Continuous intravenous infusion of recombinant human endostatin using infusion pump plus chemotherapy in non-small cell lung cancer
INTRODUCTION
Lung cancer is one of the malignancies with the highest incidence and mortality worldwide[1].By pathological typing,lung cancer is divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC,accounting for about 80%)[2].Approximately 60%-70% of NSCLC patients are diagnosed at a late stage.The median survival of stage IV patients is less than nine months.Chemotherapy regimens for NSCLC mainly include platinum-containing double-drug chemotherapy or gemcitabine and docetaxel monotherapy[3].However,the efficacy and safety of the current regimens is unsatisfactory.Therefore,the development and upgrade of treatments that are more effective,better tolerated,and less toxic is urgently warranted.
In 1971,Professor Folkman from the Harvard Medical School first proposed that the growth and spread of malignancies depended on tumor angiogenesis[4,5].Vascular endothelial growth factor (VEGF) and its receptor are important factors in tumor angiogenesis.A synergistic effect may result from the combined use of antiangiogenic drugs and chemotherapy.Recombinant human endostatin (Rh-endostain,Endostar) was approved by the National Medical Products Administration in September 2005 for the treatment of NSCLC.Previous report revealed that Rh-endostain could inhibit tumor angiogenesis,the proliferation and migration of endothelial cells by downregulating various angiogenic factors,such as VEGF[6-8].Besides,Rh-endostain could also regulate tumor microenvironment normalization,thereby promoting the proliferation of endothelial cells and podocytes and increasing the blood supply.As a result,tumor cells have an increased sensitivity to chemotherapy and radiotherapy[9,10].In a clinical study of Rh-endostain plus chemotherapy for the treatment of advanced NSCLC,the patients' prognosis was noticeably improved,and the anti-tumor effects of this regimen were demonstrated.Rh-endostain was usually administered by intermittent intravenous infusion,3-4 times per day for 14 consecutive days.However,patients might have poor adherence to this dosing regimen,which remained to be optimized.Several clinical studies were conducted based on stability tests of the continuous intravenous infusion of Rh-endostain using an infusion pump[11-13].In brief,the results showed that this administration regimen of Rh-endostain was convenient and guaranteed patient adherence.In addition,this administration regimen was conducive to maintaining the steady-state concentration of Rh-endostain in the blood,which was widely accepted and used clinically[14-19].
“I can see myself, I can see myself,” said the narcissus. “Oh, how sweet is my perfume! Up in a little room with a bow window, stands a little dancing girl, half undressed; she stands sometimes on one leg, and sometimes on both, and looks as if she would tread the whole world under her feet. She is nothing but a delusion31. She is pouring water out of a tea-pot on a piece of stuff which she holds in her hand; it is her bodice. ‘Cleanliness is a good thing,’ she says. Her white dress hangs on a peg32; it has also been washed in the tea-pot, and dried on the roof. She puts it on, and ties a saffron-colored handkerchief round her neck, which makes the dress look whiter. See how she stretches out her legs, as if she were showing off on a stem. I can see myself, I can see myself.”
The present study observed the efficacy and safety of 5-d continuous intravenous infusion of Rh-endostain in advanced NSCLC patients,which may provide further valuable clinical data for the treatment of advanced NSCLC.
MATERIALS AND METHODS
Baseline characteristics
I left baseball in 2005, with a Triple-A contract on the table from the San Diego Padres. I left not for physical reasons — I d had a torn hamstring tendon in 2003, but it hadn t affected4 my speed — but because it was my season for change. So I decided5 to walk away and once I did, like the vast majority of players, I was lost. It would be the first time since I learned to swing a bat that I would spend an entire summer without ever putting on a uniform. Even if you get a going-away party like the one the Phillies gave me on June 25th, 2005, when I threw out the first pitch of the Philadelphia-Boston game on a national TV, once the last partygoer walks out the door it s no longer you against that fastball, it is you against yourself.
Several studies evaluated the efficacy and safety of Rh-endostain plus platinumcontaining double-drug chemotherapy.However,there were only limited data on the combination of Rh-endostain plus monodrug chemotherapy or platinum-containing double-drug chemotherapy as the second-line regimen and below in advanced NSCLC patients.Patients generally showed lower adherence to intravenous drip infusion in previous studies[21,22].In the present study,the efficacy and safety of Rh-endostain administered by continuous intravenous infusion for five days using an infusion pump in retreated advanced NSCLC were assessed.
