Transcranial focused ultrasound stimulation reduces vasogenic edema after middle cerebral artery occlusion in mice
Li-Dong Deng, Lin Qi, Qian Suo, Sheng-Ju Wu, Muyassar Mamtilahun,Ru-Bing Shi, Ze Liu, Jun-Feng Sun, Yao-Hui Tang, Zhi-Jun Zhang,Guo-Yuan Yang, , Ji-Xian Wang,
Abstract Blood-brain barrier (BBB) disruption underlies the vasogenic edema and neuronal cell death induced by acute ischemic stroke.Reducing this disruption has therapeutic potential.Transcranial focused ultrasound stimulation has shown neuromodulatory and neuroprotective effects in various brain diseases including ischemic stroke.Ultrasound stimulation can reduce inflammation and promote angiogenesis and neural circuit remodeling.However, its effect on the BBB in the acute phase of ischemic stroke is unknown.In this study of mice subjected to middle cerebral artery occlusion for 90 minutes, low-intensity low-frequency (0.5 MHz) transcranial focused ultrasound stimulation was applied 2, 4, and 8 hours after occlusion.Ultrasound stimulation reduced edema volume, improved neurobehavioral outcomes, improved BBB integrity (enhanced tight junction protein ZO-1 expression and reduced IgG leakage), and reduced secretion of the inflammatory factors tumor necrosis factor-α and activation of matrix metalloproteinase-9 in the ischemic brain.Our results show that low-intensity ultrasound stimulation attenuated BBB disruption and edema formation, which suggests it may have therapeutic use in ischemic brain disease as a protector of BBB integrity.
Key Words: blood-brain barrier; brain edema; cerebral blood flow; ischemia; matrix metalloproteinase-9; neurobehavioral outcomes; tight junction; transcranial ultrasound
Introduction
Stroke, including ischemic and hemorrhagic types, is the second leading cause of death worldwide and has a high risk of disability, of which ischemic stroke accounts for approximately 76% (Campbell et al., 2019; Zhong et al., 2020; Virani et al., 2021).Tissue plasminogen activator is the only FDA-approved medication for ischemic stroke but is limited by a short therapeutic window and has a high risk of hemorrhage (Miller et al., 2011; Liu et al., 2016).Other therapeutic approaches are necessary, including ones that can attenuate ischemic brain injury.
One of the pathological phenomena that occurs during ischemic stroke is blood-brain barrier (BBB) dysfunction (Li et al., 2019;Alamri et al., 2021).The BBB consists of endothelial cells, pericytes,astrocytic end-foot processes, the basement membrane, and tight junctions (Pan et al., 2020).It separates the central nervous system(CNS) from the blood circulation, maintaining a microenvironment appropriate for neurons and glial functioning.When ischemic stroke occurs, BBB disruption occurs and cytotoxic edema develops, which leads to infiltration of hematogenous fluid from the blood circulation into the brain via disrupted tight junctions (vasogenic edema), as well as regional neuroinflammation (Obermeier et al., 2013; Zhang et al.,2020).To date, several potential therapeutic approaches have been developed to attenuate BBB disruption (Abdulkadir et al., 2020).In animal studies, researchers have discovered drugs that reduce hyperpermeability by inhibiting inflammatory mediators, decreasing matrix metalloproteinases (MMPs), and increasing angiopoetin-1 to enhance tight junctions (Jiang et al., 2018; Zhang et al., 2020).MMP-9 is a gelatinase found in blood and microvessel wells that can cause degradation of tight junction proteins during ischemic brain injury.Inhibiting hyperexpression of MMP-9 might attenuate BBB disruption(Qi et al., 2016).However, such a treatment would be difficult in clinical practice because of the narrow therapeutic time window and safety issues.
Increasing evidence has shown that physical treatments may also facilitate BBB repair after ischemic brain injury.Such treatments include transcranial magnetic stimulation, transcranial direct current stimulation, optogenetics, and focused ultrasound stimulation(FUS) (Klomjai et al., 2015; Bergmann et al., 2016; Jiang et al., 2017;Wang et al., 2021).Among these, FUS has obvious advantages as a non-invasive method with superior spatial specificity and deeper penetration.Early application mainly focused on high-intensity FUS in surgery; however, over the past decade, use of low-intensity FUS for neuromodulation has increased (Bystritsky and Korb, 2015).Compared to high-intensity, low-intensity FUS has lower power and minimal thermal effects (ter Haar, 2007; Jiang et al., 2019).Lowintensity FUS has been demonstrated to activate neuronal circuits in hippocampusin vitroandex vivowithout tissue damage (Tyler et al., 2008; Jiang et al., 2019).Furthermore, low-intensity FUS can stimulate intact brain circuits without surgery in mice (Tufail et al., 2010).Low-intensity FUS has also been applied in CNS disease models.After ischemic stroke in rats, its application results in a significant neuroprotective effect (Schuhmann et al., 2018).However,its effect on the BBB after ischemic brain injury remains unknown.
