A Stretchable Ionic Conductive Elastomer for High-Areal-Capacity Lithium-Metal Batteries
Kejia Li,Zhenglu Zhu,Ruirui Zhao,Haoran Du,Xiaoqun Qi,Xiaobin Xu,and Long Qie*
Developing high-areal-capacity and dendrite-free lithium(Li)anodes is of significant importance for the practical applications of the Li-metal secondary batteries.Herein,an effective strategy to stabilize the high-arealcapacity Li electrodeposition by modifying the Li metal with a stretchable ionic conductive elastomer(ICE)is demonstrated.The ICE layer prepared via an instant photocuring process shows a promising Li+-ion conductivity at room temperature.When being used in Li-metal batteries,the thin ICE coating(~0.27 μm)acts as both a stretchable constraint to minimize the Li loss and a protective layer to facilitate the uniform flux of Li ions.With this ICE-modifying strategy,the reversibility and cyclability of the Li anodes under high-areal-capacity condition in carbonate electrolyte are significantly improved,leading to a stable Li stripping/plating for 500 h at an ultrahigh areal capacity of 20 mAh cm-2in commercial carbonate electrolyte.When coupled with industry-level thick LiFePO4electrodes(20.0 mg cm-2),the cells with ICE-Li anodes show significantly enhanced rate and cycling capability.
Keywords
high areal capacity,ionic conductive elastomer,lithium anode,lithium-metal battery,protective layer
1.Introduction
The rapid development of the portable electronic devices and electric vehicles places increasing demands for advanced batteries with high energy density.[1-4]However,the energy density of the current intercalation-based lithium-ion batteries(LIBs)is approaching to their theoretical energy density values.[5]Under this circumstance,lithium(Li)-metal anode has been revived and attracted more and more attention[6-9]due to its high theoretical capacity(3860 mAh g-1)and the lowest reduction potential(-3.04 V vs standard hydrogen electrolyte).[10-13]Nevertheless,the wide applications of Li-metal anode in secondary batteries are still hindered by the uncontrolled lithium dendrite growth and the poor reversibility,especially when the commercial carbonate electrolytes are used.[14-16]
Up till now,a string of methods is proposed to cope with these challenges,such as creating three-dimensional(3D)Li hosts to alleviate the uneven local current density,[17]substituting solid-state electrolyte for conventional liquid electrolytes to prevent lithium dendrite penetration,[18-20]introducing additives to the electrolyte to stabilize the solid electrolyte interface(SEI) films of the Li anodes,[16]constructing robust protective layers[21-22]to reduce the side reactions between the electrolyte and Li anodes,and so on.[23-28]Although these countermeasures suppress the formation/growth of the Li dendrites and reduce the side reactions between Li metal and liquid electrolytes,it remains challenging to realize the dendrite-free and high-efficiency Li depositions under high-areal-capacity conditions in conventional carbonate electrolytes,[29-32]which is one of the most critical and urgent challenges for the practical realization of Limetal batteries.
Herein,we discovered a perfect protective layer(ionic conductive elastomer,as-called ICE)to stabilize the Li-metal anodes.The ICE,which is prepared via a facile instant photocuring method,possesses excellent stretchability,high stability in electrolytes,good adhesion to Li metal,and high room temperature(RT)Li+-ion conductivity.With a layer of as thin as ~0.27 μm,the ICE coating effectively inhibits the formation of lithium dendrites and improves the reversibility of Li anodes,thus enabling efficient Li utilization under high areal capacity during long-term cycling in the commercial carbonate electrolytes.At an ultrahigh areal capacity of 20 mAh cm-2,the assembled symmetric cell with ICE-coated Li electrodes(ICE-Li)shows a long cycling life of 500 h,three times of the control cells.When being coupled with the industry-level high-capacity LiFePO4cathode(LFP,20.0 mg cm-2),the ICE-Li||LFP full cells achieve>4 times longer lifespan(200 cycles).Moreover,the synthetic process is convenient and cost-effective,thus having a great potential to be industrially scaled-up.
