INRA82 extender enhances semen quality in ram under cooled and cryopreserved stages
Aya M. Fadl,Elshymaa A. Abdelnaby,Hossam R. El-Sherbiny
Theriogenology Department,Faculty of Veterinary Medicine,Cairo University,Egypt
ABSTRACT
Objective:To investigate the effect of INRA82 extender on ram semen quality preserved in cooled and frozen conditions in comparison with Tris-based extenders and to select the suitable extender for ram semen preservation.
Methods:Semen samples were collected and pooled from Awassi rams (n=5) and divided into three aliquots. Each aliquot was diluted with one of the following extenders:1) Tris-citrate glucose,2) Triscitrate fructose,and 3) INRA-82. For liquid storage,diluted samples were kept at 5 ℃ for 72 h. Progressive motility was measured at 0,24,48 and 72 h after cooling. Besides,viability,morphology,membrane,acrosome and DNA integrities were evaluated at 24 h post-cooling. For cryopreservation,diluted samples were cooled,equilibrated,loaded and frozen in liquid nitrogen. Thawed samples were evaluated in the same manner as cooled conditions.
Results:Seminal characteristics were improved after cooling and thawing in samples diluted with INRA-82 compared to those diluted with Tris-citrate glucose or Tris-citrate fructose (P<0.05).
Conclusions:Dilution of ram semen with INRA-82 improves semen parameters. Hence,INRA-82,as a stimulating diluent,can be successfully used to reserve viability and sustainability of cooled and cryopreserved ram semen.
KEYWORDS:Cryopreservation; Dilution; INRA-82; Ram;Tris-citrate glucose; Tris-citrate fructose
Significance
Semen extenders are utilized as a medium for sperm preservation in order to facilitate the fertilization process,as those extenders could mantain semen metabolic processes.Hence,the success of semen storage is determined by the type of extender. This study could be helpful in estimating the best extender for viability of cryopreserved ram semen,and revealed the effect of INRA-82 extender on sperm cell viability.
1. Introduction
Sheep breeding plays a vital role in the economy of many countries because of their highly productive value for meat,milk and wool[1]. In sheep,artificial insemination is the most powerful technique for breeding improvement using fresh or cooled (4 ℃)or cryopreserved ram semen of high genetic merit[2]. Moreover,the proficiency of production hinges highly on reproductive performance[3]. During preservation,sperm cells suffer from cold shock and generation of reactive oxygen species that leads to oxidative stress by time[4,5]. Alternatively,the success of semen storage is determined by the type of extender and its constituents[6].Semen needs to be extended with an appropriate extender,which can provide an appropriate physiological and metabolically environment to spermatozoa.
Various studies have been conducted to determine the proper extender for storage of ram spermatozoa[1,2,7,8]. Tris-based extender is one of the most common diluent in ram-preserved semen.Moreover,Tris-based fructose and glucose extenders resulted in higher sperm motility during 72 h of storage compared with citratebased extender[1]. INRA-82,another extender,which has been used with satisfactory results for both equine and rabbit buck semen dilution during the preservation process[9,10]. INRA-82 extender has not been previously used in ram semen preservation. Moreover,studies on the cooled stored and cryopreserved ram spermatozoa using different extenders are scanty. Therefore,the current study was to compare various extenders and determine the ideal extender for ram semen preservation.
2. Material and methods
2.1. Animals
Five fertile and clinically healthy Awassi rams (weighing 55-75 kg and aged 5-6 years old) were preserved in a natural environment in the Faculty of Veterinary Medicine at Giza square (30.0276° N,31.2101° E) with ambient temperature 22 ℃-29 ℃,humidity and daylight. All rams were provided with an equal amount of feed in accordance with National Research Council (NRC 2007). The current study was conducted for a period of two months (January to March,2021) at the Faculty of Veterinary Medicine,Cairo University.
