Advancement of chimeric antigen receptor-natural killer cells targeting hepatocellular carcinoma
Kai Dai,Yin Wu,Sha She,Qian Zhang
Kai Dai,Yin Wu,Sha She,Qian Zhang,Department of Infectious Diseases,Renmin Hospital of Wuhan University,Wuhan 430060,Hubei Province,China
Abstract With the advance of genome engineering technology,chimeric antigen receptors(CARs)-based immunotherapy has become an emerging therapeutic strategy for tumors.Although initially designed for T cells in tumor immunotherapy,CARs have been exploited to modify the function of natural killer (NK) cells against a variety of tumors,including hepatocellular carcinoma (HCC).CAR-NK cells have the potential to sufficiently kill tumor antigen-expressing HCC cells,independent of major histocompatibility complex matching or prior priming.In this review,we summarize the recent advances in genetic engineering of CAR-NK cells against HCC and discuss the current challenges and prospects of CAR-NK cells as a revolutionary cellular immunotherapy against HCC.
Key Words:Chimeric antigen receptors;Natural killer cells;Hepatocellular carcinoma;Immunotherapy;Genome engineering
INTRODUCTION
Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer,the sixth most commonly diagnosed cancer,and the third leading cause of cancer death worldwide[1,2].The key risk factors of HCC include infection with hepatitis B virus or hepatitis C virus,alcohol consumption,non-alcoholic fatty liver disease,and aflatoxin exposure[3].Surgery is the prevalent treatment for HCC.However,recurrence of HCC severely decreases the survival rate.
Locoregional therapies,including percutaneous ablation and intra-arterial chemotherapy,are alternative treatment approaches and rely on the grade of HCC.Unfortunately,more than 70% of patients with advanced HCC have little chance for transplant,surgery,or locoregional therapy.In the recent decade,cancer immunotherapy has become a novel emerging therapeutic strategy,improving treatment effectviamobilizing patients’ immune systems to launch efficient anti-tumor reactions against cancer progression.Adoptive transfer of immune cells,as an immunotherapeutic approach,is expected to improve HCC prognosis by passively infusing autologous or allogenic leukocytes afterex vivoexpansion and activation.Natural killer (NK) cells are a persistent research focus in the field of immunotherapy,due to their significant anti-tumor activity.
NK CELL BIOLOGY
NK cells,which make up 5%-15% of human blood leukocytes and 50% of hepatic innate immune cells,are the major innate lymphocytes executing anti-tumor and antiviral immunity[4].Based on the expression of cluster of differentiation (CD)16 and CD56,human NK cells can be divided into two subpopulations.The majority (85%-95%) of the peripheral blood NK cells are CD56dimCD16high,showing a developmentally mature phenotype,and mediate high cytotoxicity upon encountering target cells.The remaining minority of NK cells are the CD56brightCD16low/-subset,which have an immature phenotype and lower cytotoxicity upon activation[5].
Unlike T cells,the cytotoxicity of NK cells does not rely on T cell receptor (TCR)-mediated specific antigen recognition and is thus independent of major histocompatibility complex (MHC) expression.Instead,NK cell activity is regulated by a wide spectrum of activating and inhibitory receptors (Table 1) that induce positive and negative signals to comprehensively control NK cell behavior upon contacting target cells[6].In this regard,tumor cells that downregulate MHC expression are sensitive to NK cell cytotoxicity,owing to weaker killer cell immunoglobulin-like receptor(commonly known as KIR)-mediated inhibitory signals.Similar to cytotoxic T cells,activated NK cells express and release lytic molecules,such as perforin and granzyme B,to kill tumor cells.Additionally,NK cells secrete an array of cytokines,including interferon-g,tumor necrosis factor (TNF),and granulocyte-macrophage colonystimulating factor,to induce the activation of T cells and innate immune cells(dendritic cells,macrophages,and neutrophils),and result in the promotion of immunity against malignant cells and the tumorigenic microenvironment[4].