Treatment regimens
Rh-endostain (Shandong Simcere-Medgenn Bio-pharmaceutical Co.,Ltd.,15 mg/bottle) was administered concomitantly with chemotherapy.Rh-endostain was given by continuous intravenous infusion using an infusion pump at a dose of 210 mg for 5 consecutive days.Each treatment cycle lasted 21 d (q21d).The chemotherapy regimens included the following:(1) AP regimen:Pemetrexed 500 mg d1+carboplatin Area under roc curve (AUC)=5-6 (or cisplatin 75 mg/m) d1 q21d;(2) GP regimen:Gemcitabine 1000-1250 mg/md1+cisplatin 75 mg/m(or carboplatin AUC=5-6) d1 q21d;(3) Pemetrexed monotherapy:Pemetrexed,500 mg/md1 q21d;and (4) Docetaxel monotherapy:Docetaxel,60-75 mg/md1 q21d.Tumors were assessed as planned until disease progression.
Clinical efficacy and adverse event evaluation
Limitations in this retrospective analysis should never be neglected.For one thing,this was a retrospective study with a small sample size,and prospective clinical randomized controlled trials will be conducted for further validation of the efficacy and safety of continuous intravenous infusion of Rh-endostatin combined with chemotherapy in retreated advanced NSCLC.For another,heterogeneity of patients enrolled in this study should be further considered to reduce potential selective bias.
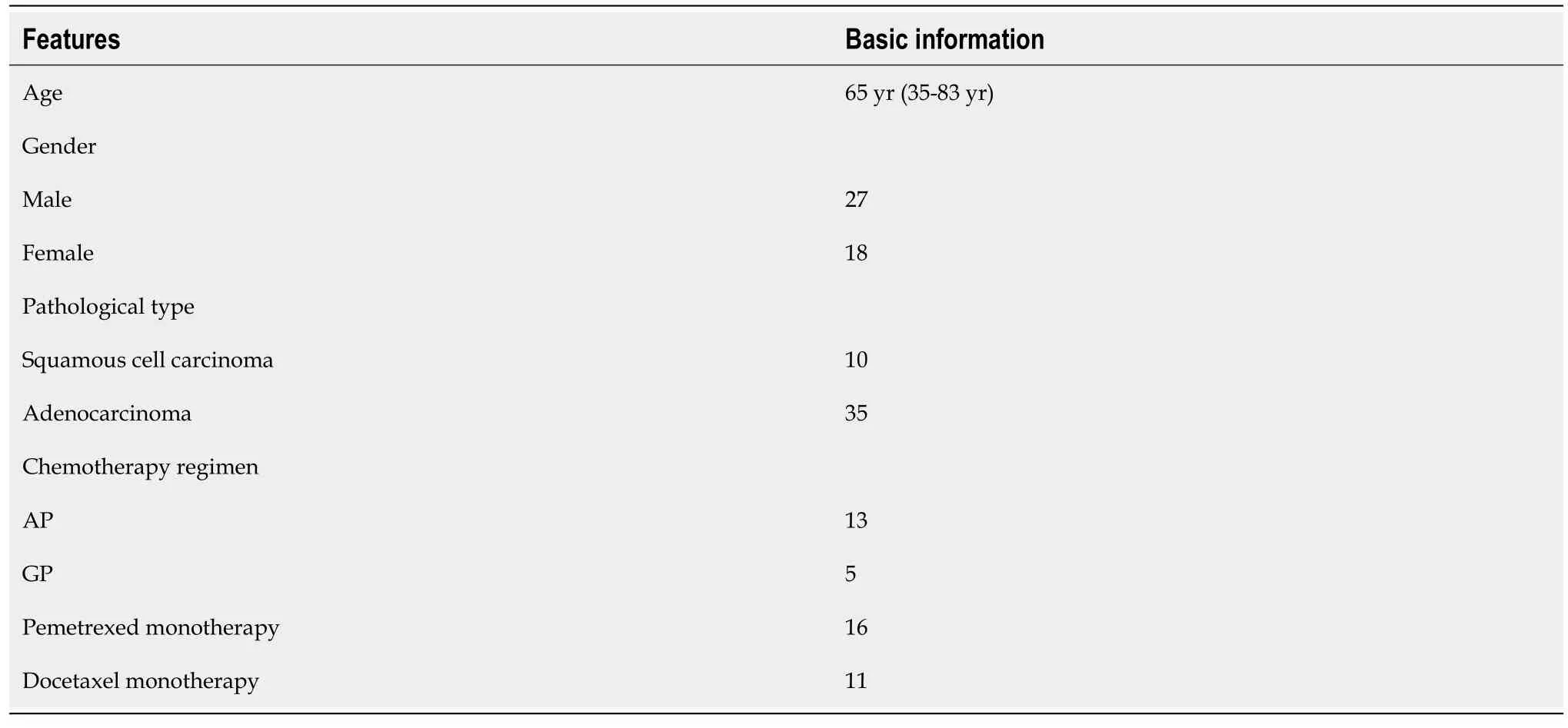
The medical records of 45 NSCLC patients who were treated at Zhejiang Provincial People's Hospital from October 2017 to April 2019 were retrospectively analyzed.Eligibility of the patients was assessed using the following inclusion criteria:(1) NSCLC confirmed by pathohistology or cytology;(2) Retreated advanced NSCLC (stage IV according to the American Joint Committee on Cancer staging system);(3) Eastern Cooperative Oncology Group performance status score,0-2;(4) Measurable and evaluated lesions without contraindications;and (5) Data on the following examinations were available:routine blood and urine tests,liver and kidney function tests,cardiac enzyme profile,and electrocardiogram,computed tomography scan of the chest,abdomen and brain,and whole-body bone scan after two cycles of treatment.The patients were excluded if any of the following exclusion