Lymphocyte count predicts the severity of COVID-19:Evidence from a meta-analysis
Yi-Si Zhao,Ying-Xi Yu
Abstract BACKGROUND In December 2019,coronavirus disease 2019 (COVID-19) was reported firstly in Wuhan,China. COVID-19 is currently a global pandemic.AIM To assess the suitability of lymphocyte count as a biomarker of COVID-19 severity.METHODS Five literature databases (PubMed/MEDLINE,Web of Science,Google Scholar,Embase,and Scopus) were searched to identify eligible articles.A meta-analysis was performed to calculate the standard mean difference (SMD) and 95%confidence interval (CI) of lymphocyte counts in coronaviral pneumonia cases.RESULTS Eight studies,including 1057 patients,were integrated in the meta-analysis.Lymphocyte counts were associated with severe coronavirus (CoV) infection(SMD=1.35,95%CI:1.97 to 0.37,P < 0.001,I2=92.6%).In the subgroup analysis stratified by prognosis,lymphocytes were associated with CoV infection mortality(n=2,SMD=0.42,95%CI:0.66 to 0.19,P < 0.001,I2=0.0%),severity (n=2,SMD=0.93,95%CI:1.20 to 0.67,P < 0.001,I2=0.0%),and diagnostic rate (n=4,SMD=2.32,95%CI:3.60 to 1.04,P < 0.001,I2=91.2%).CONCLUSION Lymphocyte count may represent a simple,rapid,and commonly available laboratory index with which to diagnosis infection and predict the severity of CoV infections,including COVID-19.
Key Words:COVID-19;Lymphocyte count;Coronavirus;Severe of disease;Meta-analysis
INTRODUCTION
In December 2019,in Wuhan,China,a novel CoV was identified as the causative agent of a novel pneumonia.The disease and the virus were subsequently termed coronavirus disease 2019 (COVID-19) and severe acute respiratory syndrome coronavirus 2(SARS-CoV-2),respectively,by the World Health Organization (WHO)[1,2].To date,COVID-19 has infected over 465 000 people in 199 countries,with nearly 21 000 deaths[3].These numbers continue to increase.As a CoV hypotype,SARS-CoV-2 is similar to the CoVs causing severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)[4,5].Both SARS and MERS spread rapidly worldwide,and have led to more than 10 000 human infections and 1000 deaths[4,6].The largest epidemiological study of COVID-19 performed by the Chinese CDC showed that 13.8% of all COVID-19 cases were severe,and 4.7% were critical[7].Critical patients,with a 49% case fatality rate,are at the most risk of death from COVID-19[7].Therefore,it is important to develop a rapid,simple clinical method to identify severe COVID-19 cases.
Lymphocytes play a decisive role in the maintenance of immune homeostasis and the inflammatory response[8].During the progression of an infectious disease,lymphocyte count reflects immune function and inflammatory state.SARS-CoV-2 invadesviathe respiratory mucosa,triggering a series of immune responses,including a cytokine storm,and affecting immune components,such as peripheral blood leukocytes and lymphocytes[9].Consistent with this,studies have shown that lymphopenia,particularly the depletion of CD4 and CD8 lymphocytes,is a clinical characteristic of COVID-19 patients,especially those with severe infections[10-12].Indeed,previous studies have shown that the decrease in lymphocyte count can be used to indicate the severity of SARS and MERS,both of which are CoV infections[13,14].However,due to the lack of analytical data,as might be provided by case–control or cohort studies,it is unclear whether lymphocyte count can also be used to reflect COVID-19 severity.Although Tanet al[15] had reported that lymphocyte count predicted the severity of COVID-19,this study had too few samples.However,relying on the association of CoV infection,we found some evidence in a small number of observational studies of COVID-19.Here,we performed a systematic review and meta-analysis to evaluate the diagnostic and prognostic utility of lymphocyte count in patients with viral pneumonia caused by CoV infections.Our aim was to explore the possibility that lymphocyte counts can predict COVID-19 severity and provide associated evidence.