We hypothesized that transcranial FUS (tFUS) attenuates brain edema in a mouse model of transient middle cerebral artery occlusion(tMCAO).To investigate this, we utilized a unique tFUS technique to treat tMCAO mice and explore the effect of tFUS on: 1) neurological deficits and cerebral edema in the acute phase of occlusion; 2) BBB injury and tight junction proteins; and 3) activity of MMP-9.
Materials and Methods
Experimental design
Experimental animal studies were reported according to the Animal Research: Reporting ofin vivoExperiments guidelines (Percie du Sert et al., 2020).Animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University, Shanghai, China (approval No.BME-ll-2019052)on May 20, 2019.Animals were housed in a specific pathogen free level animal facility at 26 ± 1°C in a 12-hour dark/light cycle.The individual ventilated cages contained four to five mice per cage.Adult male C57BL/6J mice weighing 25 to 30 g were used in the study experiments (n= 76, purchased from Charles River Laboratories,China [license No.SCXK (Jing) 2019-0009]).tMCAO was performed for 90 minutes to induce a focal ischemic brain injury.To assess the general condition of mice, body weight was measured using an electronic scale before surgery and 1 and 3 days after surgery.The mice in the experimental groups underwent 10 minutes of tFUS 2, 4,and 8 hours after occlusion, respectively.Neurobehavioral outcomes were evaluated in a double-blinded fashion using the modified neurological severity score (mNSS) on days 1 and 3 after tMCAO.Brain infarct volume and edema formation were determined using cresyl violet staining.BBB permeability was measured using IgG leakage and tight junction protein expression.MMP-9 activity was detected using a gelatin zymography assay.
tFUS in a mouse model of tMCAO
Seventy-six mice were divided into the following 6 groups (12–13 mice per group) using the random number table method: sham,sham/tFUS, tMCAO alone, tMCAO/tFUS at 2 hours, tMCAO/tFUS at 4 hours, and tMCAO/tFUS at 8 hours.Mice were anesthetized with 1.5% isoflurane (RWD Life Science, Shenzhen, China) in air under spontaneous breathing conditions.After the mice were fixed on the stereotaxic apparatus, tFUS was applied to the left hemisphere(ipsilateral to the side of occlusion) for 10 minutes with the probe attached to the overlying skull (Figure 1A).The pulsed wave bursts were delivered from a single-element focused ultrasound transducer(diameter, 2.5 cm; geometrical focal length, 12.44 mm) (Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences,China).Ultrasound was induced using the following parameters:fundamental frequency, 500 kHz; spatial peak temporal average intensity, 39 mW/cm2; pulse width, 500 μs; pulse interval,1 ms;stimulation duration, 300 ms; and stimulation interval, 3 seconds(Figure 1B and C).
tMCAO procedure
The detailed tMCAO procedure has been described previously with minor modification (Lu et al., 2017).Animals were anesthetized with 1.5% isoflurane in air under spontaneous breathing conditions.Body temperature was maintained at 37.0 ± 0.5°C during surgery using a heating pad (RWD Life Science).The entire surgical procedure was performed under a surgical microscope (Leica, Wetzlar, Germany).A midline incision was made in the neck to expose and separate the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA).A round head 6-0 suture (Dermalon,1741-11, Covidien, OH) coated with silicone was gently inserted into the ICA from the ECA stump to induce MCA occlusion.The suture insertion distance from the bifurcation to the left MCA opening was 9.5 ± 0.5 mm.Successful occlusion was defined as a 20% reduction in cerebral blood flow (CBF) as determined by laser speckle imaging (LSI,Figure 2A).After 90 minutes of occlusion, the suture was withdrawn,the CCA restored, and blood flow returned to at least 70% of baseline blood.Sham surgery mice underwent the same procedure without suture insertion.
LSI
To confirm the success of tMCAO, we monitored surface blood flow using LSI.The detailed procedure has been described previously with minor modification (Wang et al., 2019).Mice were fixed on the stereotaxic apparatus and anesthetized with 1.5% isoflurane in air under spontaneous breathing conditions.After scalp shaving and incising the skin to expose the skull, a camera and laser beam (785 nm) of the LSI system (RWD Life Science) were placed straight above the mouse brain.Imaging of CBF was obtained before tMCAO, during tMCAO, 10 minutes before tFUS treatment, and 10 minutes after tFUS treatment.