2.Results and Discussion
2.1.Fabrication and Characterization of ICE-Li Electrodes
ICE was prepared by a salt-in-polymer strategy via an instant photocuring process.[33]As illustrated in Figure 1a,the synthesis process includes two steps: first,polyethyleneglycol diacrylate(PEGDA,crosslinker),1-hydroxycyclohexyl phenyl ketone(photo-initiator 184),and lithium bis(trifluoromethane sulfonimide)(LiTFSI,salt)were dissolved in butyl acrylate(BA,monomer)liquid to form a clear solution,which is used as the precursor solution;then the as-obtained ICE precursor solution was dripped onto the surface of Li chips,after being photocured by an ultraviolet(UV)light irradiation(395 nm)for 90 min,ICE film was successfully polymerized on the surface of Li chips.To verify the successful polymerization reaction of ICE precursor,we used the Fourier-transform infrared(FT-IR)method to compare ICE precursor with ICE membrane after curing by the UV light.As shown in Figure S1,after the UV irradiation,the disappearance of unsaturated C=C stretching vibration at 1610-1680 cm-1,which belongs to the acrylic groups from the BA monomers,certifies the successful formation of ICE.Moreover,the peaks between 1300 and 1000 cm-1are primarily ascribed to the C-O stretching vibration,which may facilitate the faster mobility of Li ions.[34]Compared to other methods for the construction of protective layers,[35]this UV-irradiation method is much more comfortable and might be industrially scaled-up easily.
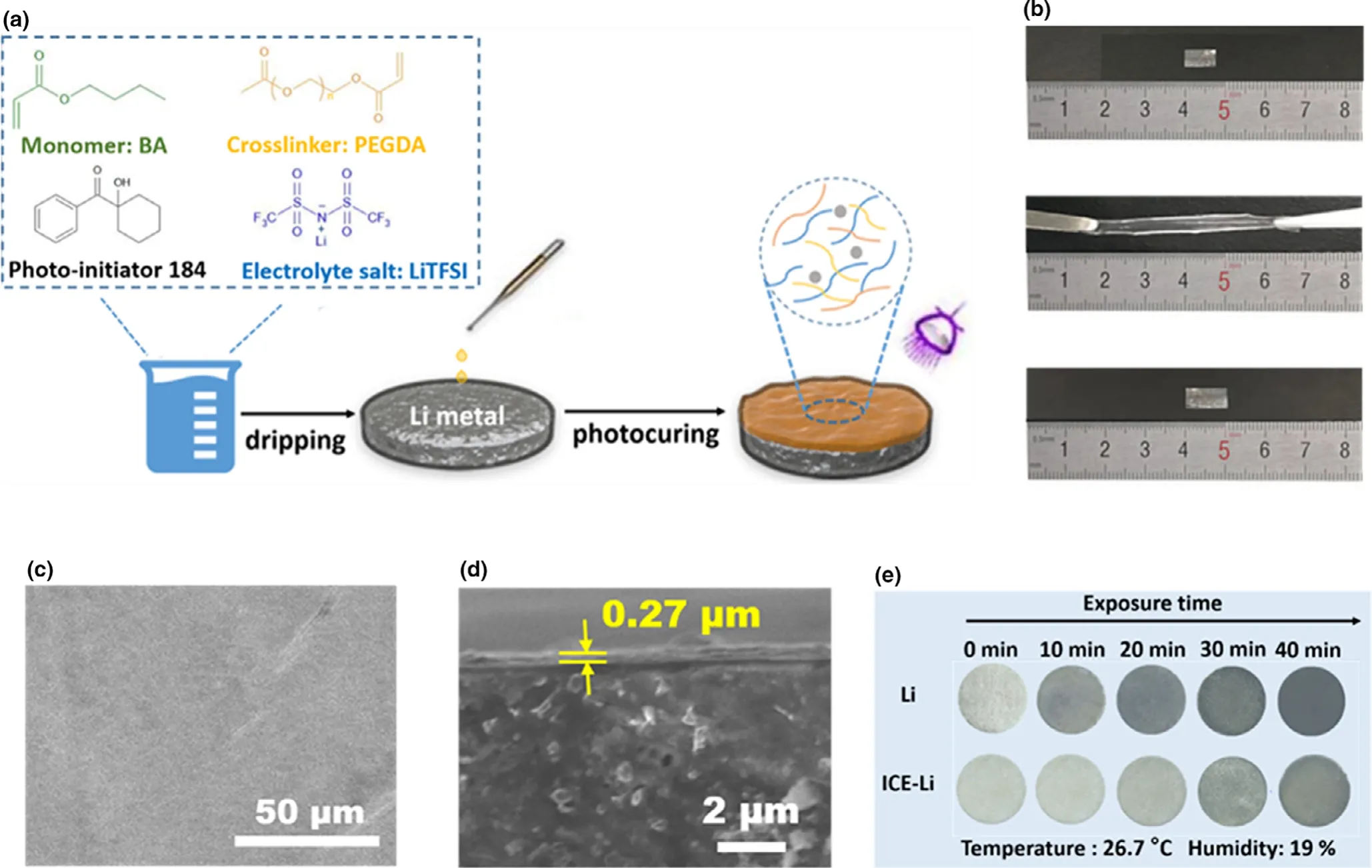
Figure 1.a)Preparation processes of the ICE membrane through UV irritation.b)Optical images of the as-prepared(top),stretched(middle),and recovered(bottom)ICE membrane.c)Top-view and d)Cross-sectional SEM images of ICE-Li.e)Photographs of the pristine Li and ICE-Li chips exposed to the atmosphere.