MATERIALS AND METHODS
Search strategy and selection criteria
This protocol was registered with the PROSPERO international prospective register of systematic reviews (CRD42020177132,available from:https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=177132) and followed the recommendations established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[16].We conducted a systematic review across five literature databases:PubMed/MEDLINE,Web of Science,Google Scholar,Embase,and Scopus.The search terms used were as follows:“l(fā)ymphocyte count,” and“pneumonia,viral” or “SARS” or “MERS” or “COVID-19”.All of the searches were concluded by March 21,2020,and two researchers independently evaluated the search results.
Eligibility criteria
We included published peer-reviewed articles describing case–control,cohort,or cross-sectional studies,where lymphocyte counts were measured in peripheral blood samples from humans of any age with CoV infections.Case reports,conference abstracts,and review articles were excluded.
COVID-19 infections were diagnosed using next-generation sequencing or real-time reverse transcription polymerase chain reactions (RT-PCRs)[17].MERS-CoV was diagnosed according to the WHO criteria:a confirmed case was defined as a suspected case that was positive for MERS-CoV based on RT-PCR results[18].SARS infections were confirmed based on a definite exposure history,as well as either a positive RTPCR test during acute infection or detectable CoV-specific antibodies during convalescence[19].We excluded studies conducted exclusively in patients with active cancer,chronic liver disease,HIV,or immunosuppression.When an article reported duplicate information from the same patient,the reports were combined to obtain complete data,but the case was only counted once.
Study selection
All of the titles and abstracts returned by the database search (Figure 1) were reviewed by first author (Zhao YS) independently to assess the need for a full-text review.Any disagreements were resolved through discussion between the same author and another author (Yu YX).Reasons for exclusion were recorded.
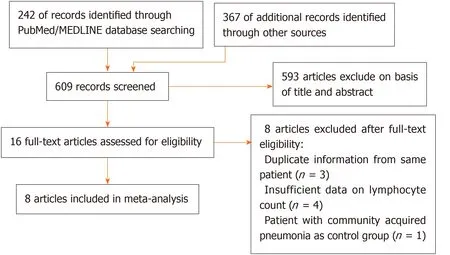
Figure 1 Article selection process.
Assessment of risk of bias
Publication bias was assessed using a funnel plot,Begg’s test and Egger’s test.
Statistical analysis
According to a study by Hozoet al[20],we estimating the mean and standard deviation from the median and range,and the size of a sample as data extrapolation.When sample size was small (n< 25),we used a simple formula:x=(a+2m+b)/4 to estimate the mean (x) using the values of the median (m),low and high end of the range (a and b,respectively).As soon as sample size exceeded 25,the median itself was the best estimator.When sample size was small (n< 15),we used the formula:S2=1/12 × (((a -2m+b)2)/4+(b -a) 2) to estimate the standard deviation (S2).When the sample size increased (15 <n< 70),Range/4 was the best estimator for S2.For large samples (n> 70) Range/6 was the best estimator for S2[20].To indicate the severity of CoV infection,we designed a cohort that combined three different measures of prognosis with respect to the control group:diagnosedvsnondiagnosed,severevsnonsevere,and deathvssurvival.The control group included nondiagnosed,nonsevere and survival groups.The case group was severe,including diagnosed,severe and death groups.
We used the random-effects model to calculate the standard mean difference (SMD)and 95% confidence interval (CI) for lymphocyte count in the CoV-infection patients and to draw a forest plot.Subgroup analysis was performed based on the study definition of severity.Heterogeneity between pairs of studies was quantified using theI2statistic.We investigated potential sources of heterogeneity,including prognosis and data source (original datavsextrapolated data),by performing subgroup analyses.One was prognosis subgroup that was divided into mortality,severity and diagnostic rate subgroups according to different prognosis investigated in included studies.One was data source subgroup that was divided into original data and extrapolated data subgroups.The criterion was whether the data extrapolated according to the study by Hozoet al[20].All of the analyses were performed using Stata version 14 (Stata Corp.,College Station,TX,USA).
RESULTS
Results of the electronic search
We identified 609 articles using database searches.After screening,16 articles remained.After examination of the full-text articles,we excluded eight of these articles:three contained duplicate information from same patient;four articles had insufficient data on lymphocyte count;and one article chose a patient with community-acquired pneumonia as a control group.Therefore,eight studies were included in our meta-analysis[10,13,14,19,21-24].