Western blot analysis
To explore tight junction function, we determined expression of zonula occludens-1 (ZO-1), occludin, and claudin-5 using Western blot analysis.The ipsilateral cortex was collected into a protein lysate to extract protein.The protein (30 μg) was loaded onto a 10% Acryl/Bis-acryl (Shanghai Epizyme Biotechmology Co., Ltd., Shanghai,China) gel for electrophoresis.Proteins were transferred to PVDF membranes (Merck Milliopore, Tullagreen, Ireland) activated by methanol.Membranes were incubated with 5% non-fat milk for 1 hour at room temperature followed by overnight incubation with primary antibodies to ZO-1 (Cat# 61-7300, anti-mouse, 1:1000 dilution, Thermo Fisher Scientific, Waltham, MA, USA), occludin (Cat#33-1500, anti-mouse, 1:1000 dilution, Thermo Fisher Scientific),claudin-5 (Cat# 35-2500, anti-mouse, 1:1000 dilution, Thermo Fisher Scientific), and β-actin (Cat# 66009 , anti-mouse, 1:2000 dilution,Proteintech, Wuhan, China) at 4°C on the shaker.Membranes were washed with tris-buffered saline with 0.1% Tween 20 (pH 7.5) 3 times and then incubated with horseradish peroxidase-conjugated secondary antibodies (Cat# HA1001, donkey anti-rabbit, 1:5000 dilution; Cat# HA1006, donkey anti-mouse, 1:5000 dilution, HuaAn Biotechnology Co., Ltd., Hangzhou, China) at room temperature for 1 hour on the shaker.The membranes were reacted with an enhanced chemiluminescence substrate (Meilunbio, Dalian, China)and then imaged (Criterion Stain Free System Instruction Manual,https://www.bio-rad.com/webroot/web/pdf/lsr/literature/10014472.pdf, Bio-Rad, Hercules, CA, USA).
Gelatin zymography assay
To determine MMP activity, we measured MMP-2 and MMP-9 activity using a gelatin zymography assay.The detailed assay procedure has been described previously with minor modification(Cai et al., 2017).An equal amount of protein from the perifocal ischemic region (60 μg) was loaded onto 10% Acryl/Bis-acryl gel with 15% gelatin (Sinopharm Chemical Reagent Co., Ltd., Shanghai,China) for electrophoresis.The sodium dodecyl sulfate was removed from the gels with 2.5% Triton X-100 for 1 hour.Gels were incubated in 1× incubation buffer (10×: 0.5 M Tris Base, 2.0 M NaCl, 0.05 M CaCl2, 0.2% (w/v) Brij-35, pH 7.6, Sinopharm Chemical Reagent Co.,Ltd.) for 72 hours at 37 °C.Then, they were stained with Coomassie stain (Sinopharm Chemical Reagent Co., Ltd.) for 1 hour followed by destaining in 10% acetic acid for 2 hours.Results were recorded using a scanner (Bio-Rad, Hercules, CA, USA).
Infarct and edema volume measurement
Brain infarct volume was measured by cresyl violet (Meilunbio)staining as described in a previous study (Mamtilahun et al., 2020).The mouse brains were rapidly removed and frozen at –80°C.A series of coronal sections were cut (20 μm thickness) using a cryostat(Leica Biosystems, Shanghai, China) and collected.Sixteen sections were calculated in total.The following formula was used to calculate infarct volume:
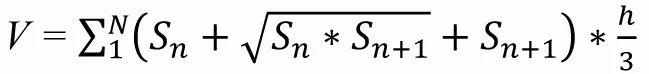
wherehis the distance (μm) between two adjacent sections (200μm) and S is the infarct area (mm2) in each section as determined by ImageJ software (64-bit Java 1.8.0_172, NIH, Bethesda, MD, USA;Schneider et al., 2012).Edema volume was determined using the same method.Both infarct and edema volume are expressed as a percentage of the total volume of the ipsilateral hemisphere.
Hematoxylin and eosin staining to evaluate brain injury
Hematoxylin and eosin (HE) staining was performed to examine the histopathology following brain tissue injury.Frozen coronal sections were fixed with 4% paraformaldehyde for 10 minutes, stained using a HE kit (#MB9898, Meilunbio), and examined using bright-field microscopy (Leica).
