Methodological quality assessment of meta-analyses of cognitive interventions among Alzheimer's disease
Guang-Hong Han, Xiao-Li Pang, Wei Wang, Hui-Li Sun
1 Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, China.
2 School of Nursing, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, China.
Abstract: Aims: The current study is designed to assess the methodological quality of the meta-analyses of cognitive interventions among Alzheimer's disease, and to investigate the compliance with 16 AMSTAR 2 items.Method:We searched Web of Science, Sinomed, PubMed, Embase, and Cochrane Library from 2016 to 2021, to get the meta-analyses of cognitive interventions in Alzheimer's disease.The AMSTAR 2 was used to assess the methodological quality.Furthermore, we also explored the compliance with AMSTAR.Results:We included 9 studies in our research.Of them, 6 articles were rated as "extremely low", 2 articles as "low" and 1 article as "high".Furthermore, the reporting rates for 16 AMSTAR 2 items ranged from 22.22% to 100%.Conclusion:The methodological quality of meta-analyses of cognitive interventions in Alzheimer's disease is not ideal, and there is still room for improvement.Future studies are supposed to explore the relevant factors that possibly influence the methodological quality.
Keywords:Alzheimer’s disease, AMSTAR, Methodological quality, Meta-analyses, Cognitive interventions
Introduction
Alzheimer's disease (AD) is the most common type of dementia, accounting for 60%~70% of all dementia, and has become the third most life-threatening disease after cardiovascular diseases and tumors [1].The World Health Organization reports that the total number of dementia patients worldwide in 2020 is about 50 million, with 10 million new cases occurring every year [1].The elderly population will reach 451 million in China by 2050, with 19.4 million people suffering from dementia [2, 3].Alzheimer's disease is characterized by a decline in memory function and cognitive ability that gradually worsen with age and affect thinking ability [4].However, there is no effective medicine to cure Alzheimer's disease [5].Cognitive intervention is a psychological intervention method, which affects individual behavior by improving individual thinking ability, to improve the outcome of the disease [6].Cognitive intervention is an essential nonpharmacological therapy for Alzheimer's disease, consisting of cognitive training, cognitive stimulation as well as cognitive rehabilitation [7].
Meta-analyses of cognitive interventions for Alzheimer's disease are gradually increasing.However, the methodological quality has not been reported.Poor methodological quality will hinder the evidence- based transformation of cognitive interventions for Alzheimer's disease.Therefore, this study will conduct a rigorous methodological quality assessment for meta-analyses of cognitive interventions in Alzheimer's disease.
Methods
Search Strategy
Five databases, consisting of Web of Science, Sinomed, PubMed, Embase, and Cochrane Library, were retrieved from 2016 to 2021, to get the meta-analyses of cognitive interventions in Alzheimer's disease.The search terms included: (Alzheimer Disease* OR Dementia* OR AD OR Alzheimer's Disease OR dementia*) AND ("cognitive stimulation" OR "cognitive retraining" OR "reality orientation*" OR "cognitive rehabilitation" OR "cognitive intervention*" OR "cognitive training") AND (systematic review and meta-analyses OR meta-analysis* OR Systematic Review*).
Inclusion and Exclusion Criteria
We developed the following inclusion criteria: (a) The people were suffering from AD; (b) The intervention approach was cognitive interventions; (c) The study type was systematic reviews or meta-analyses.We also developed the following exclusion criteria: (a) The people were suffering from mild cognitive impairment, temporo-frontal dementia or vascular dementia; (b) Cognitive interventions were not temporo-conducted; (c) The study type was overviews of systematic reviews or reviews.
Study Selection
Our two authors independently selected the included studies.They read the title and the abstract first.Then they read the full text.Finally, they identified the articles to be included in our study.In the whole literature screening process, any disagreement would be resolved through mutual negotiation or consultation with other authors.
Data Extraction
Our authors extracted the following information from the included studies, consisting of "sample size", "the type of original study", "first author", "the number of databases retrieved", "publication year", as well as "number of original studies".
Methodological Quality Assessment
Our authors used AMSTAR 2 (a measurement tool to assess systematic reviews),which was developed specifically to assess the methodological quality of systematic reviews and meta-analyses [8], to appraise the methodological quality of included meta-analyses.AMSTAR 2 consists of sixteen items, of which 7 items were considered key items.AMSTAR 2 divides the quality of meta-analyses into 4 grades, including high, medium, low as well as very low.AMSTAR item 11 and AMSTAR item 12 are merely fit for a meta-analysis, not a systematic review.
In our study, one point was given for "fully reported items" and 0 for "unreported items".Hence, the maximum score for systematic reviews was 14; the maximum score for meta-analyses was 16.The minimum score was 0.
Results
Search Results
We searched 1172 studies at first.843 studies remained after eliminating duplicates.15 studies remained after reading their titles as well as abstracts.At last, 9 studies remained after reading their full texts.The study selection process was demonstrated in Figure 1.