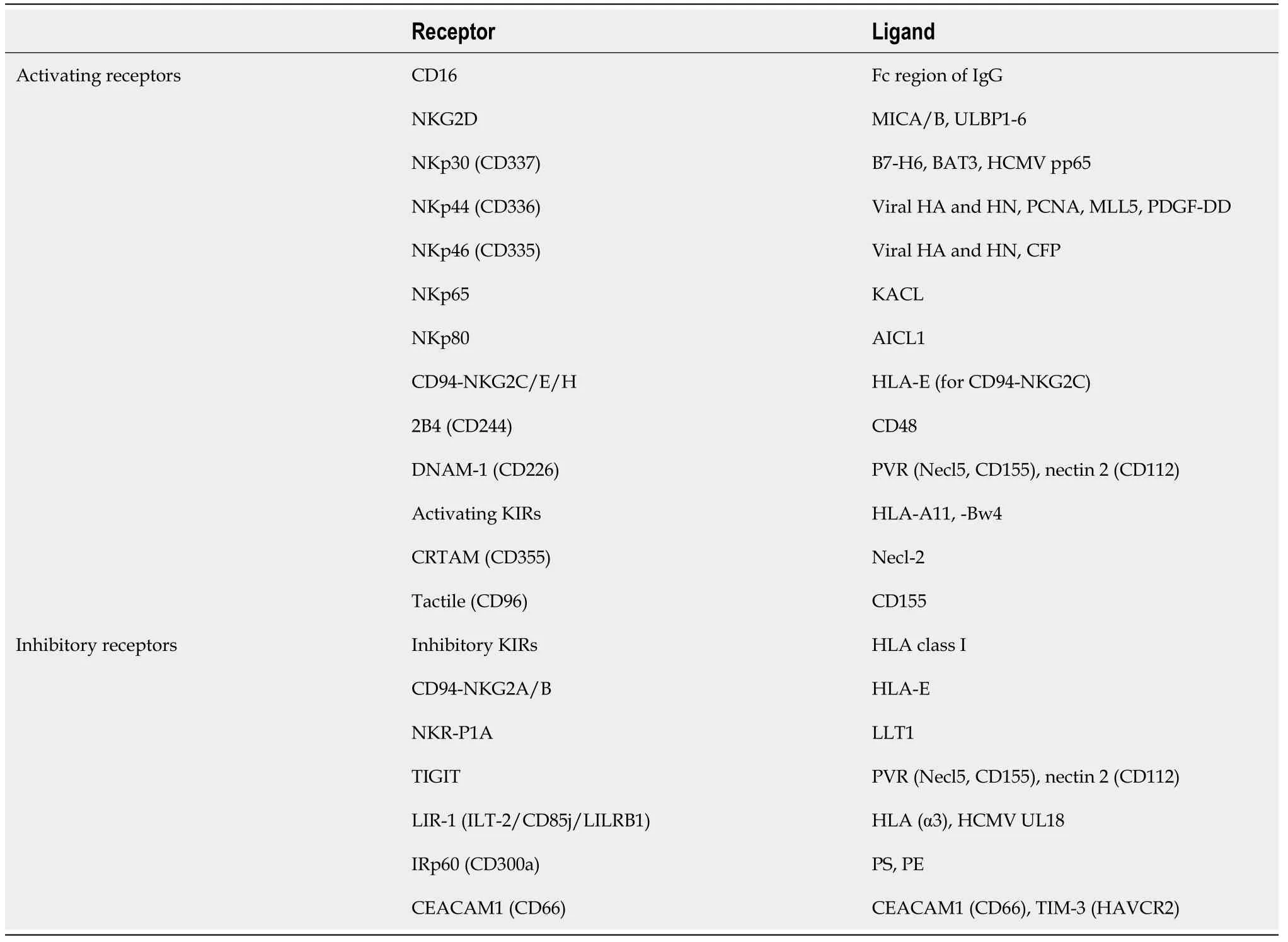
Table 1 Activating and inhibitory receptors on human natural killer cells
CARS FOR TUMOR TREATMENT
Thanks to genome editing technology,CARs were initially designed for T cellmediated immunotherapy against relapsed or refractory B-cell malignancies[7].A CAR is an antibody-derived antigen-recognizing domain,linked with T cell signaling domains.When expressed by a T cell,the CAR targets a specific antigen and transduces activating signals to that T cell.A CAR consists of three domains,namely an ectodomain,a transmembrane domain,and an intracellular signaling domain[8].The ectodomain is a single-chain variable fragment (scFv) comprising the variable regions of heavy and light chains of an antibody connected with a short linker peptide of 10-25 amino acids.The intracellular domain is conventionally derived from the TCR CD3ζ chain and/or other key accessory proteins for relaying activating signals.Three generations of CARs have been developed to date.The first-generation CARs contain CD3ζ alone.The second-generation CARs integrate an extra costimulatory domain from CD28 or 4-1BB.The third-generation CARs contain more than one costimulatory domain[9].The latest ? fourth-generation ? CARs possess supplementary exogenous proteins,such as cytokines,to enhance the lifespan and effector function of NK cells[10].
Although T cells armed with CARs (hereinafter referred to as “CAR-T cells”) have demonstrated unprecedented efficacy in boosting anti-tumor immunity,but they have intrinsic shortcomings[11,12].First,CAR-T cells require prior antigen priming,which depends on the expression of MHC molecules on target cells.However,various types of tumor cells downregulate MHC expression and therefore escape from the attack