Immunostaining
To further explore tight junction function, ZO-1, occludin, and claudin-5 expression were also examined using immunostaining.Frozen coronal sections were fixed with ice methanol at –20°C for 10 minutes followed by washing with phosphate buffered saline three times.Sections were incubated with 10% bovine serum albumin for 1 hour at room temperature and then incubated overnight with primary antibodies to CD31 (Cat# AF3628 , anti-mouse, 1:200 dilution, R&D Systems, Minneapolis, MN), ZO-1 (Cat# 61-7300, anti-mouse, 1:100 dilution, Thermo Fisher Scientific), occludin (Cat#33-1500, anti-mouse, 1:100 dilution, Thermo Fisher Scientific),and claudin-5 (Cat# 35-2500, anti-mouse, 1:100 dilution, Thermo Fisher Scientific) at 4°C.The primary antibodies were washed with phosphate buffered saline 3 times and then incubated with donkey anti-mouse IgG H&L (Alexa Fluor? 594, Cat# A21203, 1:500, Thermo Fisher Scientific) and donkey anti-mouse IgG H&L (Alexa Fluor? 488,Cat# A21202, 1:500, Thermo Fisher Scientific) conjugated secondary antibodies at 37°C for 1 hour.The results were observed under a confocal microscope (Leica).
IgG staining
To determine the effect of ultrasound on BBB integrity, we examined IgG leakage.Frozen coronal sections were fixed with 4%paraformaldehyde for 10 minutes and 0.3% H2O2in methanol for 30 minutes.The ABC reagent kit (Vector Labs, Burlingame, CA, USA) and DAB kit (Vector Labs) were used in the following steps.Results were examined using bright-field microscopy (Leica) and analyzed using ImageJ software to calculate mean integrated optical density (IOD)(Tang et al., 2014).
Real-time polymerase chain reaction assay
To demonstrate the inflammatory response in the brain, we examined interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α),and transforming growth factor-β (TGF-β).The ipsilateral cortex was collected and placed into TRIzol reagent (Thermo Fisher Scientific)to extract RNA.The RNA concentration was detected using a spectrophotometer (NanoDrop1000, Thermo Fisher, Wilmington, DE).RT of RNA to cDNA was performed using a RT kit (Yeasen, Shanghai,China).The cDNA was used to perform real-time PCR with the One Step RT-qPCR SYBR Green Kit (Yeasen).The RT-PCR amplification reaction was performed under the following conditions: 95°C for 5 minutes followed by 40 cycles at 95°C for 10 seconds and 60°C for 30 seconds.The primer sequences (Sangon Biotech, Shanghai, China)are listed in Table 1 (Wen et al., 2020).The primer sequence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was designed by Sangon Biotech (No.662304) and used as an internal control and the expression level of each target gene was normalized to that of GAPDH using the 2–ΔΔCtmethod, where Ct is the threshold cycle.
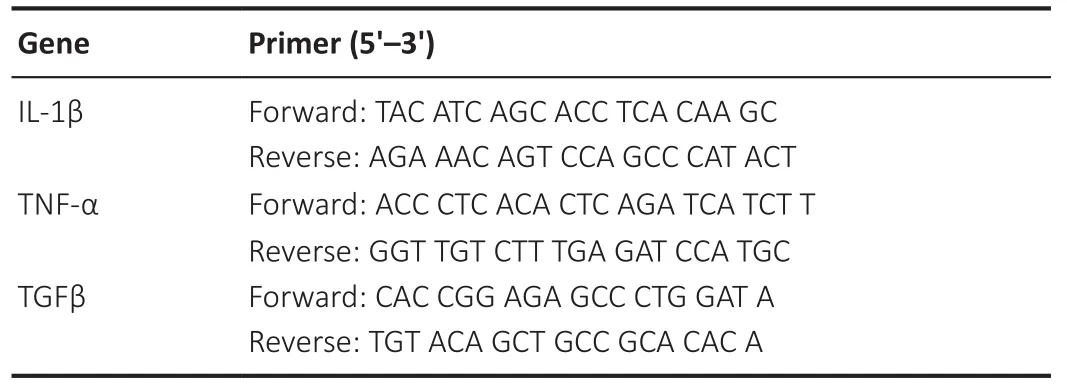
Table 1 | Primer sequences of IL-1β, TNF-α, and TGF-β used in this study
Neurobehavioral assessment
mNSS scores (Schaar et al., 2010; Tang et al., 2014) ranging from 0 to 14 were used to assess neurological deficit.The tests were performed on days 1 and 3 after tMCAO and included raising the mouse by the tail (0–3 points), walking on the floor (0–3 points),beam balance testing (0–6 points), and reflex testing (0–2 points).Higher scores represent worse neurobehavioral function.