2.2. Collection and evaluation of semen
Semen collection was achieved using lubricated and pre-warmed(40 ℃-42 ℃) artificial vagina twice/week for 2 months. Semen collection was performed at early morning at every Monday and Wednesday for two months. The freshly collected semen samples were transformed into the laboratory at 37 ℃ for further evaluation.To remove variations,the ejaculates were pooled and evaluated for basic semen characteristics including progressive motility,viability and sperm cell concentration (109/mL). Semen volume was assessed in mL using a graduated tube,while the sperm concentration was assessed with Neubauer hemocytometer. Only semen samples showing more than 80% progressive motility,85% live sperm and 3.5×109/mL were subjected to further preservation procedures[11].
The pooled semen sample was divided into three aliquots. Each aliquot was diluted at a ratio of 1:5 with one of the following extenders:1) Tris citric acid glucose [Tris–(hydroxymethyl)-aminomethane 3.028 g,citric acid monohydrate 1.700 g,glucose 1.250 g dissolved in 100 mL of distilled water]; 2) INRA-82 composed of lactose (0.150 g),raffinose (0.150 g),glucose(2.50 g),potassium citrate (0.041 g) ,Hepes (0.476 g),sodium citrate (0.025 g),skimmed milk (0.15%) dissolved in 100 mL distilled water[11]; 3) Tris citric acid fructose composed of Tris–(hydroxymethyl)-aminomethane (3.028 g),citric acid monohydrate(1.700 g),citric acid monohydrate (3.028 g) ,fructose (1.250 g)dissolved in 100 mL distilled water[1].
Each extender was supplemented with 15 mL egg yolk,7 mL glycerol,100 IU penicillin G sodium,and 100 mg streptomycin sulfate[12]. For cooling,diluted semen samples were set aside in the refrigerator at temperature 5 ℃ for 72 h. For cryopreservation,diluted semen samples were cooled to 5 ℃ for 2 h,equilibrated at 5 ℃ for 15 min,loaded in 0.25 mL French straws and sealed with polyvinyl powder. The straws were kept in a horizontal position at 6.5 cm over the liquid nitrogen level for at least 10 min and then the straws were plunged in the liquid nitrogen tank for long-term storage. After one week,frozen semen was thawed by eliminating two straws from the tank and then placing them in a water bath at 40 ℃ for 30 s[13].
2.3. Evaluation of cooled and frozen-thawed ram semen
2.3.1. Sperm progressive motility assessment
Progressive motility of the sperm was assessed subjectively using a bright field microscope (×400; Olympus BH-2,Tokyo,Japan),with a warm stage maintained at 37 ℃. A wet mount was made using a 10 μL drop of semen placed directly on a microscope glass slide and covered by a cover slip. Sperm motility percentages were performed in five different microscopic fields for each semen sample. The percentage of motile population was calculated at 0,24,48,72 h after cooling at 5 ℃ and 0,1,2,3 h after thawing at 37 ℃.
2.3.2. Viability and sperm abnormalities assessment
Eosin-nigrosin stain was used to assess the percentage of viable sperm[12]. Each sample was prepared by adding a drop of cooled or frozen-thawed semen sample mixed with 2 drops of the stain to the preheated glass slide,extended with another glass slide and air-dried.A bright field microscope (×1 000 with oil immersion) was used to randomly inspect a total of 200-sperm in five different microscopic fields in duplicated smears. Normal live sperm was white (excluding eosin stain),whereas dead sperm (lost membrane integrity) absorbed eosin stain and appeared pinkish in color. In the same slide used for viability assessment,abnormal sperm with defects including head,mid-piece and tail were evaluated,and the percentages of live and abnormal spermatozoa were calculated.
2.3.3. Osmotic resistance
The sperm functional membrane integrity of cooled or frozenthawed semen samples was evaluated using hypo-osmotic swelling test (HOST)[14]. Briefly,30 μL of a chilled or frozen-thawed semen sample was mixed with 300 μL of 100 mOsm/kg hypotonic solution(9.0 g fructose + 4.9 g sodium citrate/L distilled water). The mixture was incubated at 37 ℃ for 1 h. A drop of the incubated mixture was placed on a pre-warmed glass slide and covered with a coverslip,and then immediately evaluated under a bright field microscope(×400; Olympus BH-2,Tokyo,Japan). The tails of all membraneresistant sperm showed swelling,causing the flagella to curl and there was an inflated bubble in the membrane. A total of 200 sperm were calculated in at least five fields of the microscope in duplicated smears.
