Prevalence of non-tuberculosis mycobacteria among samples deposited from the National Tuberculous Reference Laboratory of Iran (2011-2018)
Saman Ayoubi, Parissa Farnia, Poopak Farnia, Jafar Aghajani, Jalaledin Ghanavi, Ali Akbar Velayati
1Mycobacteriology Research Center (MRC), National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Biotechnology, School of Advanced Technology in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
ABSTRACT
KEYWORDS: Diagnosis; Iran; Non-tuberculosis Mycobacterium;Prevalence
Significance
The prevalence of non-tuberculosis mycobacteria has not been taken seriously so far, and no detailed studies have been conducted on the prevalence of non-tuberculosis mycobacteria in the world. In this study, in comparison with other researches,isolation and identification of non-tuberculosis mycobacteria from other mycobacteria with primers and standard method designed in Mycobacteriology Research Center, Tehran, Iran has been done. As treatment for NTM differs from that for M.tuberculosis, accurate detection of Mycobacterium sp. is of public health significance.
1. Introduction
Tuberculosis (TB) is one of the oldest diseases of mankind[1]and still remains a major cause of morbidity and mortality of 10 million and 1.4 million people respectively in 2019 worldwide,with Mycobacterium (M.) tuberculosis the predominant causative agent[2], followed by non-tuberculosis mycobacteria (NTM)[3].NTM are found in the environment, such as food, soil and water and infections in animals are often zoonotic[4-6]. NTM infection in humans is on the rise in most countries around the world[3].Pathogenic NTM species include M. abscessus, M. avium, M.ferrititum, M. intracellularare, M. kansasii, and M. simiae[7-11]. NTM can infect a variety of human organs, such as lung, skin and soft tissue, particularly among immunocompromised individuals[12-18].Given their widespread presence in the environment and global distribution, epidemiological surveillance of NTM is of particular importance[14,19,20]. In Iran, prevalence of NTM from 2002 to 2006 was 12%[7]. However, in 2011, NTM prevalence in Isfahan was reported as high as 62%[21,22], but in Khuzestan Province in 2016, prevalence of only 30% was reported[23]. Thus, accurate identification of Mycobacterium sp. is critical as all patients with acidfast bacterial infection, especially those with respiratory symptoms,are diagnosed as having TB infection, which requires isolation and/or long term antibiotic treatment[24-26]; whereas NTM infection causes a wide range of diseases[27-30]and NTM are sensitive to a different set of antibiotics used to treat TB[31-33].
Given the importance of the growing prevalence of diseases caused by NTM worldwide, studies have been conducted to evaluate the prevalence of NTM in Iran[30,34]. Here, we report NTM prevalence and species in samples deposited from National Tuberculosis Reference Laboratory of Iran from 2011 to 2018 to determine the extent of the disease so that appropriate measures can be carried out to control and eliminate this public health problem in the country.
2. Materials and methods
2.1. Study method and sample collection
This cross-sectional descriptive study was performed on 15771 sputum samples obtained from patients presenting with pulmonary tuberculosis symptoms and sent to the Mycobacteriology Research Center, Tehran, Iran from 2011-2018. Samples came from provinces all across the country for mycobacterial tests to confirm presence of M. tuberculosis (Ethical approval: IR.SBMU.NRITLD.REC.1397.569).