Statistical analysis
Statistical analyses were performed using SPSS software version 21.0(IBM, Armonk, NY, USA).All data are presented as means ± standard deviation.The open source G*Power version 3.1.9.7 was used to estimate the minimal sample size and perform power analysis (Faul et al., 2007).Data were compared using the Student’st-test or oneway analysis of variance followed by Bonferroni correction.P< 0.05 was considered significant.
Results
tFUS improves neurobehavioral outcomes and reduces edema in tMCAO mice
We first examined HE-stained brain tissue to assess the effect of tFUS and found that tFUS-treated mouse brain had no detectable pathological changes, the same as normal control mouse brain(Figure 2B).To explore the effect of tFUS on mouse neurobehavioral outcomes, mice were treated with tFUS for 10 minutes 2, 4, and 8 hours after tMCAO.We found that mNSS on days 1 and 3 after tMCAO did not differ between mice treated with tFUS 2 hours after tMCAO (tMCAO/tFUS at 2-hour group) and tMCAO alone mice.The tMCAO/tFUS at 4-hour group performed significantly better than the tMCAO alone group on day 1 (P< 0.05) but not on day 3.mNSS score was significantly lower in the tMCAO/tFUS at 8-hour group than the tMCAO alone group on days 1 and 3 (P< 0.01; Figure 2C).These results demonstrated that tFUS attenuated neurological deficit in tMCAO mice compared to control mice.
We also examined animal body weight before surgery and on days 1 and 3 following tMCAO.Before tMCAO and 1 day after, body weight did not differ among the tMCAO alone and the three tMCAO/tFUS groups.However, on day 3 after tMCAO, body weight was significantly lower in the tMCAO alone group than the tMCAO/tFUS at 8-hour group (P< 0.05; Figure 2D), suggesting that tFUS can improve mouse general condition after ischemic brain injury.
To determine if better neurological outcome in the tMCAO/tFUS at 8-hour group was associated with less brain tissue injury, we examined brain infarct volume and edema formation (Figure 2E).Edema volume was smaller in the tMCAO/tFUS at 8-hour group than the tMCAO alone group on day 3 after tMCAO (P< 0.05; Figure 2F).However, edema volume did not differ among the tMCAO/tFUS at 2 hours, tMCAO/tFUS at 4 hours, and tMCAO alone groups.Infarct volume did not differ among groups (P> 0.05; Figure 2G).
tFUS improves CBF
To investigate the effect of tFUS on CBF, we measured CBF in the normal mouse brain using LSI 10 minutes before and 10 minutes after tFUS stimulation.tFUS caused an approximately 20% increase in CBF (Figure 3A), indicating a direct effect on CBF.We then measured CBF in tMCAO mice before and after tFUS.CBF consistently changed in tMCAO mice.Compared to the tMCAO alone group, CBF increased in the tMCAO/tFUS at 8 hours group (Figure 3B), indicating that tFUS stimulation applied 8 hours after occlusion improved CBF.
tFUS attenuates BBB disruption in tMCAO mice
To determine whether tFUS treatment reduced tMCAO-induced brain edema by protecting the BBB, we examined BBB leakage using IgG staining.We did not detect IgG leakage in the tFUS-treated normal mice, indicating that tFUS itself did not affect BBB permeability.However, degree of IgG leakage was smaller in the tMCAO/tFUS at 8-hour group compared to the tMCAO alone group on day 3 after tMCAO (P< 0.05; Figure 4A).This suggests that tFUS treatment reduced brain edema by attenuating disruption of the BBB in tMCAO mice.
We then further examined whether tFUS treatment protected the structural integrity of the BBB.Expression of the tight junction proteins ZO-1, occludin, and claudin-5 was examined in tMCAO mice using immunostaining and Western blotting 3 days after occlusion.Expression of ZO-1, but not occludin or claudin-5, was significantly higher in the tMCAO/tFUS at 8-hour group than the tMCAO alone group (P< 0.05; Figure 4B and C).These results suggest that tFUS attenuated BBB disruption by reducing tight junction protein ZO-1 degradation.

Figure 1|The low-frequency tFUS setup on the mouse ischemic brain.
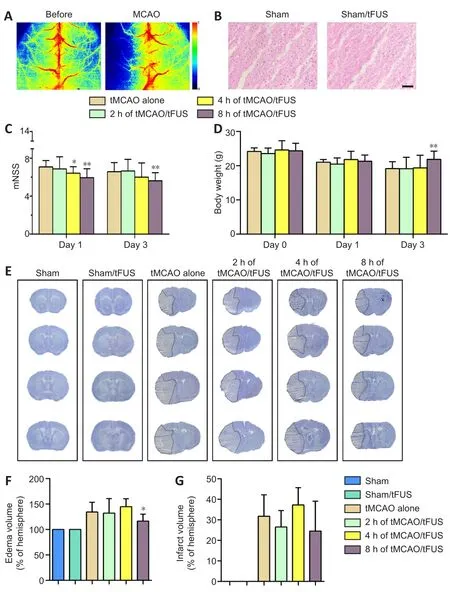
Figure 2| tFUS reduces brain edema and neurological deficit.