Characteristics of the included studies
The characteristics of the eight included studies are summarized in Table 1.All of the studies included were prospective or retrospective case–control studies,cohort studies,or cross-sectional studies.The patient population in each study ranged from 30 to 346 (1057 patients in total).The earliest publications were from 2003[22,24],and the most recent article was published in 2020[10,21,23].Most studies were based in China (n=7),including Taiwan (n=3)[13,19,24],Wuhan (n=2)[10],Beijing (n=1)[22],and Shanghai (n=1)[23].One study was based in Saudi Arabia[14].The CoV diseases studied were COVID-19 (n=3;37.5%)[10,21,23],MERS (n=1;12.5%)[14],and SARS (n=4;50%)[13,19,22,24].Four studies (50%) investigated prognosis with respect to diagnostic rate (diagnosedvsnondiagnosed)[19,22-24],two studies (25%) investigated prognosis with respect to severity (severevsnonsevere)[10,21],and two studies(25%) investigated prognosis with respect to mortality (deathvssurvival)[13,14].
Characteristics of lymphocyte count
The means and standard deviations of lymphocyte counts from four studies[10,14,21,23] were extrapolated from sample size,median,and IQR (Table 1).In the forest plot,if the SMD (95%CI) was < 0,it showed that the mean and standard deviation of lymphocyte counts in the case group were less than those in the control group.That meant lymphocyte counts were less in severe CoV infection.Analysis showed that lymphocyte counts were associated with severe CoV infection (SMD=1.35,95%CI:1.97 to 0.37,P< 0.001,I2=92.6%) (Figure 2).There was heterogeneity among studies.Therefore,subgroup analysis,stratified by prognosis and data source,was performed.In the subgroup analysis stratified by prognosis,lymphocytes were associated with mortality due to CoV infection (n=2,SMD=0.42,95%CI:0.66 to 0.19,P< 0.001,I2=0.0%),with severity of CoV infection (n=2,SMD=0.93,95%CI:1.20 to 0.67,P< 0.001,I2=0.0%),and with the diagnostic rate of CoV infection (n=4,SMD=2.32,95%CI:3.60 to 1.04,P< 0.001,I2=91.2%) (Figure 3).In the subgroup analysis stratified by data source,lymphocytes were associated with both the extrapolated data (n=4,SMD=0.92,95%CI:1.33 to 0.51,P< 0.001,I2=64.0%) and the original data (n=4,SMD=1.97,95%CI:3.35 to 0.60,P< 0.001,I2=96.5%) (see Supplementary Figure 1,which illustrates the forest plot of subgroup analysis stratified by data source),explaining the observed overall heterogeneity among studies.In addition,because few studies were included in our analysis,it was unclear whether the funnel plot was symmetrical(Figure 4),but Begg’s test and Egger’s test showed that publication bias had no significant effects on the results of the meta-analysis (P=0.174).
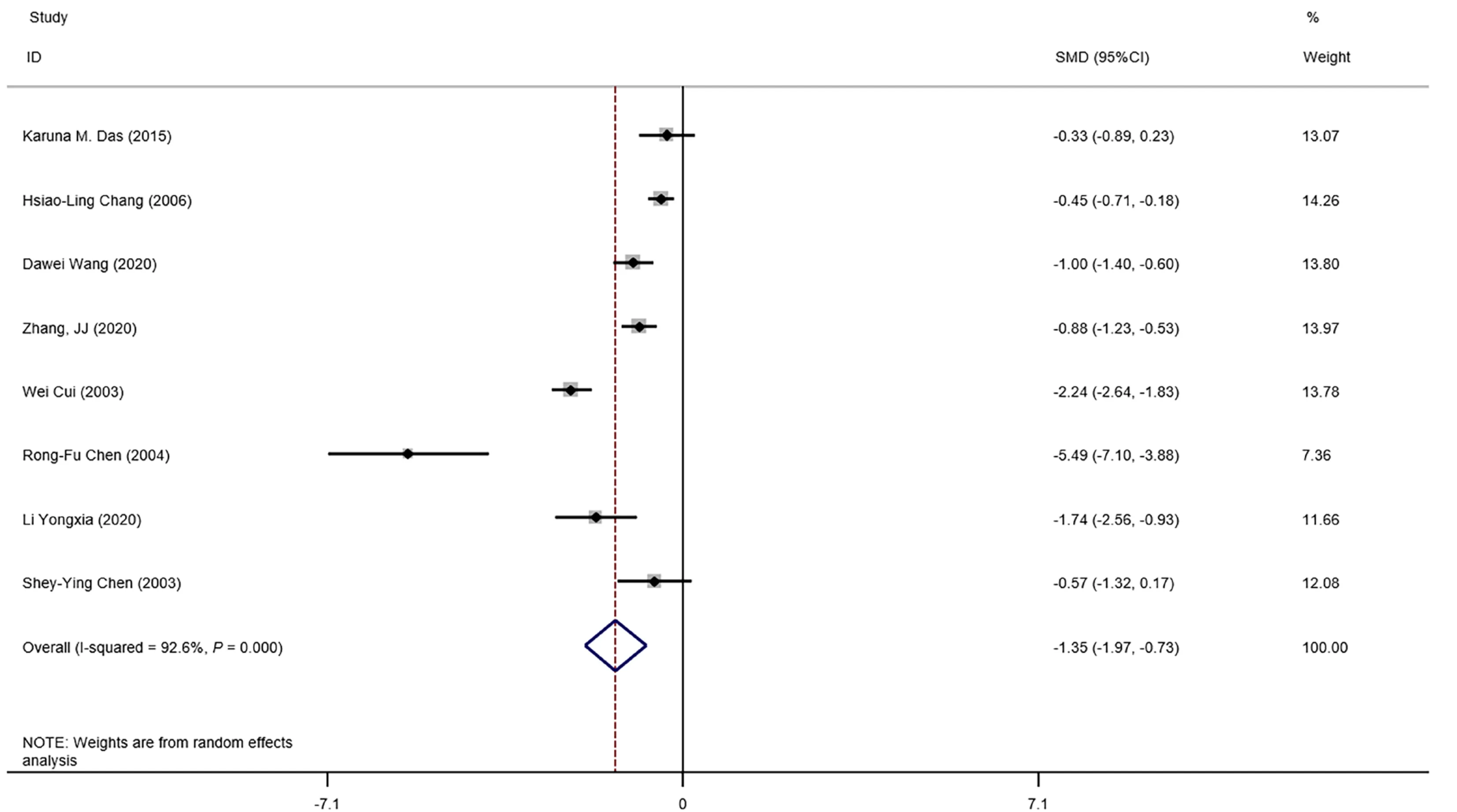
Figure 2 Forest plot:overall identification of lymphocyte count in patients with coronaviral pneumonia.P =0.000 means P < 0.001.CI:Confidence interval.
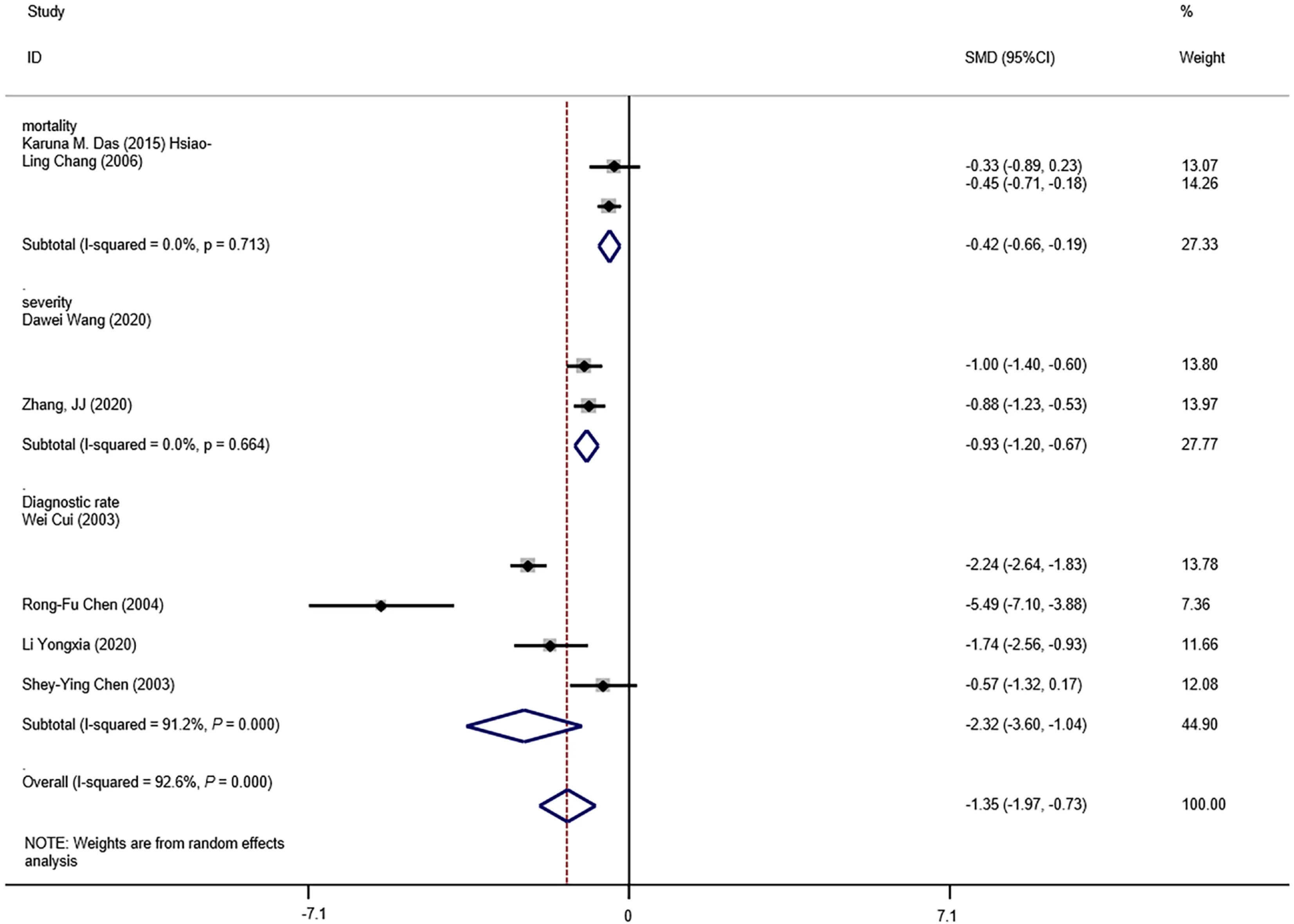
Figure 3 Forest plot:overall identification of lymphocyte count in patients with coronaviral pneumonia,subgroup analysis stratified by prognosis.P =0.000 means P < 0.001.CI:Confidence interval.
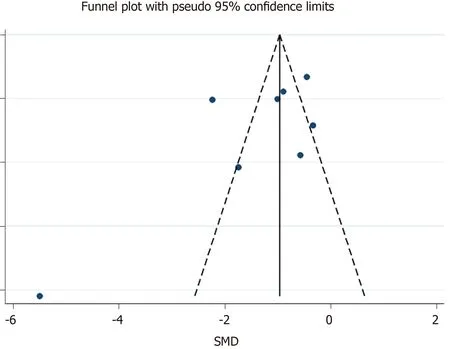
Figure 4 Funnel plot:publication bias of eight studies included.