2.2. Laboratory procedures
Initial isolation of Mycobacterium spp. was performed on Lowenstein-Jensen media (Bio-Rad, Marnes-la-Coquette,France) [25]. Subsequently, mycobacterial DNA was extracted from samples using a QiAamp DNA kit (QIAGEN, Germantown,MD)[26]. In order to identify M. tuberculosis, a 190 bp fragment of IS6110 insertion sequence was amplified using primers Tb1 (5′-ATCCTGCGAGCGTAGGCGTCGG-3′) and Tb2 (5'-CAGGACCACGATCGCTGATCCGG-3′) in a reaction mixture(50 μL) containing 8 pmol each of Tb1 and Tb2 primers, 10×buffer (QIAGEN, Germantown, MD), 1.5 mM MgCl, 0.2 mM dNTPs, 2% (v/v) dimethylsulfoxide (DMSO), 1 U Taq polymerase(QIAGEN, Germantown, MD), and 1 μg of DNA[35]. Thermocycling was done as follows: 95 ℃ for 10 min; 35 cycles of 93 ℃ for 20 sec, 65 ℃ for 60 sec and 72 ℃ for 20 sec; and a last step of 72 ℃ for 5 min. Amplicons were analyzed by 1.5% agarose gelelectrophoresis and visualized under UV illumination by ethidium bromide staining. M. tuberculosis strain H37Rv[28]was used as reference control. PCR-negative specimens were analyzed for NTM species using restriction fragment length polymorphism(RFLP)-PCR of a hsp65 fragment[36]. Nested-PCR was performed using primers Tb15 (5′-CGTAYGACGAAGAGGCCCGT-3′) and Tb17 (5′-WASGGRTCCTCSAGGACSGC-3′) were employed in the first PCR step in a reaction mixture (50 μL) containing 4 pmol each of primers Tb15 and Tb17, 10× buffer (QIAGEN,Germantown, MD), 1 mM MgCl, 1 mM dNTPs, 1% (v/v)DMSO, 1 U Taq polymerase (QIAGEN, Germantown, MD),and 1 μg of DNA, with thermocycling conditions as described above, except that 30 cycles and annealing temperature of 60 ℃were used. The second PCR step was performed using primer pairs Tb11 (5'-ACCAACGATGGTGTGTCCA-3') and Tb12 (5'-CTTGTCGAACCGCATACCCT-3') in a reaction mixture (50 μL)containing 8 pmol each of primers Tb11 and Tb12, 10× buffer(QIAGEN, Germantown, MD), 1.5 mM MgCl, 0.2 mM dNTPs,2% (v/v) DMSO, 1 U Taq polymerase (QIAGEN, Germantown,MD), and 1.5 μL of first-step reaction solution. Thermocycling was performed in the above described instrument as follows: 95 ℃ for 5 min; 30 cycles of 94 ℃ for 30 sec, 56 ℃ for 60 sec and 72 ℃ for 40 sec; with a last step of 72 ℃ for 10 min. Amplicons (439 bp) were digested with BstEⅡand with HaeⅢ(Sigma-Aldrich, Darmstadt,Germany) (1.5 U/μL, 60 ℃, 5 min) and analyzed by 8% acrylamide gel-electrophoresis and ethidium bromide staining. Fragment sizes were compared with patterns of reference NTM species and M.tuberculosis strain H37Rv (Table 1).
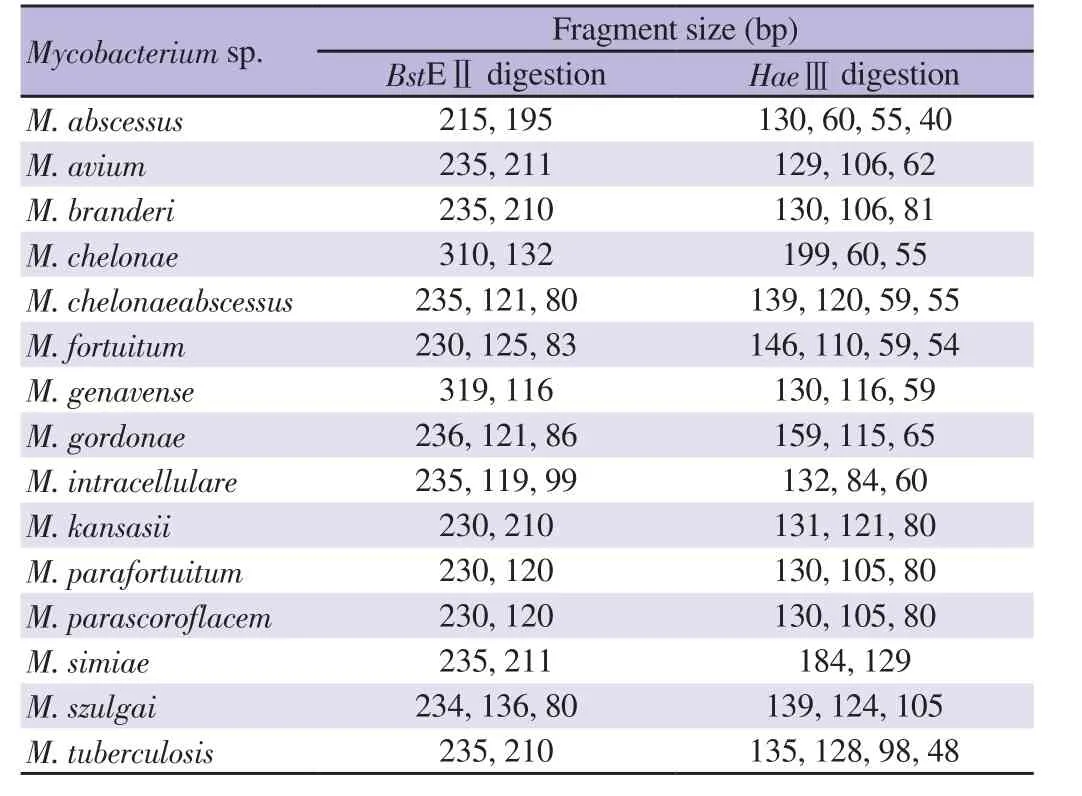
Table 1. Sizes of Mycobacterium spp. hsp65 amplicon fragment digested with BstEⅡ and HaeⅢ.