Figure 3| tFUS improves CBF.
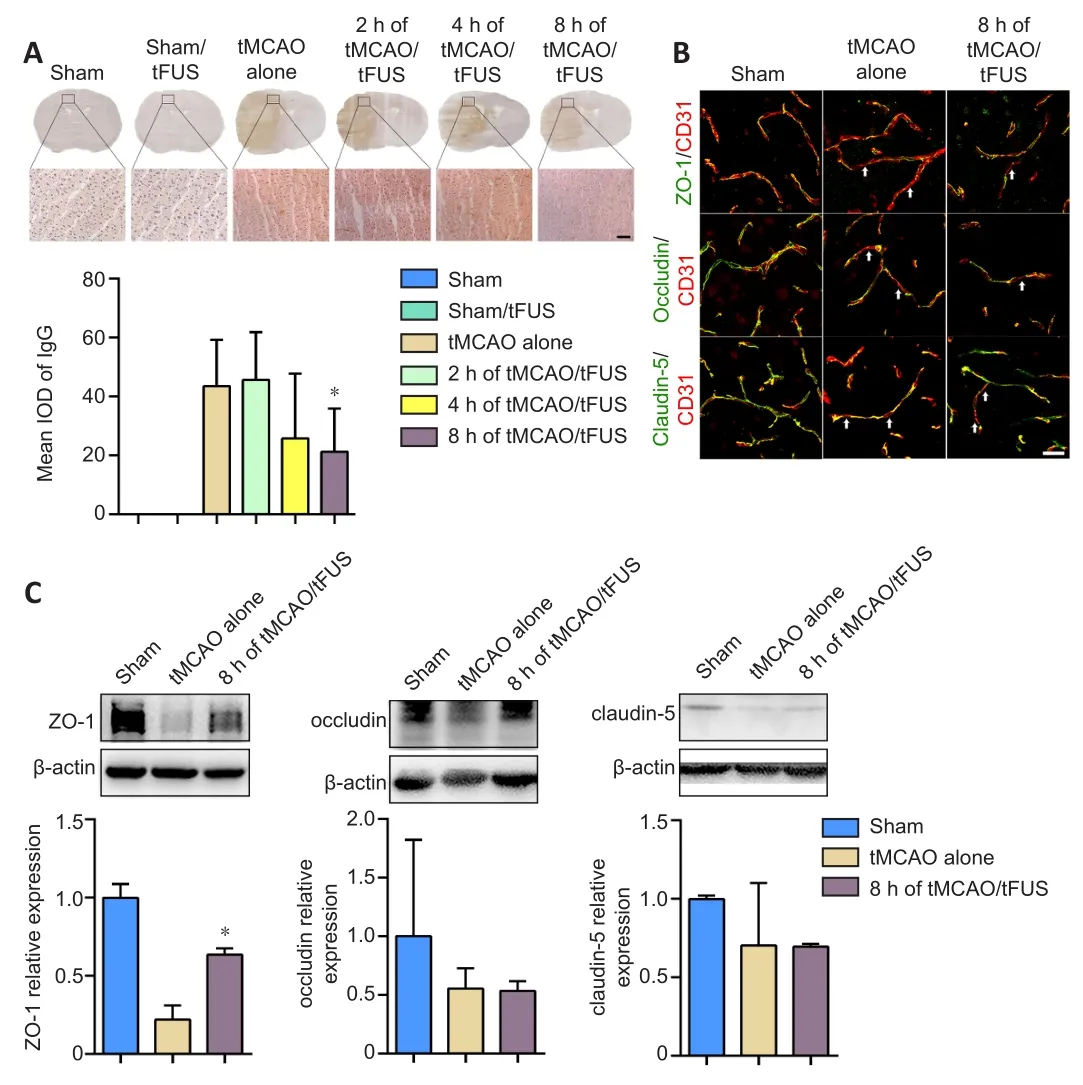
Figure 4| tFUS treatment reduces IgG leakage and enhances ZO-1 expression in tMCAO mice.
tFUS decreases MMP-9 activity and TNF-α expression
To demonstrate whether the protective effects of tFUS on the BBB in ischemic mice were associated with decreased MMP-9 activity,we examined MMP-9 enzyme activity in tMCAO mice 3 days after occlusion using the gelatin zymography assay.MMP-9 activity was lower in the tMCAO/tFUS at 8-hour group than the tMCAO alone group (P< 0.05, Figure 5A), suggesting that the decrease in tight junction degradation induced by tFUS may be related to reduction in MMP-9 activity.