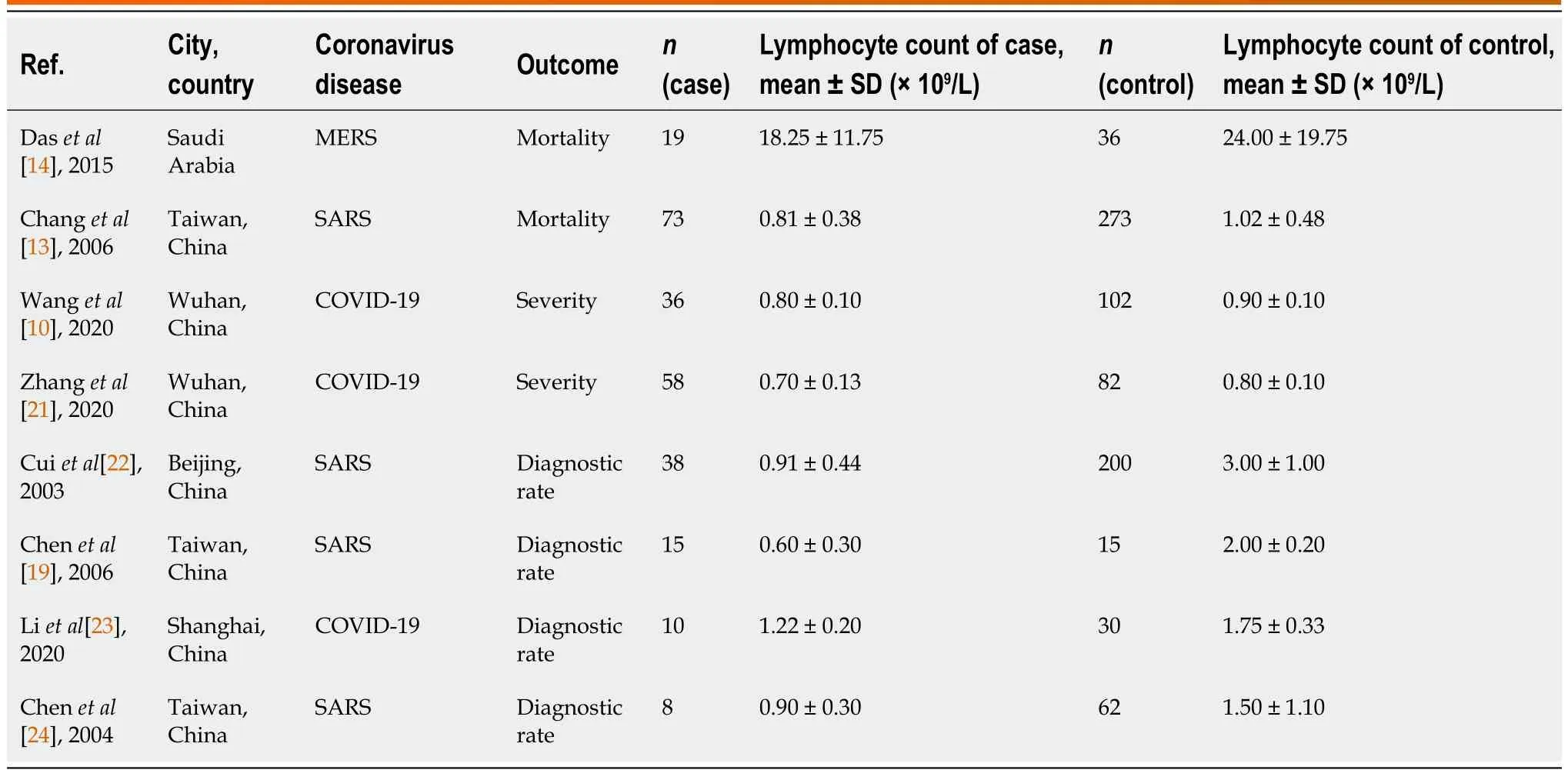
Table 1 Characteristics of the included studies
DISCUSSION
COVID-19 is rapidly infectious and highly severe,with a high mortality rate[3].Patients with COVID-19 exhibit a wide range of variability in disease severity.In clinical practice,we believe that low levels of lymphocytes are disadvantageous for COVID-19 patients.Because lymphocyte count reflects disease characteristics,it might potentially help in the evaluation of COVID-19 severity.Usefully,this measure is easily available in laboratory tests.
Lymphocytes are vital cells that maintain immune function and execute the immune response in the body[8].In the best-case scenario,the cellular immune response rapidly clears CoV with little or no clinical signs of infection.Alternatively,the virus causes a state of immunosuppression,which debilitates and sometimes overwhelms the host’s defenses[25].Currently,no detailed study of the immunological response to SARS-CoV-2 is available.Thus,we must rely on previous studies of other CoVs,especially SARS-CoV and MERS-CoV[26].Based on other CoVs,SARS-CoV-2 might induce a T-lymphocyte-mediated protective immune response[25].However,lymphopenia is associated with many types of infections,including coronavirus[14,27].Studies have shown that lymphopenia is related to cell apoptosis in SARS patients[19,28].In addition,hospitalized patients infected with SARS-CoV-2 frequently manifest lymphopenia,suggesting that cellular immune responses may be suppressed[10,21].However,lymphopenia in MERS but not SARS cases could be a result of direct infection of T cells and infection-induced apoptosis[29].Although the lymphopenia in patients with SARS and those with MERS may have different mechanisms,they forecast similar outcome.Furthermore,lymphocyte count as a biomarker to predicting severity in other non-CoV diseases (such as measles,herpes and vaccinia) is effective[19].Therefore,the hypothesis which predictive role of lymphopenia in COVID-19 is reasonable,and some recently study also provide some evidence to prove this hypothesis[15].However,studies of the role of lymphocytes in COVID-19 are rare.