3. Results
Out of 15771 sputum specimens sent for examination at the Mycobacteriology Research Center, Tehran, Iran from 2011 to 2018, 6637 were identified as containing M. tuberculosis (presence of IS6110190-bp amplicon) while 9134 were negative, and of the latter samples, RFLP-PCR of hsp65 fragment identified 658 as positive with NTM and 670 with M. tuberculosis, resulting in a total of 42.08% (6637/15771) of the samples positive for Mycobacterium spp, consisting of 7307 (46.33%, 7307/15771) M. tuberculosis and the remaining NTM species. The most frequent NTM was M. simiae(56.08%, 369/658) followed by M. intracellulare (10.49%, 69/658),M. abscessus (10.18%, 67/658) and M. chelonae (8.97%, 59/658)(Table 2). Some representative RFLP-PCR amplicon profiles are shown in Supplementary Figure 1.
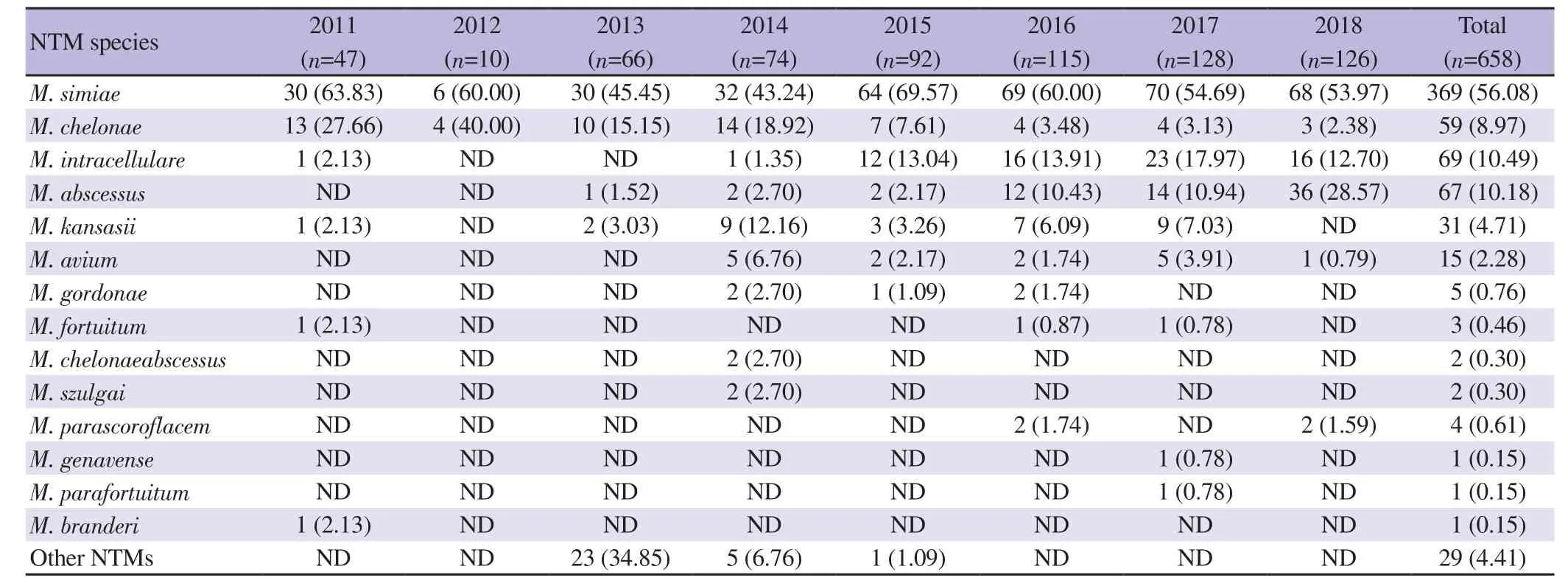
Table 2. Non-tuberculosis mycobacteria (NTM) detected in specimens at Mycobacteriology Research Center, Tehran, Iran (2011-2018) [n (%)].