As shown in Figure 1b,when being stretched,the ICE membrane(0.8 cm×0.5 cm)deforms accordingly without crack or fracture and recovers to its original form,indicating its excellent ductility and elasticity to withstand the deformation.Such an excellent stretchability endows the ICE film the capability to move with Li metal during the fluctuation displacement of Li surface during the repeated stripping/plating process and thus suppress the formation of Li dendrites and minimize the loss of the Li metal.The top-view scanning electron microscopy(SEM)images of ICE-Li are shown in Figure 1c,compared to bare Li which has a rough surface(Figure S2),the surface of ICE-Li is smooth and dense after the treatment with ICE membrane.The crosssectional SEM investigation(Figure 1d)shows that the ICE membrane is uniformly coated on the surface of Li metal with a thickness of~0.27 μm,much thinner than the reported ones,[36-38]such a small thickness has negligible influence on the overall energy density of the battery.Benefiting from the salt-in-polymer synthesis,the as-prepared ICE is Li+-ion conductive.As shown in Figure S3,the RT conductivity of ICE is about 4.98× 10-7S cm-1at 25°C,higher than those of the poly(ethylene oxide) (PEO)-based electrolytes (10-8to 10-7S cm-1).[39]Also,when tested with electrolyte,the conductivity of ICE is about 1.32× 10-5S cm-1at 25°C(Figure S4),indicating the swelling of ICE facilitates the transport of Li ions.The reason is that during the static process of the battery,the polymer coating firstly absorbs part of the electrolyte and swells,resulting in Li-ion transportation like gel electrolytes through polymer chains.
The air stability and sensitivity of the bare Li and ICE-Li chips were further compared by exposing them to the ambient atmosphere(temperature:26.7°C,humidity:19% ).As the photographs shown in Figure 1e,the shiny surface of both samples can be observed after taking them out of the glove box.However,the bare Li metal rapidly oxidizes and turns to black after its exposure to air and eventually turns to total black in 40 min while the ICE-Li metal exhibits relatively better air stability in the same condition.The improved air stability after ICE coating makes the industrial applications of Li-metal anodes possible.Moreover,the ICE membrane also shows excellent chemical stability in commercial carbonate electrolyte 1 M lithium hexafluorophosphate(LiPF6)in ethylene carbonate(EC),dimethyl carbonate(DMC),and diethyl carbonate(DEC)with the volume ratio of 1:1:1.After being soaking in the carbonate electrolyte for one week,the ICE membrane maintains the same size without obvious swelling,cracking,or mass loss(Figure S5a,b).Moreover,there is no disparity in peak position or density for the FTIR of the ICE membrane before and after immersion test(Figure S5c),thus indicating the superb chemical stability of ICE membrane in carbonate electrolyte and the potential to be further used in batteries.
