween-20 for 1 h at room temperature (about 25 °C). Membranes were incubated with primary antibodies [mouse monoclonal anti-osteopontin (OPN) (1:500), anti-glucose transporter 1 (GLUT1, 1:200), anti- GLUT3 (1:200), anti-phosphorylated c-Jun N-terminal kinase (p- JNK, 1:200), anti-JNK, anti-p-alpha kinase threonine (Akt) (1:500), anti-Akt (1:50 0), anti-p-p38 (1:20 0), anti-p38 (1:30 0) and anti- glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:10 0 0)] for 1 h at room temperature. Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). After 3 washes, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (Zhongshan Golden Bridge, Beijing, China) for 1 h at room temperature. The protein expression was visualized with enhanced chemiluminescence reagent (ECL kit, Amersham, UK). The proteins were quantitated by the amount of inner control by software Image J ( https://imagej.nih.gov/ij/ ). The counting data and measurement data were exhibited by the ratio (%) and mean ±standard deviation, respectively. Statistical analysis was conducted using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The statisti- cal significance of differences among three groups was determined by Fisher’s exact test and Student’st-test. Animal survival was an- alyzed with Kaplan-Meier survival analysis (Log-rank test). All data were analyzed using two-tailed tests unless otherwise specified, and aPvalue<0.05 was considered statistically significant.
As showed in Table 1 , survival rate in the nsPEF-treated group was significantly higher than that in the control group (4/6 vs. 0/6,P<0.05). The surgery group exhibited the highest survival rate at 83.3% (5/6). The occurrence of residual tumor in the nsPEF-treated group was much lower compared with that in the control group (2/6 vs. 6/6,P<0.05). The surgery group exhibited the best out- come, with a lower residual tumor rate (1/6), while the survival showed no statistical significance compared with the nsPEF-treated group (P>0.05). The occurrence of lung metastasis tumor in the surgery group and nsPEF-treated group was much lower compared with the control group (0/6 and 1/6 vs. 6/6,P<0.05, Table 1 ).
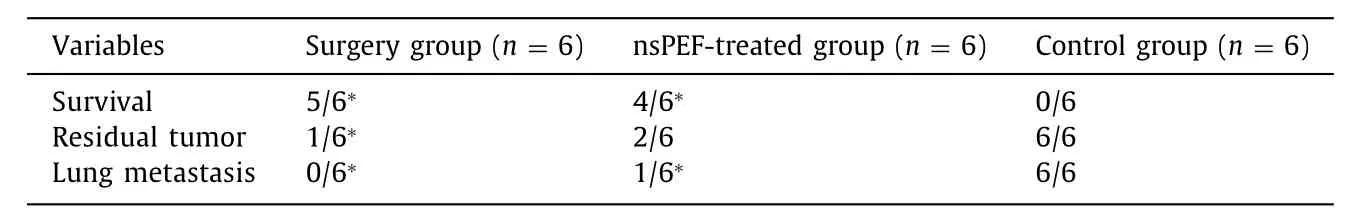
Table 1 The tumor progression and survival of mice after 3-month follow-up.
As shown in Fig. 2 , nsPEF treatment reduced the expression of OPN, GLUT1 and GLUT3, which meaned that the tumor growth was inhibited. In addition, the phosphorylation levels of JNK, AKT and p38 in tumor were also detected. nsPEF reduced the phosphoryla- tion of AKT and JNK, but not the phosphorylation of p38 in HCC tissue. These results indicated that JNK and AKT signal pathways were involved in nsPEF-induced OPN reduction and the following downstream alterations.
In conclusion, our study indicated that locoregional nsPEF is feasible in ablating HCC as a curative therapy. OPN might be used as a non-invasive biochemical marker for glycogen metabolism ex- haustion to monitor the ablation efficacy in order to adjust nsPEF ablation strategy. Our study provides a novel insight into an OPN pathway involved in nsPEF-caused HCC eradication.
Acknowledgments
Authors thank the kind support on animal experiment from Liang-Jie Hong and Dan-Jing Guo from Ready Bio-technology Ltd., Hangzhou, China.
CRediTauthorshipcontributionstatement
ShenHu:Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.YanZhu:Formal analysis, Inves- tigation, Writing - review & editing.YuChen:Investigation, Re- sources, Writing - review & editing.PuCheng:Conceptualization, Supervision, Writing - review & editing.Gui-RongWang:Data curation, Investigation.Li-QuanWang:Conceptualization, Funding acquisition, Supervision, Writing - review & editing.Xin-MeiChen:Conceptualization, Supervision, Writing - review & editing.
Funding
This study was supported by grants from National S&T Major Project (2018ZX10301201), National Science Foundation of China (8202780 0 08, 82070516 and 82027803), Zhejiang Provincial Nat- ural Science Foundation (LZ21H160 0 02 and LY19H040014), and Medical Health Science and Technology Project of Zhejiang Provin- cial Health Commission (2021KY719).
Ethicalapproval
Animals received appropriate humane care from certificated professional staff in compliance with both the Principals of Lab- oratory Animal Care and the Guide for the Care and Use of Labo- ratory Animals approved by the Animal Care and Use Committees of Zhejiang University.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub- ject of this article.
 Hepatobiliary & Pancreatic Diseases International2022年2期
Hepatobiliary & Pancreatic Diseases International2022年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Meetings and Courses
- Information for Readers
- NAFLD or MAFLD: That is the conundrum
- Relevant Content
- Neutrophil-to-lymphocyte ratio or platelet-to-lymphocyte ratio is a predictive factor of pancreatic cancer patients with type 2 diabetes
- Isolated pancreatic metastasis from a malignant pleural mesothelioma diagnosed using endoscopic ultrasonography-guided fine needle aspiration biopsy