criteria were met:(1) Women who were pregnant or lactating;(2) Hemorrhagic tendency,history of thrombosis,or currently taking anticoagulant medication;(3) Abnormal organ functions and unable to tolerate the side effects of Rh-endostain and chemotherapy;and (4) The presence of other malignancies.All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).The study was approved by ethics committee of Zhejiang Provincial People’s Hospital (People’s Hospital of Hangzhou Medical College) (2021QT290).Individual consent for this retrospective analysis was waived.
Statistical analysis
Data analysis were performed using SPSS 22.0 software.The ORR and CBR in patients with different pathological types of NSCLC who received different treatment regimens were compared using thetest.<0.05 indicated a significant difference.All tests were two-sided.The survival curve was estimated using the Kaplan–Meier method.
I am grateful for your goodwill25, but take back your gift! The fairy had pity on his youth and want of faith, and took care that one end of the thread remained in his hand
RESULTS
General information
31.Began to laugh quite loudly: Bettelheim believes that making the princess laugh is to free her emotionally (186 and that this is frequently achieved by the hero s making persons who normally command respect look ridiculous (186).Tater sees the refusal to laugh as comic relief (286).Return to place in story.
The following is an annotated1 version of the fairy tale. I recommend reading the entire story before exploring the annotations2, especially if you have not read the tale recently.
Analysis of short-term clinical effects on NSCLC patients
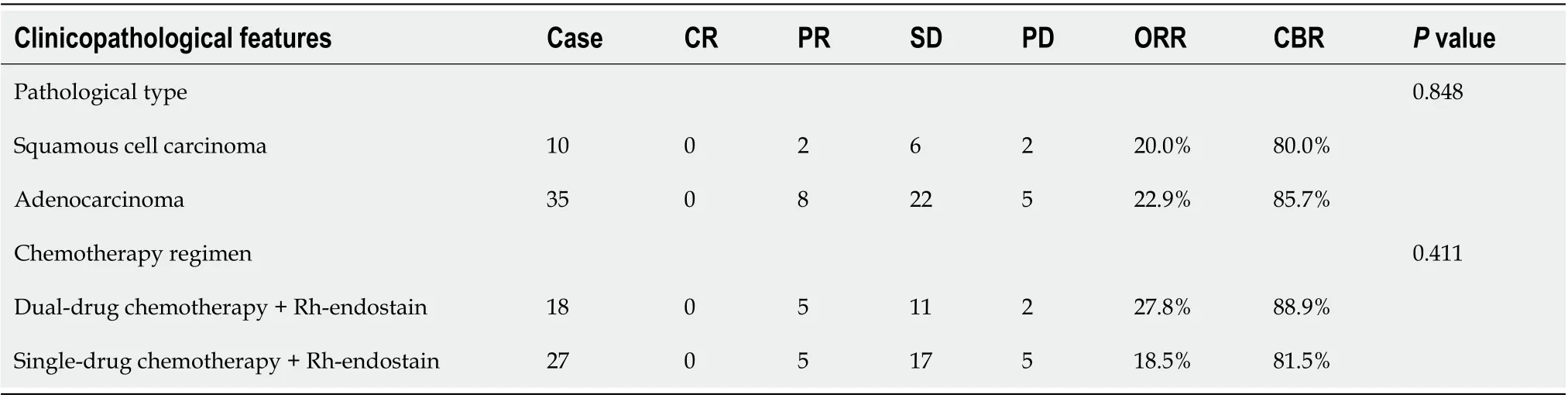
The clinical data of all 45 enrolled patients were evaluated.There were no cases of CR,10 cases of PR,28 cases of SD,and 7 cases of PD.ORR was 22.2%,CBR was 84.4%,and median progression-free survival (mPFS) was 5.3 mo (Figure 1).The patients were also stratified by pathological type and chemotherapy regimens.No significant differences were observed in patients with different types of NSCLC who received different treatments (>0.05).Further details were provided in Table 2.