of CAR-T cells.Second,before infusion of CAR-T cells,patients have to receive lymphodepleting treatment to facilitate thein vivopersistence of the CAR-T cells.However,a large number of CAR-T cells in the recipient increases the risk of graftvshost disease (commonly known as GVHD).Third,activated CAR-T cells produce enormous amounts of cytokines,including interleukin (IL)-1a,IL-2,IL-6,TNF-α,MCP-1,IL-8,IL-10 and IL-15,rendering the cytokine release syndrome (otherwise known as CRS) and neurotoxicity.Compared to T cells,NK cells overcome these disadvantages because they function in an MHC-independent manner,without triggering GVHD,and produce a distinct spectrum of cytokines.
CAR-NK CELLS
CAR constructs for NK cells
For NK cells,the CAR structure is quite similar to that of T cells.The extracellular scFv is also derived from an antibody against a tumor antigen.So far,the tumor antigens targeted by engineered scFv involve CD19 and CD20 (B-cell acute and chronic leukemia antigen)[13],human epidermal growth factor receptor 2 (commonly known as HER2;breast cancer)[14],GD2 (neuroblastoma and melanoma)[15],CD138 (multiple myeloma)[16],CD4 (T cell lymphoma)[17],and epidermal growth factor receptor(EGFR) which is overexpressed in multiple tumors[18].Some researchers have designed CAR-NK cells targeting certain immunosuppressive cells,such as alternatively-activated macrophages and myeloid-derived suppressor cells,to enhance antitumor immunity[19,20].Moreover,a recent endeavor has been made to create a bispecific CAR featuring an scFv that targets two antigens simultaneously.NK cells equipped with this bispecific CAR are supposed to execute cytotoxicity more efficiently,in case one antigen is weakly expressed or absent on certain tumor cells[21].