In this study,we designed a meta-analysis to explore the feasibility of using lymphocyte count to predict COVID-19 severity.All of the eight studies included in our meta-analysis involved SARS,MERS or COVID-19,and were prospective or retrospective case–control,cohort or cross-sectional studies (Table 1).Existing research results do not always rank the severity of COVID-19 infections using the same criteria.Even during the earliest part of the Chinese outbreak,the guidelines for COVID-19 diagnosis and treatment issued by the Chinese National Health Commission were revised seven times based on clinical experiences[30].Therefore,we roughly evaluated disease severity using paired concepts:diagnosedvsnondiagnosed [19,22-24];severevsnonsevere[10],and deathvssurvival[13,14].
We found that lymphocyte count was associated with severe CoV infection(Figure 2).Lymphocytes were also associated with CoV mortality,severity and diagnostic rate (Figure 3).Thus,lymphocyte count may represent a simple,rapid,and commonly available diagnostic and severity-prediction tool for CoV infections,including COVID-19.Because we included studies of SARS-CoV-2 infections,our results indicate the value of lymphocyte count for the assessment of COVID-19 severity.A recent related study suggested that lymphocyte percentage might be a reliable indicator of moderate,severe or critical infections,independent of auxiliary indicators[15].However,this study included few severe (n=39) and critical (n=28)cases[15].In combination,our analysis and the most recent research results suggested that lymphocyte counts potentially reflect COVID-19 severity.Meanwhile,some research has shown that the viral load of SARS-CoV-2 is associated with the lymphocyte count:A study of interleukin (IL)-3 (a cytokine produced by T lymphocytes) discovered that patients with high viral load presented had lower plasma IL-3 levels than low viral load[31].CD4:CD8 ratio and T regulatory cells significantly decrease in mice with high viral load[32].Not only T cells,but also B cells and natural killer cells in patients with severe COVID-19 were significantly lower than those in patients with the mild form[33,34].Lymphopenia in COVID-19 may have several underlying causes,including the destruction of lymphatic organs,the direct attack on lymphocytes by SARS-CoV-2[35],lymphocyte apoptosis due to the continual release of inflammatory cytokines,or,in severe cases,the inhibition of lymphocytes by hyperlactacidemia[36].It is clear that hyperlacticacidemia-and cytokine-storm-related acute respiratory distress syndrome is the primary presentation of patients with critical COVID-19[30].Thus,lymphocyte count is an important indicator of severe or critical COVID-19.
For the acute outbreak of COVID-19,the meta-analysis has limitations.We faced a shortage of data sources during the research.Even now,the data on COVID-19 are insufficient.The research results in the previous response to CoVs should be used for reference.The most mainstream of these were SARS and MERS.As other important CoV diseases,they have similarities to COVID-19.From the perspective of the important characteristics of respiratory infections,SARS and MERS could be included in the reference category.The prevention and treatment methods of COVID-19 all often refer to methods of SARS and MERS.A meta-analysis about prevention of person-to-person transmission of COVID-19 also included SARS and MERS coincidentally[37].Studies have shown that lymphopenia is related to apoptosis in SARS[28].Lymphopenia is the result of direct T cell infection and infection-induced apoptosis in MERS[29].Although we think that this conclusion is insufficient,because there are some treatments (for example:hormone use,antibody titer,etc.) that affect the lymphocyte count in the clinic.However,from the current situation of prevalence,spread and treatment,it is of reference value.In studies that were included in the meta-analysis,five referred to lymphocyte counts that were obtained on the day of hospital admission,and two studies did not report clearly the time that lymphocyte counts were obtained.In clinical research,because it is difficult to make a complete review of the course of disease admission,it is generally accepted to choose the day/time of hospital admission as the sampling point[10].It has been shown that the degree of lymphopenia is steady during the first week[38],and lymphocyte counts progressively decrease and reach their lowest point on day 14 in most cases[38].Therefore,our analysis did not divide these studies by time of lymphocyte count acquisition.One week of hospital admission could be recognized as a stable interval for lymphocyte counts.However,a recent study showed that lymphocyte count varied at different times of hospitalization[39].Thus,further study of lymphocyte counts at varied times could increase the predicted efficiency in COVID-19.