2.2.Electrochemical Performance of Symmetric Cells
The advantages of the ICE coating on the electrochemical performance were first verified by symmetric cells with carbonate electrolyte.To confirm the Li+ion transport performance of ICE layer,electrochemical impedance spectroscopy(EIS)was performed.It can be seen in the Nyquist plots that symmetric cell with ICE-Li electrodes shows a slightly lower overall impedance than the control cell,illustrating that the ICE membrane has negligible influence on Li+ion transportation ability(Figure S6a).The symmetric cells were further cycled at various current densities and capacities.When the capacity is fixed at 1 mAh cm-2,the cells with bare Li electrodes exhibit a rapidly increased polarization voltage after cycling about 200 h and are soon short-circuited under the relatively low current densities of 0.5 mA cm-2(Figure S6b)and 1 mA cm-2(Figure 2a).By contrast,the cells with ICE-Li electrodes show flatter voltage profiles and can achieve the longer lifespan for 1000 h at the current density of 1 mA cm-2.And the same tendency can be observed when the current density is increased to 2.5 mA cm-2(Figure S6c),5 mA cm-2(Figure S6d),and 10 mA cm-2(Figure S7a).Furthermore,the contrast in cycling stability is more obvious when the symmetric cells are tested at a higher current density of 20 mA cm-2with an areal capacity of 10 mAh cm-2(Figure S7b)and 20 mAh cm-2(Figure 2b)respectively.As shown in Figure S7b and Figure 2b,bare Li electrodes undergo a rapidly increased polarization voltage while ICE-Li electrodes are stable for 500 h.To the best of our knowledge,such a long lifespan is superior to those of many previously reported Li anodes under similar current density and areal capacity test conditions.[40-42]It should not be ignored that the excellent electrochemical performance mentioned above is obtained with a thin ICE coating of only ~0.27 μm.
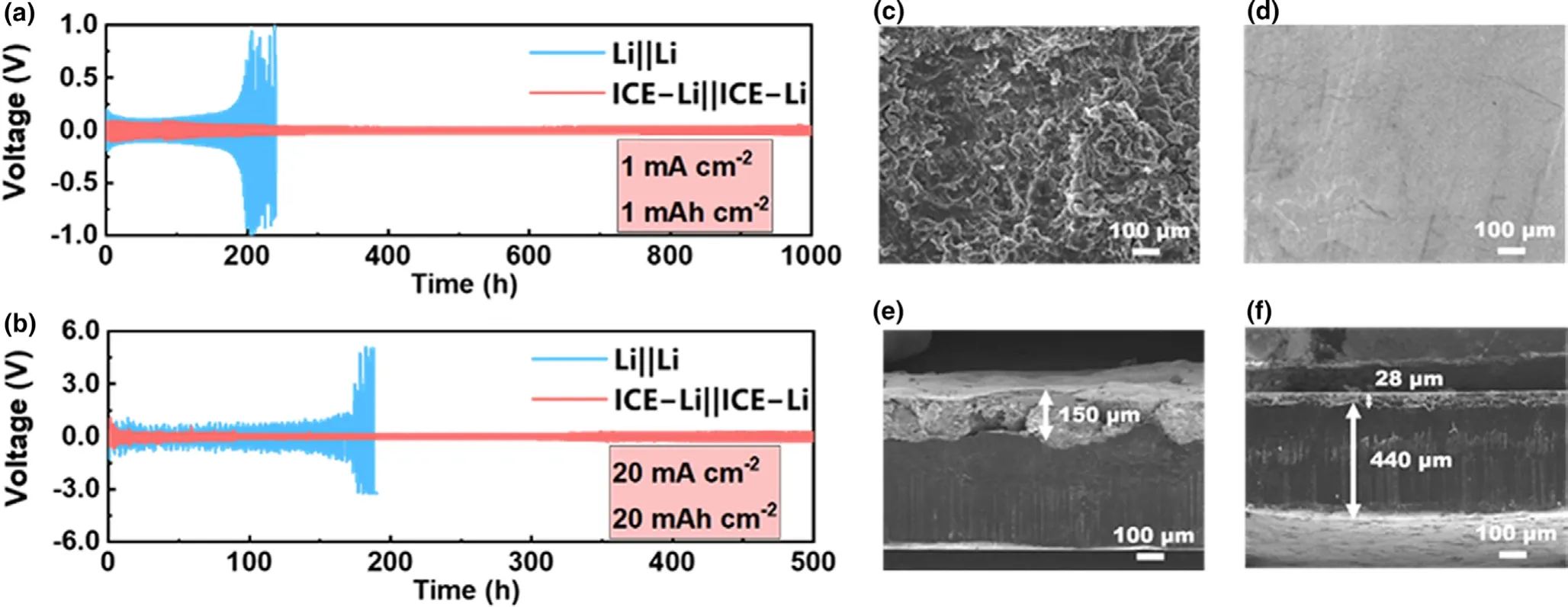
Figure 2.The cycling stability of the symmetric Li||Li and ICE-Li||ICE-Li cells with carbonate electrolyte at current densities and areal capacities of a)1 mA cm-2and 1 mAh cm-2and b)20 mA cm-2and 20 mAh cm-2.Top-view SEM images of c)Li and d)ICE-Li,and cross-sectional SEM images of cycled e)Li and f)ICE-Li after 100 cycles at a current density of 1 mA cm-2and an areal capacity of 1 mAh cm-2.