AEs
The following AEs were observed as follows decreased hemoglobin (34 cases,75.6%),nausea/vomiting (32 cases,71.1%),elevated transaminase (24 cases,53.3%),leukopenia (16 cases,35.6%),thrombocytopenia (14 cases,31.1%),and constipation (1 case,3.4%).None of the patients had leukopenia,nausea/vomiting,and constipation of grade III and above.Further details are provided in Table 3.Overall,the toxicity profile of the combination treatment in this study was acceptable and manageable.
A total of 45 NSCLC patients who were treated at Zhejiang Provincial People's Hospital from October 2017 to April 2019 were retrospectively analyzed in this study,and baseline characteristics were shown in Table 1.The median age was 65 years (Interquartile range:35-83 years).Besides,27 of the NSCLC patients in this study were male,with the rest 18 of the patients being female.Among them,10 NSCLC patients (22.2%) were Squamous cell carcinoma,with the rest of them being Adenocarcinoma type.Besides,the chemotherapy drugs combined with Rh-endostain included AP (=2),GP (=2),Pemetrexed monotherapy (=16) and Docetaxel monotherapy (=11).

DISCUSSION
By this time he was somewhat alarmed, and did his best to put the ship about and get back to the river, but wind and tide were too strong for him, and he began to think of the number of times, from his childhood up, that he had been warned not to meddle82 with water
Our study was observational in nature.All the enrolled patients had stage IV NSCLC in which no driver genes were identified.The chemotherapy regimens used were primarily the platinum-containing double-drug regimen and monodrug therapy (monodrug therapy was favored as a later-line treatment or for patients in a poor general condition (PS≥2),such as gemcitabine and docetaxel monotherapy).The above chemotherapy regimens combined with rh-endostain,as a targeted antiangiogenic agent,can normalize tumor vessels,sensitize tumor cells to chemotherapy,and improve patient prognosis.Our results showed that in the 45 enrolled patients,ORR was 22.2%,CBR was 84.4%,and mPFS was 5.3 mo.The incidences of hematological and non-hematological toxicities of grade III and above were low.No Rhendostain-related cardiac functional abnormalities,as reported previously,occurred in our study.Furthermore,the efficacy was compared in patients with different pathological types and receiving double-drug or monodrug chemotherapy.However,no significant differences were identified.Rh-endostain combined with either doubledrug or monodrug chemotherapy improved the efficacy in both lung adenocarcinoma and NSCLC.Our research findings lay the foundation for making clinical decisions on different chemotherapy regimens.
Taken together,5-d continuous intravenous infusion of Rh-endostain using an infusion pump improved patient adherence,bringing significant clinical benefits to the patients.Further clinical studies were warranted to further confirm the efficacy and safety of this regimen,in order to improve the prognosis of patients with advanced NSCLC.