2.3.4. Acrosome integrity
The integrity of acrosome was evaluated post cooling and post thawing by using specific stain (FertiPro N.V. Belgium) called Spermac as previously measured by Ghallab et al[9]. In short,dried sperm smears prepared for each sample were fixed in 10% formalin solution for 10 min. Then each slide was passed through staining solutions A,B,and C for 1 min at room temperature. The slides were inspected using bright field microscope (×1 000,in oil immersion).200 sperm were counted in at least five different microscopic fields and the percentage of sperm with intact acrosomes (with normal apical ridge) was recorded in duplicated smears.
2.3.5. DNA integrity
To test the DNA integrity of cooled and frozen-thawed ram spermatozoa,gel electrophoresis (Comet) with single-cell under high alkaline environments was used. Diluted semen samples were processed by centrifugation,encapsulation,lysis and electrophoresis to denature double-stranded DNA. After that,the slides were stained with ethidium bromide. The processed glass slide was examined under a fluorescence microscope at ×400. The sperm head with fragmented DNA showed that the DNA relocated to the anode and formed a "Comet" tail[15,16].
2.4. Statistical analysis
Data analyses were made using SPSS program (SPSS,V16.0,SPSS Inc.,USA). After arcsine transformation,all data were distributed normally. One-way analysis of variance was used to compare the sperm quality parameters between extenders. Data were expressed as mean±standard deviation (mean±SD). P<0.05 was considered a statistically significant difference.
2.5. Ethics statement
This study was conducted according to the approval number of the Animal Use Ethical Committee (VET-IACUC-2021).
3. Results
3.1. Sperm progressive motility of cooled ram semen for 72 h
The data of the impact of INRA82 extender on the cooled (5 ℃)ram sperm progressive motility compared to Tris-citrate fructose and Tris-citrate glucose at 0,24,48 and 72 h are presented in Table 1. Progressive motility was recorded highest in INRA82 extender compared to Tris-citrate fructose and Tris-citrate glucose extenders at all the time points (all P<0.05).

Table 1. Progressive motility of cooled (5 ℃) ram semen for 72 h using different extenders (%).
3.2. Quality parameters of cooled ram semen
The data of the impact of INRA82 extender on the cooled (5 ℃)ram semen quality parameters (viability,functional membrane integrity,acrosome integrity,normal sperm and non-fragmented DNA) compared to Tris-citrate fructose and Tris-citrate glucose extenders at 24 h are presented in Table 2. All the fore-mentioned semen quality parameters were recorded highest in INRA82 extender compared to Tris-citrate fructose and Tris-citrate glucose extenders(P<0.05)
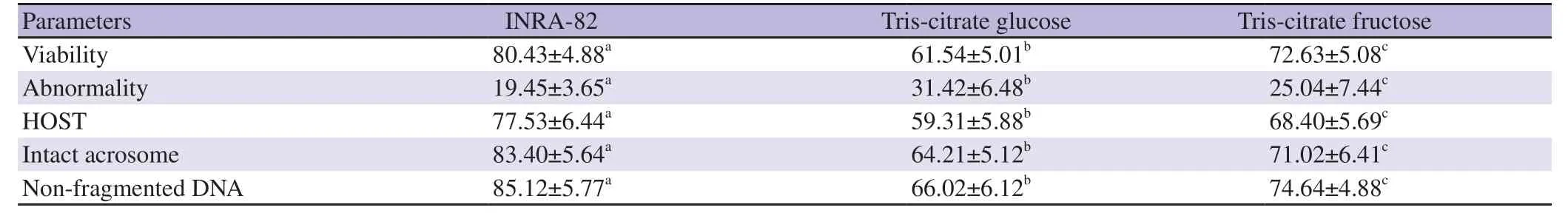
Table 2. Quality parameters of cooled ram semen (5 ℃) extended with different extenders (%).