During the period of the survey, the number of NTM species increased over the years, with M. simiae the major NTM species,ranging from 43%-70% of NTM-positive annual samples (Table 2).It is worth noting M. abscessus was first identified in 2013 (2% of NTM samples) and rose to 28% in 2018.
4. Discussion
The prevalence of NTM infection in several Asian and European countries have increased over the years, from 8.2 per 100000 population in 1999 to 16 per 100000 in 2013[2], a trend observed in the present study. The predominant NTM species varies depending on country. In USA, a survey in 2017 reported M. avium and M.fortuitum as the two major NTM species[37,38], while in Asian countries (India, Japan, Singapore, and Thailand), M. avium and M.kansasii are predominant[36,39]and in Turkey, M. fortuitum has the highest prevalence[40,41]. In the present study, the most major NTM was M. simiae, accounting for 56.08% of the samples examined.However, there are variations in the major NTM species identified in the country depending on location and period of the survey:from 2006 to 2008 in Isfahan, M. fortuitum is the most common NTM sp[38,39]; an earlier survey in 2010 of samples at the National Tuberculosis Reference Laboratory noted M. simiae as the most common[35,42]; and in Zahedan in 2014, based on conventional biochemical methods, the most common NTM species reported is M. kansasii[30,36,37]. As the samples examined in the present study came from all regions of the country, the data should be more representative of the national NTM prevalence in the human population, at least for bronchial infection. In USA, between 2008 and 2012, the prevalence of NTM has been noted to be on the rise among pulmonary patients[32,41].
In the present study, the second most abundant NTM species was M. intracellulare, a fast-growing mycobacterium (including other fast growing NTM species) that is found in water source and soil[32-35].Simple precautionary measures such as boiling drinking water can prevent from infection and subsequent pulmonary colonization[38,39].
Given the similarity of NTM species to M. tuberculosis,molecular methods are more accurate, faster and more sensitive than conventional tests for differentiating NTM species from M.tuberculosis[35-38]. However, absence of PCR amplification of IS6110 sequence does not mean absence of M. tuberculosis; in the present study, RFLP-PCR analysis of hsp65 revealed slightly more samples positive for M. tuberculosis compared to those NTM-positive[38,40]. As treatment of NTM infection differs from that of M.tuberculosis, their detection and identification are of great importance to physicians because choice of medication is species specific and certain drug combinations can lead to deleterious side effects,which can pose problems due to the lengthy dosage regimen (12-24 months) required for complete cure[40,41].
In conclusion, the study highlights the usefulness of molecular detection of not only M. tuberculosis but also non-tuberculosis mycobacteria that was present with significant prevalence throughout Iran. Despite the mistaken belief of certain physicians,these mycobacteria pose an important public health problem and their detection and identification should assist in appropriate antibiotic combination therapy and reduce the country’s burden of mycobacterial infection.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
We thank Professor Parissa Farnia for guiding and supporting our project.
Funding
This work was supported by funding from the Mycobacteriology Research Center, National Tuberculosis and Lung Research Institute(NRITLD), Masih Daneshvari Hospital (Grant No. 16597).
Authors’ contributions
Ali Akbar Velayati and Parissa Farnia conceived of the presented idea. Jalaledin Ghanavi developed the theory and performed the computations. Poopak Farnia verified the analytical methods. Saman Ayoubi and Jafar Aghajani carried out the experiment. Saman Ayoubi wrote the manuscript with support from Jafar Aghajani and Parissa Farnia. All authors discussed the results and contributed to the final manuscript.
 Asian Pacific Journal of Tropical Medicine2021年10期
Asian Pacific Journal of Tropical Medicine2021年10期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Clinical, biochemical and imaging characteristics of adrenal histoplasmosis in immunocompetent patients in a non-endemic area: A case series
- Prediction of malaria cases in the southeastern Iran using climatic variables: An 18-year SARIMA time series analysis
- Detection of dengue virus serotype 3 in Cajamarca, Peru: Molecular diagnosis and clinical characteristics
- Efficacy and safety of ivermectin for COVID-19: A systematic review and meta-analysis
- Favipiravir and its potentials in COVID-19 pandemic: An update
- Nix-TB and ZeNix trials: Paving the way for shorter regimens for drug-resistant tuberculosis