We also examined mRNA expression of the cytokines IL-1β, TNF-α,and TGF-β on day 3 after occlusion and found that expression of TNF-α, but not IL-1β or TGF-β, was significantly lower in the tMCAO/tFUS at 8-hour group than the tMCAO alone group (P< 0.05; Figure 5B).This suggests that tFUS can attenuate focal inflammation.
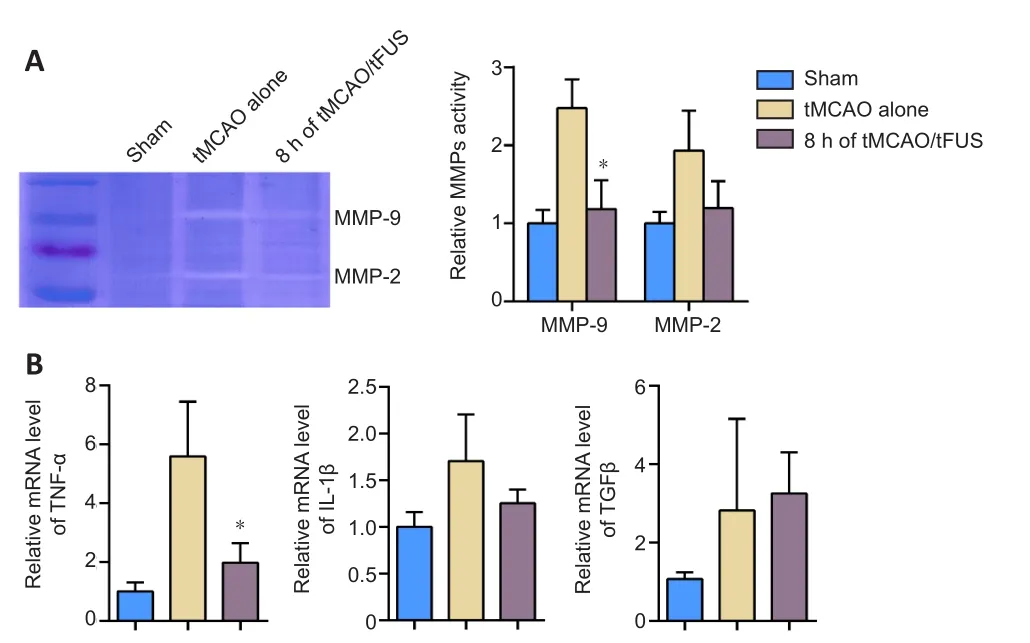
Figure 5 | MMP-9 activity and TNF-α expression decrease in ischemic brain after tFUS treatment.
Discussion
In this study, we demonstrated that tFUS can modulate the sensorimotor circle to promote improved neurobehavioral outcome in a mouse model of tMCAO.tFUS reduced vasogenic edema volume, which may result from improved cortical blood flow.tFUS also reduced BBB leakage and attenuated tight junction protein ZO-1 loss, which may be related to reduction of MMP-9 activity and TNF-α expression.
tFUS is a relatively new technique and determining the best stimulation parameters is important to maximize beneficial effects while limiting side effects.Ultrasound can be divided into low (20 kHz–1 MHz) and high frequencies (1–20 MHz) (Zhao et al., 2019).Low-frequency ultrasound waves have deeper penetration (Chen et al., 2015).Focused ultrasound is used in various tissue therapies and has shown promise in transcranial neuromodulation.Unfocused ultrasound is commonly used for imaging and diagnostic purposes(Xin et al., 2016; Zhao et al., 2019).tFUS frequencies generally range from 0.2 to 1.5 MHz (Mehic et al., 2014).For the best neuromodulatory effect in brain disease treatment, tFUS intensity should range from 30 to 500 mW/cm2(Tengfei et al., 2015; Oh et al., 2019; Liu et al., 2020).After several tests, we finally selected a frequency and intensity of 0.5 MHz and 39 mW/cm2, respectively, for our study.We did not observe any visible behavioral abnormalities after tFUS in normal mice, nor did we detect morphological changes in the brain tissue.At this frequency and intensity, tFUS exhibited high spatial resolution, deep penetration, and no thermal brain injury, indicating appropriateness for small animal application.
tFUS applied immediately after occlusion can reduce edema volume in a rat model of distal MCAO (Guo et al., 2015).In addition, tFUS treatment protects BBB integrity in a mouse model of traumatic brain injury (Su et al., 2017).Furthermore, earlier tFUS intervention after ischemic stroke in rats provides a stronger neuroprotective effect(Liu et al., 2019, 2020).The duration of ultrasound application in the above studies ranged from 5 to 60 minutes; therefore, after our preliminary experiment, we selected a duration of 10 minutes and applied it 2, 4, and 8 hours after tMCAO.