The transmembrane domain of a CAR belongs to CD8a,CD28,CD3,or ICOS,with some exceptions derived from NKG2D[22].Recent findings suggest that the sequences and locations of the transmembrane domain should be delicately designed to achieve the best function[23].The intracellular domain is key to the signal transduction activity and consequent NK cell activation upon the engagement of the scFv with tumor antigens.Most CAR-NK cells exploit CD3ζ as a signaling domain to transduce activating signals because the immunoreceptor tyrosine-based activation motif (also known as ITAM) of CD3ζ initiates NK cell killing[24-26].Additional costimulatory domains derived from CD28,CD137 (4-1BB),or CD244 (2B4) are usually required for efficient killing[22,27].In recent research,replacing CD3ζ with the intracellular region of DAP12 has resulted in a better effect on glioblastoma[28].The most common CAR constructs used in CAR-NK cells have been summarized elsewhere[8].
Sources of CAR-NK cells
Currently,clinical-grade NK cells are manufactured on a large scale from the following sources.
NK92 cell line:The NK-92 cell line was established from a patient with large granular lymphoma in 1994.It does not attack recipients’ allogeneic cells but kills a wide range of tumor cells,such as leukemia and melanoma.The cell line is easy to growin vitro,making it an attractive agent for adoptive cancer immunotherapy.However,its tumorigenicity potential,deficiency in CD16 and NKp44 expression,and the requirement for lethal irradiation before adoptive transfer pose concerns about clinical safety and therapeutic efficiency[29].
Peripheral blood mononuclear cells (PBMCs):Sufficient allogeneic NK cells can be enriched from PBMCs of a patient or healthy donor,followed byex vivostimulation,expansion,and genetic engineering.PBMC-derived CAR-NK cells are predominantly CD56dimCD16+mature NK cells with high cytotoxicity,making them especially suitable for either autologous or allogeneic transfer[8].
Umbilical cord blood (UBC):As an alternative NK cell source,UBC with identified MHC types can be obtained from UBC banks.Nonetheless,the NK cell abundance in UBC is relatively low,so it takes a longex vivoexpansion and activation procedure before sufficient NK cells are generated.Another issue involves UBC-derived NK cells exhibiting an immature phenotype and being less cytotoxic to target cells[30].
Hematopoietic progenitor cells (commonly known as HPCs):CD34+HPCs are sorted from bone marrow,embryonic stem cells,mobilized peripheral blood,or UBC.They are expanded and differentiated into NK cellsex vivo,under the effect of a set of cytokines.HPC-derived NK cells are mature and significantly cytotoxic to leukemia cells[31].
Induced pluripotent stem cells (iPSCs):iPSCs are derived from mature somatic cells that have been reprogrammed back into a pluripotent stem cell state.They enable the development of an unlimited source of multiple cell types needed for therapeutic purposes.Theoretically,one iPSC is competent to generate a large quantity of homogeneous CAR-NK cells.However,iPSC-derived NK cells are relatively immature and less cytotoxic,posing the challenge of inducing both phenotypically and functionally mature NK cells[8].A recent study developed a robust and efficient manufacturing system for the differentiation and expansion of high-quality iPSCderived NK cells that produced inflammatory cytokines and exerted strong cytotoxicity against an array of hematologic and solid tumors[32].
CAR-NK CELLS IN HCC THERAPY
Both academia and industry have made rapid progress in developing CAR-engineered immune cells for HCC[33,34].One of the major challenges for CAR-based immunotherapy for HCC is to target a specific,safe,and effective tumor antigen.Currently,the following tumor antigens are considered candidate targets of CAR-based immunotherapy for HCC:glypican-3 (GPC3),α-fetoprotein,epithelial cell adhesion molecule(commonly known as EpCAM),and mucin-1 (commonly known as MUC1)[35,36].Of note,glypican-3 is a glycoprotein overexpressed on the cell surface of HCC tissues but not in healthy liver[37].A bispecific CAR targeting GPC3 and the asialoglycoprotein receptor 1 (commonly known as ASGR1) and featuring CD3ζ and 28BB (containing CD28 and 4-1BB signaling domains) has been tested in an HCC xenograft mouse model[38].