CONCLUSION
Our meta-analysis provides information on three simple and common interventions to combat the immediate threat of COVID-19,while new evidence on pharmacological treatments,vaccines,hematology monitoring and other personal protective strategies is being generated[37].It showed that lymphocyte count may represent a simple,rapid and commonly available laboratory index with which to diagnosis infection and predict the severity of CoV infections,including COVID-19.However,our study was limited by the lack of COVID-19 studies,especially those with homogeneous or uniform standards.Therefore,it was necessary to include SARS and MERS in our study;both of which can lead to coronaviral pneumonia.Thus,these diseases might represent a reference for COVID-19.During the current severe COVID-19 pandemic,we hope to provide a framework for the future predictions of COVID-19 severity.Indeed,if critical infections could be predicted and treated earlier,mortality would be lower.Lymphocyte count is a potential indicator of COVID-19 severity and deserves further examination.However,the high heterogeneity among studies suggests that additional research,including case–control and cohort studies,is required for detailed analyses of the relationship between lymphocyte count and COVID-19 severity.
ARTICLE HIGHLIGHTS
Research background
In December 2019,coronavirus disease 2019 (COVID-19) was reported first in Wuhan,China.COVID-19 is currently a global pandemic.
Research motivation
COVID-19 with high morbidity is a life-threatening disease globally.It is important to develop a rapid,simple clinical method to identify severe COVID-19 cases.
Research objectives
The aim of this study was to assess the suitability of lymphocyte count as a biomarker of COVID-19 severity.
Research methods
We searched five literature databases (PubMed/MEDLINE,Web of Science,Google Scholar,Embase,and Scopus) to identify eligible articles.A meta-analysis was performed to calculate the standard mean difference (SMD) and 95% confidence interval (CI) of lymphocyte counts in coronaviral pneumonia cases.
Research results
Our research integrated eight studies,including 1057 patients.Lymphocyte counts were associated with severe coronavirus (CoV) infection (SMD=1.35,95%CI:1.97 to 0.37,P< 0.001,I2=92.6%).In the subgroup analysis stratified by prognosis,lymphocytes were associated with coronavirus infection mortality (n=2,SMD=0.42,95%CI:0.66 to 0.19,P< 0.001,I2=0.0%),severity (n=2,SMD=0.93,95%CI:1.20 to 0.67,P< 0.001,I2=0.0%),and diagnostic rate (n=4,SMD=2.32,95%CI:3.60 to 1.04,P< 0.001,I2=91.2%).
Research conclusions
Lymphocyte count may represent a simple,rapid and commonly available laboratory index with which to diagnosis infection and predict the severity of CoV infections,including COVID-19.
Research perspectives
As a CoV hypotype,severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is similar to the CoVs causing severe acute respi-ratory syndrome and Middle East respiratory syndrome.Hence,we performed a systematic review and meta-analysis of the literature to evaluate the diagnostic and prognostic utility of lymphocyte count in patients with viral pneumonia caused by CoV infections.Our aim was to explore the possibility that lymphocyte counts predict COVID-19 severity and provide associated evidence.
ACKNOWLEDGEMENTS
This article also commemorates the Chinese medical staff who gave their lives in the fight against COVID-19.
 World Journal of Clinical Infectious Diseases2021年3期
World Journal of Clinical Infectious Diseases2021年3期
- World Journal of Clinical Infectious Diseases的其它文章
- Prevalence of anal human papillomavirus infection in patients with human immunodeficiency virus infection:A systematic review
- Can a radioimmunoass a y kit be developed for accurate detection of the S proteinof sev ere ac ute respirator y syndrome coronavirus 2?