2.3.Morphology of the Cycled Li Electrodes
The morphology of bare Li and ICE-Li electrodes was studied after 100 cycles of stripping/plating in symmetric cells at a constant current density of 1 mA cm-2and areal capacity of 1 mAh cm-2by top-view and cross-sectional scanning electron microscope(SEM)images(Figure 2c-f).As depicted in Figure 2c,d,due to the uncontrolled Li electrodeposition and the poor compatibility between the Li metal and the carbonate electrolyte,porous and loose products can be apparently seen on the rough surface of bare Li electrode after 100 cycles,and this result explains the poor performance of symmetric batteries using bare Li-metal anode mentioned above.On the contrary,the surface of the cycled ICE-Li electrode is relatively smooth and uniform,indicating that the interfacial layer successfully leads to a homogeneous plating and stripping of Li and suppresses the undesirable lithium dendrites.Such conclusions could also be confirmed by the cross-sectional SEM images.The bare Li metal exhibits a loose and porous structure,with the accumulated SEI or as-called “dead”Li(~150 μm)on the top(Figure 2e).On the other hand,there is almost no bulk expansion and lithium dendrite formation after the long-term cycling for the ICE-Li electrode(Figure 2f),suggesting the ICE membrane effectively suppresses the growth of lithium dendrites and the accumulation of“dead”Li.Also,the morphology of bare Li and ICE-Li electrodes after 20 cycles of stripping/plating in symmetric cells at a constant current density of 20 mA cm-2and areal capacity of 20 mAh cm-2was displayed in Figure S8.We can see that a large number of irregular lithium dendrites are observed on the surface of bare Li electrode,while the surface of the cycled ICE-Li electrode is relatively smooth and uniform.According to the cross-sectional images,the accumulation of“dead” Li is significantly decreased for ICE-Li than that of the Li electrode.This result is in accordance with the performance of symmetric batteries.
2.4.Electrochemical Performance of Li||Cu and ICE-Li||Cu Cells
The effect of ICE on the reversibility of the Li plating/stripping process was investigated with Li||Cu half cells using bear Cu and ICE-coated Cu(ICE-Cu)foils as working electrodes with carbonate electrolyte.The charge of Li stripping with respect to that of Li deposition on Cu(namely Coulombic efficiency,CE)is used as the performance index to evaluate the reaction reversibility and the stability during cycling.As is shown in Figure 3a,when being cycled at a current density of 0.5 mA cm-2with a fixed areal capacity of 0.5 mAh cm-2,the CE of the cell with ICE-Cu electrode is stabilized at 93.6% for 100 cycles,while the control cell shows a gradual CE fading to<80% after 60 cycles.Figure 3b shows the voltage profiles of the initial Li plating/stripping cycle as a function of Li deposition capacity.The initial CEs of the cells with the pristine Cu and ICE-Cu are 93.1% and 94.2% ,respectively.Also,the voltage curves of Li||Cu cells during Li electrodeposition are given in Figure 3c,and the voltage profiles of Li plating/stripping on ICE-Cu at the 1st cycle and 100th cycle are given in Figure S9a.As can be seen,ICE-Cu electrode has a lower nucleation overpotential(68.5 mV)than Cu electrode(89.9 mV),indicating that ICE coating provides more uniform nucleation sites and facilitate the Li deposition.
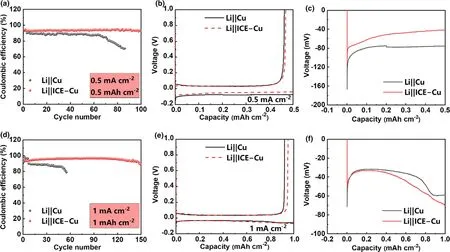
Figure 3.Electrochemical performance of the Li||Cu and Li||ICE-Cu cells with carbonate electrolyte:a)CE,b)voltage profiles of Li plating/stripping,and c)enlarged voltage curves of Li plating at a current density of 0.5 mA cm-2and an areal capacity of 0.5 mAh cm-2;d)CE,e)voltage profiles of Li plating/stripping,and f)enlarged voltage curves of Li plating at a current density of 1 mA cm-2and an areal capacity of 1 mAh cm-2.