Although CAR-NK cells have been tested in a few clinical trials against leukemia,lymphoma,and several solid tumors,to our knowledge,no clinical trials have been performed to evaluate the efficacy of CAR-NK cells in HCC treatment.Several laboratory studies of CAR-NK cells against HCC have been conducted and showed the bright prospect of CAR-NK-based HCC immunotherapy.
The first report of CAR-NK cells against HCC was published in 2018.Yuet al[39]developed GPC3-CAR-NK-92 cells and demonstrated the potent anti-tumor properties of these cells.The CAR construct comprised a CD8α signal peptide,a humanized GPC3-specific scFv (known as “hu9F2”),a CD8α hinge region,and a CD28 transmembrane region followed by the intracellular domains of CD28 and CD3ζ.The GPC3-CAR-NK-92 cells showed potent cytotoxicity and cytokine production upon encountering HCC cells with either high or low GPC3 expression,bothin vitroandin vivo.Notably,the GPC3-CAR-NK-92 cells were cytotoxic to GFP3-negative HCC cells.Huanget al[40] made a further modification to GPC3-CAR-NK-92 cells by replacing the intracellular domains of CD28 and CD3ζ with the costimulatory domains of DNAM1 and 2B4.DNAM1,also known as CD226,is a costimulatory receptor in cytotoxic T cells,NK cells,and monocytes[41].DNAM1 cross-linking results in phosphorylation of its cytoplasmic tyrosine residues and drives NK cell cytotoxicity[42].2B4,as above-mentioned,is known as CD244 and harbors a cytoplasmic domain containing immunoreceptor tyrosine-based switch motifs (commonly known as ITSMs) that are responsible for interacting with multiple signaling adaptors and transmitting activating signals[43].This modification endowed GPC3-CAR-NK-92 cells with faster expansion,lower apoptosis and higher cytotoxic abilities than their original counterparts[40].
Tsenget al[44] reported that NK cells transduced with a CAR that targets CD147,a cell surface marker that is significantly upregulated in HCC,can effectively kill various malignant HCC cell linesin vitro,as well as HCC tumors in xenograft and patient-derived xenograft mouse models.In this study,the CD147-CAR contained the scFv of the anti-CD147 antibody derived from clone 5F6 with optimization,a human IgG1-CH2CH3 spacer,a transmembrane domain of CD28,the intracellular domain of CD28-4-1BB,and intracellular signaling domains of the TCR-ζ chain.The CARencoding retrovirus was transduced into the NK-92MI cell line,which is an IL-2-dependent NK cell line derived from PBMCs obtained from a patient with rapidly progressive non-Hodgkin's lymphoma.These CD147-CAR-NK-92MI cells were effectively activated by CD147+ HCC cell lines and demonstrated impressive cytotoxicity against the target HCC cell lines.When using primary PBMC-derived NK cells to prepare CD147-CAR-NK cells,the researchers observed efficient killing effects on susceptible target cells,including SKHep1,Huh7,and HepG2,etc.Furthermore,primary NK cells isolated from different zones of HCC liver tissue and engineered to express CD147-CAR can kill CD147+ HCC cell lines selectively and specifically.This study offers a valuable insight into manufacturing CD147-CAR-NK cells as either an autologous or an allogeneic off-the-shelf cell-based product.
Bouattouret al[45] developed c-MET-CAR-NK cells and tested the efficacy against HCC cell lines.c-MET is the product of the proto-oncogeneMETand acts as a tumorigen in HCC,since enhanced c-MET activity initiates and contributes to the progression of HCC.The CAR construct contained an scFv of an anti-c-MET antibody fused with truncated human EGFR (referred to as huEGFRt).The intracellular domain of the CAR comprised the 4-1BB and the DAP12 cytoplasmic domain.c-MET-CARencoding lentivirus was then prepared and transduced into human PBMC-derived NK cells.The resultant c-MET-CAR-NK cells remarkably killed HepG2 cellsin vitro;however,no data were generated to demonstrate the effect of c-MET-CAR-NK cellsin vivo.Of note,c-MET-CAR-NK cells could be potentially used to treat other solid tumors,because the upregulation of c-MET has been found in breast cancer,lung cancer,and colorectal cancer[46-48].