Some previous studies have reported similar findings.For example,five days of intravenous infusion of Rh-endostain using an infusion pump as first-line treatment achieved similar efficacy to a continuous intravenous drip in advanced NSCLC patients (PFS:6.0 mo3.8 mo,=0.10).In addition,the incidences of AEs did not increase[23].The use of an infusion pump improved the adherence of patients to rhendostain treatment.The short-term efficacy and tolerance of Rh-endostain using the above treatment regimen with concurrent radiochemotherapy in unresectable stage Ⅲ NSCLC were satisfactory[24].Rh-endostain administered by continuous intravenous infusion using an infusion pump plus concurrent radiochemotherapy was associated with a low incidence of AEs in advanced NSCLC patients.These patients also reported a higher level of comfort and demonstrated better adherence.Therefore,the quality of medical care and nursing was improved[25].
Treatment efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria[20].The efficacy indicators were as follows:complete response (CR,defined as disappearance of all target lesions,no new lesions,and return of tumor markers to normal,for at least 4 wk),partial response (PR,defined as the sum of the decrease in the maximum diameters of the target lesions by more than 30%,for at least 4 wk),stable disease (SD,defined as the sum of the decrease in maximum diameters of the target lesions,yet not reaching the standard of PR or being increased yetnot reaching the standard of progressed disease),and progressed disease (PD,defined as the sum of the increase in the maximum diameter of the target focus by at least 20%,or the appearance of new lesions).Objective response rate (ORR)=(CR+PR)/(total number of cases in each group),and clinical benefit rate (CBR) were determined.Progression-free survival (PFS) was defined as the time from the first administration to disease progression confirmed by objective evidence or death due to any cause.Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCICTCAE version 5.0),and were recorded.
CONCLUSION
ORR:Objective response rate;CBR:Clinical benefit rate;CR:Complete response;PR:Partial response;SD:Stable disease;PD:Progressed disease.
ARTICLE HIGHLIGHTS
Research background
To date,current available treatment options for non-small cell lung cancer (NSCLC)are associated with significant limitations in safety and efficacy.Therefore,development and achievement of potential therapeutic therapies for NSCLC is necessary.
Research motivation
This study mainly evaluated the efficacy and safety of continuous intravenous infusion of recombinant human endostatin (Rh-endostain) using an infusion pump in patients with retreated advanced NSCLC.
60.Beg pardon:The sisters do not always beg for forgiveness in the tale. Sometimes their jealousy grows with Cinderella s good fortune and they are ultimately punished for their lack of charity. In the Grimm s Aschenputtel, they are filled with rage and scheme to capitalize on Cinderella s good fortune.Return to place in story.
Research objectives
This study aimed to investigate the efficacy and safety of continuous intravenous infusion of Rh-endostain in retreated advanced NSCLC patients.
Research methods
Forty-five patients from Zhejiang Provincial People's Hospital received continuous intravenous infusion of Rh-endostain using an infusion pump.Objective response rate (ORR),clinical benefit rate (CBR),median progression-free survival (mPFS),and adverse events were analyzed after treatment.
Research results
In these 45 patients,ORR was 22.2%,CBR was 84.4%,and mPFS was 5.3 mo.The following AEs were observed as follows,decreased hemoglobin (34 cases,75.6%),nausea/vomiting (32 cases,71.1%),elevated transaminase (24 cases,53.3%),leukopenia (16 cases,35.6%),thrombocytopenia (14 cases,31.1%),and constipation (1 case,3.4%).None of the patients had leukopenia,nausea/vomiting,and constipation of grade III and above.
Research conclusions
Five-day continuous intravenous infusion of Rh-endostain using an infusion pump improved patient adherence,and brought about favorable efficacy and safety in retreated advanced NSCLC.
Research perspectives
Prospective clinical randomized controlled trials will be conducted for further validation of the efficacy and safety of continuous intravenous infusion of Rhendostatin combined with chemotherapy in retreated advanced NSCLC.
 World Journal of Clinical Cases2022年4期
World Journal of Clinical Cases2022年4期
- World Journal of Clinical Cases的其它文章
- Surgical treatment of acute cholecystitis in patients with confirmed COVID-19:Ten case reports and review of literature
- Rituximab as a treatment for human immunodeficiency virusassociated nemaline myopathy:What does the literature have to tell us?
- Eustachian tube involvement in a patient with relapsing polychondritis detected by magnetic resonance imaging:A case report
- Endoscopic clipping for the secondary prophylaxis of bleeding gastric varices in a patient with cirrhosis:A case report
- Inflammatory myofibroblastic tumor after breast prosthesis:A case report and literature review
- Langerhans cell histiocytosis presenting as an isolated brain tumour:A case report