Electrochemical impedance spectroscopy(EIS)was also performed on the cells(Figure S9b).Obviously,both the 2nd and 50th cycle of the cell with ICE-Cu electrode exhibit lower resistances than those of the control cell.To further investigate the cycling stability of the ICE-Cu electrode,cells were measured under a high current(1 mA cm-2)with the areal capacity of 1 mAh cm-2.As shown in Figure 3d,the cell with ICE-Cu electrode exhibits a high CE retention and cycling stability(150 cycles).In contrast,the CE of the control cell fades quickly,and the cell died after only 60 cycles.As shown in Figure 3e,the initial CEs of the cells with Cu and the ICE-Cu electrodes are 92.1% and 95.7% ,respectively.Also,the voltage curves of Li||Cu cells during Li electrodeposition(Figure 3f)adequately indicate that the ICE coating enables higher Li reversibility,which could be explained as that the ICE membrane helps to homogenize the Li+ion deposition and inhibit the formation of“dead”Li.
SEM images of Cu electrodes before and after plating for the one cycle with a current density of 0.5 mA cm-2for a total of 0.5 mAh cm-2of Li are given in Figure S10.Compared to Cu which has a rough surface in(Figure S10a),the surface of ICE-Cu is smooth and dense(Figure S10b).As demonstrated in Figure S10c,d,obvious porous and loose lithium dendrites can be apparently seen on the rough surface of the bare Cu electrode.In contrast,the surface of ICE-Cu metal electrode is relatively smooth and uniform,indicating that the interfacial layer can successfully lead to a homogeneous plating of lithium.This result is in accordance with the electrochemical performance of the Li||Cu cells.
2.5.Electrochemical Performance of Full Cells
In order to evaluate the potential of ICE-Li for practical applications,full cells were assembled using industry-level LFP cathodes(loading:20.0 mg cm-2).It can be seen in the EIS spectrum(Figure 4a)that the ICE-Li||LFP cell shows a smaller overall impedance than that of the Li||LFP cell,indicating the positive effect of surface layer on decreasing the interfacial resistance,which could be explained by improved wettability of the ICE-Li to the carbonate electrolyte.As the contact angle results shown in the inset of Figure 4a,after dropping the carbonate electrolyte vertically onto the pristine Li and ICE-Li chips,the contact angles of electrolyte droplet on the Li and ICE-Li chips are 18.3°and 6.2°respectively.After 5 s,the contact angles change to 11.2°and 3.6°respectively,implying that electrolyte droplets could spread onto the surface of the ICE-Li anode more rapidly.The enhanced affinity of ICE layer with electrolyte decreases the concentration gradient of Li+ion near the Li surface and facilitates the Li+-ion transport.
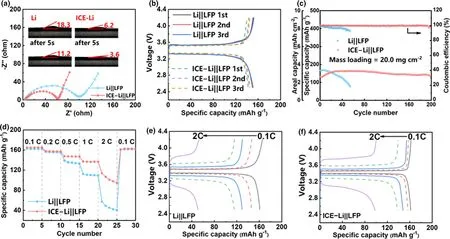
Figure 4.Electrochemical performance of Li||LFP and ICE-Li||LFP cells with carbonate electrolyte.a)Nyquist plots with insert showing the contact angles of the carbonate electrolyte with the Li and ICE-Li electrodes.b)Charge/discharge curves for the first three cycles.c)Long-term cycling performance and CE at 0.5 C.d)Rate capability at various rates from 0.1 to 2.0 C and corresponding charge/discharge curves from 0.1 to 2.0 C of e)Li||LFP and f)ICE-Li||LFP cells.
Figure 4b compares the charge and discharge curves of both cells for the first three cycles,and it is noticed that the voltage differences between the charge and discharge platforms of ICE-Li||LFP cell are slightly smaller than those of the Li||LFP cell,suggesting that ICE coating onto the Li anode reduces the polarization of the full cell.Figure 4c shows the long-term cycling performance of the cells at 0.5 C(1 C=170 mAh g-1).Obviously,the cell with ICE-Li remains stable in the long-term cycling,and the coulombic efficiency maintains>99% even after 200 cycles.In contrast,although the Li||LFP cell exhibits a high initial discharge capacity(165 mAh g-1),it decreases rapidly to<125 mAh g-1after 40 cycles.