3.3. Effect on progressive motility of frozen-thawed semen
The data of the impact of INRA82 extender on the frozen-thawed ram sperm progressive motility compared to Tris-citrate fructose and Tris-citrate glucose extenders at 1,2 and 3 h after thawing at 37 ℃are presented in Table 3. Progressive motility was recorded highest in INRA82 extender compared to Tris-citrate fructose and Triscitrate glucose extenders at all the time points (P<0.05).
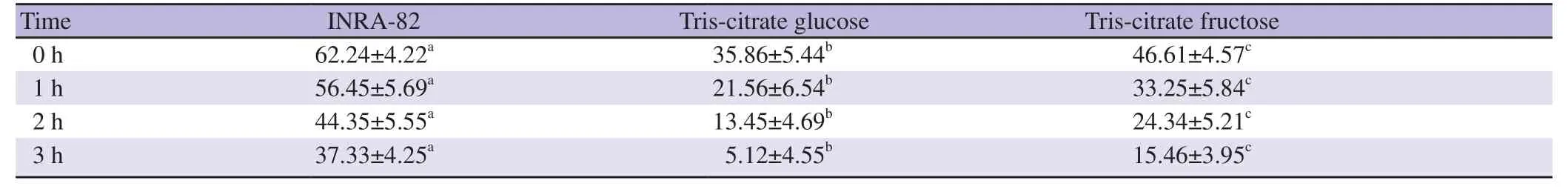
Table 3. Effect of different extenders on the individual motility of frozen-thawed ram semen after thawing at 37 ℃ for 3 h (%).
3.4. Quality parameters of frozen-thawed ram semen
The data of the impact of INRA82 extender on the frozen-thawed ram semen quality parameters (viability,functional membrane integrity,acrosome integrity,normal sperm and non-fragmented DNA) compared to Tris-citrate fructose and Tris-citrate glucose extenders are presented in Table 4. All the fore-mentioned semen quality parameters were recorded highest in INRA82 extender compared to Tris-citrate fructose and Tris-citrate glucose extenders(P<0.05).
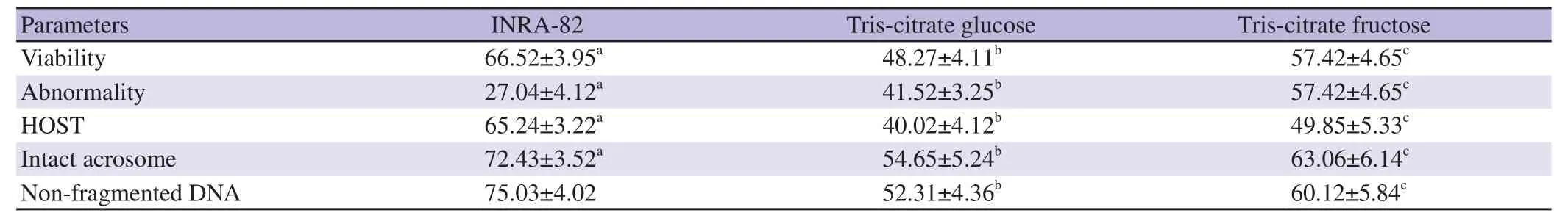
Table 4. Quality of frozen-thawed ram semen after using different extenders (%).
4. Discussion
The choice of the extender is crucial for successful sperm preservation. Semen preserved either in cooled or frozen conditions experienced both physical and chemical disruptions[16],which not only deteriorates sperm quality parameters but also decreases its fertility potential[17,18]. Several years ago,many attempts were conducted to improve semen freezing outcomes via using different extenders[19],cryoprotectants[20]and antioxidants[21,22]. Tris-based extender was extensively used in ram semen preservation[23,24].INRA82 extender showed a great success in equine and rabbit semen freezing[25,26]. Therefore,based on the composition of INRA82 extender and success in freezing equine and rabbit semen,the authors hypothesized that INRA82 extender might have a positive effect in chilling and freezing of ram spermatozoa when compared to Tris-based extenders. The findings of the present study supported the hypothesis that INRA82 extender enhances the post thawed semen quality. These results are beneficial for raising the outcomes of sheep breeding industry.