Our results demonstrated that tFUS intervention 8 hours after tMCAO resulted in the best reduction of brain injury; the earliertime points (2 and 4 hours after tMCAO) were not as effective.Brain edema occurs rapidly after ischemic stroke and includes cytotoxic and vasogenic types.Cytotoxic edema is caused by a water distribution shift from extracellular to intracellular compartments.Vasogenic edema occurs because of continuous BBB injury in the first few hours after stroke.Our results showed that tFUS at 2 and 4 hours after tMCAO had no better effect than tFUS at 8 hours, which suggests that tFUS interrupts vasogenic edema rather than cytotoxic edema.Therefore, tFUS may have a relatively long therapeutic window for treating ischemic stroke and other diseases that induce vasogenic edema.However, the mechanisms underlying the effects of tFUS on brain edema warrant further investigation.
We demonstrated that vasogenic edema decreased in the mouse brain following application of tFUS 8 hours after tMCAO.Moreover,this decrease was associated with a decrease in degradation of the tight junction protein ZO-1.Tight junctions play an important role in maintaining BBB integrity.Notably, degradation of occludin or claudin-5 was not decreased after tFUS treatment, suggesting that the effect of tFUS on the BBB is relatively minor.
Neuroinflammation interacts with activation of MMPs (Rempe et al.,2016).We found that MMP-9 activity and TNF-α expression were decreased in tMCAO mice who received tFUS 8 hours after occlusion,which is consistent with degradation of tight junction proteins.Tight junction degradation could be inhibited via decreased MMP-9 activation.We also found that CBF increased in tFUS treated mice compared to control mice; however, there was no evidence of a link between MMP-9 expression and CBF change.A previous study reported that tFUS can upregulate endothelial nitric oxide synthase(eNOS) in mouse models of bilateral common carotid artery stenosis and vascular dementia (Eguchi et al., 2018).NO concentration can be regulated by eNOS at a physical level.Low NO concentration can activate MMP-9 while a high concentration can inhibit it (O’Sullivan et al., 2014).tFUS may increase eNOS, then increase CBF, and finally inhibit MMP-9 activation.Although the results demonstrated that tFUS is a unique tool for the treatment of ischemic stroke, the optimal therapeutic time, duration, stimulation perimeters need to be further determined.In addition, molecular mechanisms of tFUS induction, such as eNOS, MMPs, growth factors, as well as signal pathways, how these factors involved in neuroprotection and neuronal repair also need be studied.
In conclusion, tFUS can decrease BBB disruption in mice with ischemic stroke.When applied 8 hours after tMCAO, tFUS reduced vasogenic edema, loss of tight junction proteins, MMP-9 activity,and expression of TNF-α.tFUS has therapeutic potential in ischemic stroke.
Acknowledgments:Li-Yuan Ren and Hang Song (both from Shanghai Jiao Tong University) are acknowledged for their kindly help with ultrasound parameter measurement.
Author contributions:Conceptualization: GYY and JXW; methodology:LDD, LQ, QS, SJW, MM, JFS and JXW; validation: LDD, LQ, QS and SJW;formal analysis: LDD, LQ and JFS; investigation: LDD, LQ, QS and SJW;resources: YHT, ZJZ, JXW and GYY; data curation: LDD, LQ, and QS;writing—original draft preparation: LDD and GYY; writing—review and editing: LDD, LQ, QS, SJW, MM, RBS, ZL, JFS, YHT, ZJZ and GYY;visualization: LDD and GYY; supervision: GYY; project administration: GYY and JXW; funding acquisition: JFS, YHT, ZJZ, JXW and GYY.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Early life stress-induced neuroinflammation and neurological disorders: a novel perspective for research
- Gut microbiome: a balancing act between degeneration and regeneration
- Aquaporin 4 deficiency eliminates the beneficial effects of voluntary exercise in a mouse model of Alzheimer’s disease
- (D-Ser2) oxyntomodulin recovers hippocampal synaptic structure and theta rhythm in Alzheimer’s disease transgenic mice
- Sustained delivery of vascular endothelial growth factor mediated by bioactive methacrylic anhydride hydrogel accelerates peripheral nerve regeneration after crushinjury
- Sustained release of exosomes loaded intopolydopamine-modified chitin conduits promotes peripheral nerve regeneration in rats