The ICE-Li||LFP cell also shows improved rate capability.As can be seen from Figure 4d,both of the two cells show similar level of initial specific capacity of~160 mAh g-1at a low current density of 0.1 C.However,the cell with ICE-Li electrode exhibits significant advantages with the increase of the current density.As shown in Figure 4e-f,the Li||LFP cell delivers specific capacities of 147,130,116,and 51 mAh g-1,respectively,from at 0.2,0.5,1.0,and 2.0 C,while the ICE-Li||LFP cell displays higher specific capacities of 157,148,139,and 99 mAh g-1,respectively,under the same condition.More importantly, the specific capacities of both cells are recovered to~160 mAh g-1when the current density is reset to 0.1 C.Such remarkable cycling stability and rate capability of the high-loading ICELi||LFP cell confirm the superb practicality of ICE-Li.
To understand the positive effect of the ICE coating on the electrochemical behaviors of Li anodes better,a schematic diagram is illustrated.As depicted in Figure 5,due to the uncontrolled Li electrodeposition and the serious side reactions between the high reactive Li metal and the carbonate electrolyte,the bare Li anode is more likely to undergo irreversible surface degradation with the repeated cracking and reparation of as-formed SEI film during the plating/stripping process,leading to the accumulation of the “dead”Li upon long-term cycling(Figure 5a).In contrast,the ICE coating with excellent stretchability and RT Li+-ion conductivity could not only serve as an a robust and chemically stable interfacial layer to withstand the enormous volume fluctuations of Li metal during the continuous plating and stripping process and minimize the side reactions between the Li anode with the electrolytes,but also works as an ionic conductor to uniformize the Li+ion flux and regulate uniform Li deposition,which in turn leads to a more homogeneous distribution of lithium ions and highly improved Li cycling efficiency(Figure 5b).In addition,this ICE-modifying strategy can also be applied to other electrolyte system to match with the high-voltage cathode(Figure S11-S13)or other metal anodes(Figure S14),thus having a great universality.
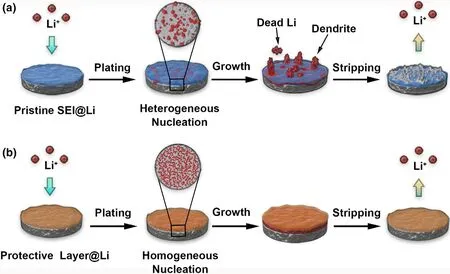
Figure 5.Schematic diagram of lithium plating/stripping process on the surface of a)Li and b)ICE-Li chips.
3.Conclusion
In conclusion,an ICE layer with excellent stretchability,high stability in carbonate electrolytes,good adhesion to Li metal,and high RT Li+-ion conductivity is proposed as a protective layer for Li anodes.An ICE film of as thin as~0.27 μm could effectively isolate the Li anodes from the corrosive electrolytes,withstand the volume fluctuations of Li metal during the repeated plating and stripping process,and inhibit the formation of lithium dendrites,and thus enabling the long-term cycling stability and the superb reversibility of Li anodes in carbonate electrolytes even with an ultrahigh areal capacity of 20 mAh cm-2.When being coupled with the industry-level high-capacity LFP cathodes,the ICE-Li||LFP full cells achieve>4 times longer lifespan(200 cycles)than the control cell.Considering the facile processability,excellent electrochemical performance,and superb universality,the as-proposed ICE-coating strategy presents the perspectives and potentials to explore advanced LMBs for practical applications.
Acknowledgements
This research was supported by the National Natural Science Foundation of China under Grant No.51802225 and the funding from State Key Laboratory of Materials Processing and Die&Mould Technology,Huazhong University of Science and Technology.
Conflicts of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Introduction of Frontier Outlook
- Sn Alloy and Graphite Addition to Enhance Initial Coulombic Efficiency and Cycling Stability of SiO Anodes for Li-Ion Batteries
- Biomass Template Derived Boron/Oxygen Co-Doped Carbon Particles as Advanced Anodes for Potassium-Ion Batteries
- Gas Generation Mechanism in Li-Metal Batteries
- Understanding the Coffee ring Effect on Self-discharge Behavior of Printed micro-Supercapacitors
- Directional Oxygen Functionalization by Defect in Different Metamorphic-Grade Coal-Derived Carbon Materials for Sodium Storage