In the current study,INRA82 extender showed a significant increase in percentages of post thaw progressive motility,structural and functional membrane integrities and acrosome intactness,while fragmented DNA and abnormal sperm percentages were decreased compared to Tris-citrate fructose and Tris-citrate glucose extenders after cooling and freezing conditions. These improvements are in line with previous studies conducted in stallions[9,11,27,28]and rabbit bucks[26]. The possible mechanisms through which INRA82 extender improves semen quality preserved in cooled and frozen conditions are explained as follow:Firstly,INRA82 extender protects the spermatozoa against cooling,freezing and thawing injuries via its content of multiple sources of sugars (raffinose,lactose and glucose),as a non-permeable cryoprotectants,which lead to better sperm plasma membrane protection against ice crystal formation more than one source of sugar in Tris-citrate fructose and Tris-citrate glucose[29,30]. Moreover,natural phospho caseinate,β-lactoglobulin,lipoprotein and casein micelles in skimmed milk provide an additional protection and resilience to the spermatozoa against cryopreservation injuries[31]. Secondly,INRA82 extender provides the sperm with antioxidants such as raffinose[32]and sulphadryl groups in skimmed milk[33]; as a non-enzymatic antioxidant,it scavenges the excess free radicals generated throughout freezing and thawing processes and alleviates the detrimental effects of oxidative stress[34]. Thirdly,INRA82 extender improves the osmotic balance and increases the energy substrates available to the spermatozoa via its sugar content which in turn protects the spermatozoa against osmotic shock and provides more available adenosine triphosphate needed for sperm biological functions[35,36].
Contrary to our results,ram semen fluid diluted with Tris-based diluents retains better sperm mobility and membrane integrity than skim milk diluents[6,33]. The discrepancy in these results may be due to the use of milk diluent only without the additives (sugar solution)found in INRA82 extender that have a positive impact during cooling and freezing conditions.
Study limitations are summarized as further studies are needed to evaluate the effect of different extenders on in vivo fertility of frozen ram semen.
In conclusion,INRA82 extender improves semen quality parameters of ram semen in cooled and frozen conditions and is considered the most suitable diluent for ram semen preservation that helps the improvement of sheep breeding industry. However,further research points need to be clarified to elucidate more mechanisms through which INRA82 extender protects spermatozoa in different levels in terms of computer assisted semen analysis motilities,mitochondrial membrane potential,lipid peroxidation assay,and antioxidant capacity.
Conflict of interest statement
The authors declare no potential conflicts of interest concerning the research,authorship,and publication of this article.
Authors' contributions
Aya M. Fadl carried out semen collection,evaluation and statistical analysis of data curation. Hossam R. El-Sherbiny carried out semen processing and freezing,data curation and formal analysis. Elshymaa A. Abdelnaby made data visualization and data curation.
 Asian Pacific Journal of Reproduction2022年2期
Asian Pacific Journal of Reproduction2022年2期
- Asian Pacific Journal of Reproduction的其它文章
- Protective effect of Scrophularia striata combined with trehalose and cysteine added to diluents on cryopreservd goat epididymal sperm
- Profiling of seminal antioxidant indices and sperm quality in Plasmodium bergheiinduced malarial mice treated with Phyllanthus amarus
- An ethnopharmacological survey of medicinal plants used in the traditional treatment of human infertility in eastern Algeria
- Spousal communication,fertility preference and other factors affecting contraceptive use among married couples in Ekiti State,Nigeria
- Determinants of emergency contraceptive pill use in Bangladesh:An analysis of national survey data
- A scoping review of SARS-CoV-2 and male infertility:Concerns and future prospects