CHALLENGES TO BE ADDRESSED
Although CAR-NK cells have demonstrated their potency and advantages in tumor killing,technical or clinical issues remain to be addressed that will optimize CAR-NKbased immunotherapy against HCC.
First and foremost,stringent screening of HCC-specific antigens is necessary to minimize or avoid severe adverse effects due to on-target/off-tumor toxicity.Ontarget/off-tumor toxicity arises from the simultaneous expression of a target antigen on both tumor and healthy tissues[49].This toxicity,sometimes lethal,can be reduced by careful design of CAR constructs that improve the recognition of tumor cells.One such approach is to affinity-tune CARs so that they detect tumor cells with a high density of surface antigens and do not react against normal cells that have low antigen densities[50].Altering the scFv binding domainviamutagenesis or recombination of heavy and light chains can genetically tune the affinity of a CAR to fulfill this purpose[51,52].The intracellular signaling domain of a CAR can also be tuned to induce a moderate activating signal to weaken CAR-mediated direct cytotoxicity.
Another issue is the promotion of CAR-NK cell expansion and persistencein vivo.Insufficientin vivoexpansion of CAR-NK cells after adoptive transfer owing to unidentified factors is a particularly serious challenge.A recent clinical trial has demonstrated that incorporating IL-15 into CAR-NK cells remarkably augmentedin vivoexpansion[53].
The third issue involves the effect of CAR-NK cells on solid tumors like HCC,which remains unsatisfactory.The heterogeneity of solid tumors,the harsh tumor microenvironment that induces NK cell exhaustion,the limited infiltration from the bloodstream into tumor sites,and the immunosuppression caused by immunosuppressive cells and metabolic disturbances comprehensively inhibit CAR-NK cell function[54].More efforts have to be made to deal with these challenges,one-by-one,to build more efficient CAR-NK cells against HCC and other solid tumors.With the advance of genetic engineering science,CAR-NK cells equipped with geneticallyincorporated cytokines,antibodies,survival factors,constitutively-active signaling molecules,etc.will greatly promote NK cell proliferation,persistence,migration,and penetration into HCC.
CONCLUSION
CAR-NK cell therapy is an emerging immunotherapy against tumors,including HCC.Its advantage in manufacturing off-the-shelf cellular therapy products with high clinical availability and safety makes it a promising anti-HCC approach.Recent breakthroughs in genome editing techniques have potentiated the production of novel CAR-NK cells with high anti-HCC specificity and activity and low on-target/offtumor toxicity.Innovative engineering tools including CRISPR-Cas9,zinc finger nucleases (referred to as ZFNs),transcription activator-like effector nucleases (referred to as TALENs),and meganucleases are expected to revolutionize the design and creation of CAR constructs for NK cells in the future.With the great efforts being made to enhance safety and activity through laboratory studies and clinical trials,we are confident in the eventual overcoming of the remaining challenges to CAR-NK cell therapy,bringing hope to HCC patients and their families.
 World Journal of Gastrointestinal Oncology2021年12期
World Journal of Gastrointestinal Oncology2021年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Critical biomarkers of hepatocellular carcinoma in body fluids and gut microbiota
- Minimally invasive surgical treatment of intrahepatic cholangiocarcinoma:A systematic review
- Anatomic resection improved the long-term outcome of hepatocellular carcinoma patients with microvascular invasion:A prospective cohort study
- Clinical features of intracerebral hemorrhage in patients with colorectal cancer and its underlying pathogenesis
- Comparison of tumor regression grading systems for locally advanced gastric adenocarcinoma after neoadjuvant chemotherapy
- Hepatocellular carcinoma surveillance and quantile regression for determinants of underutilisation in at-risk Australian patients