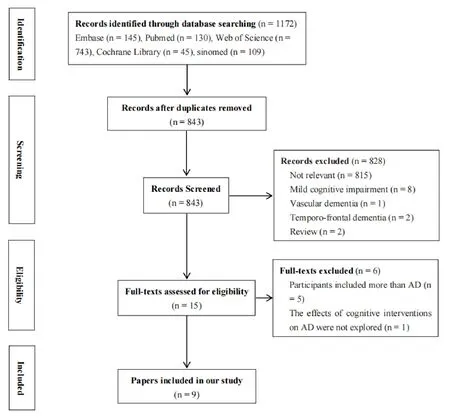
Figure 1.Flowchart of paper selection.
Essential Characteristics of Included Studies
Of the nine studies, six studies only included randomized controlled trials; One study only included cohort studies; One study included randomized controlled studies and case-control studies; And one study included randomized controlled studies and quasi-experimental studies.Essential characteristics of the studies included in our research were demonstrated in Table 1.
Methodological Quality
The AMSTAR score of the two systematic reviews included in our study was 9 and 11, respectively.The AMSTAR score of the seven meta-analyses included in our study ranged from 10 to 16.The methodological quality of 6 studies was rated as "very low", two studies as "low" and one study as "high".The methodological quality of the studies included in our study was demonstrated in Table 2.
Eleven of the 16 AMSTAR items had over 70% compliance with AMSTAR 2 statement.The reporting rates of AMSTAR 2 items were demonstrated in the Table 3.
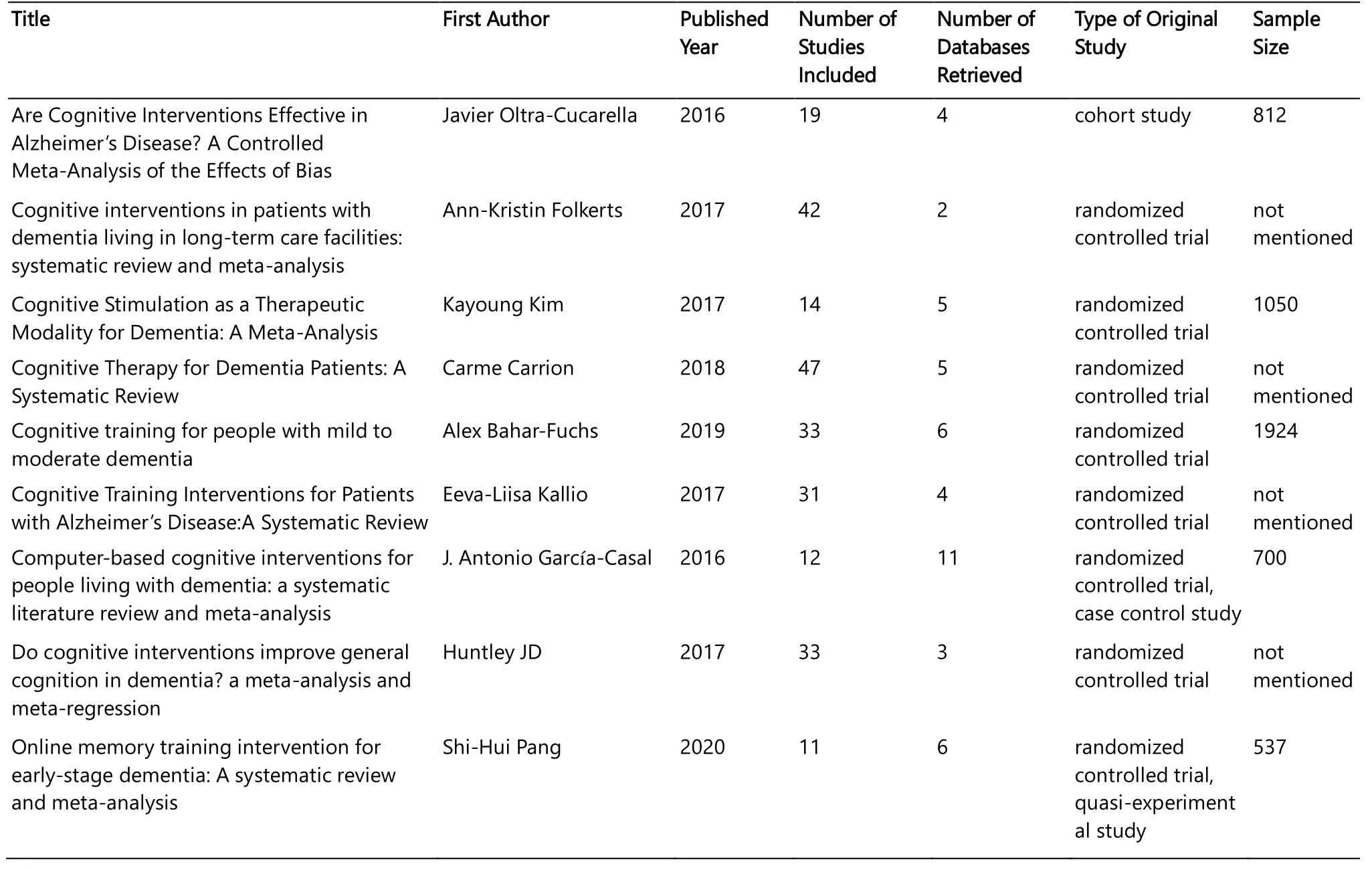
Table 1.Essential Characteristics of the Studies Included
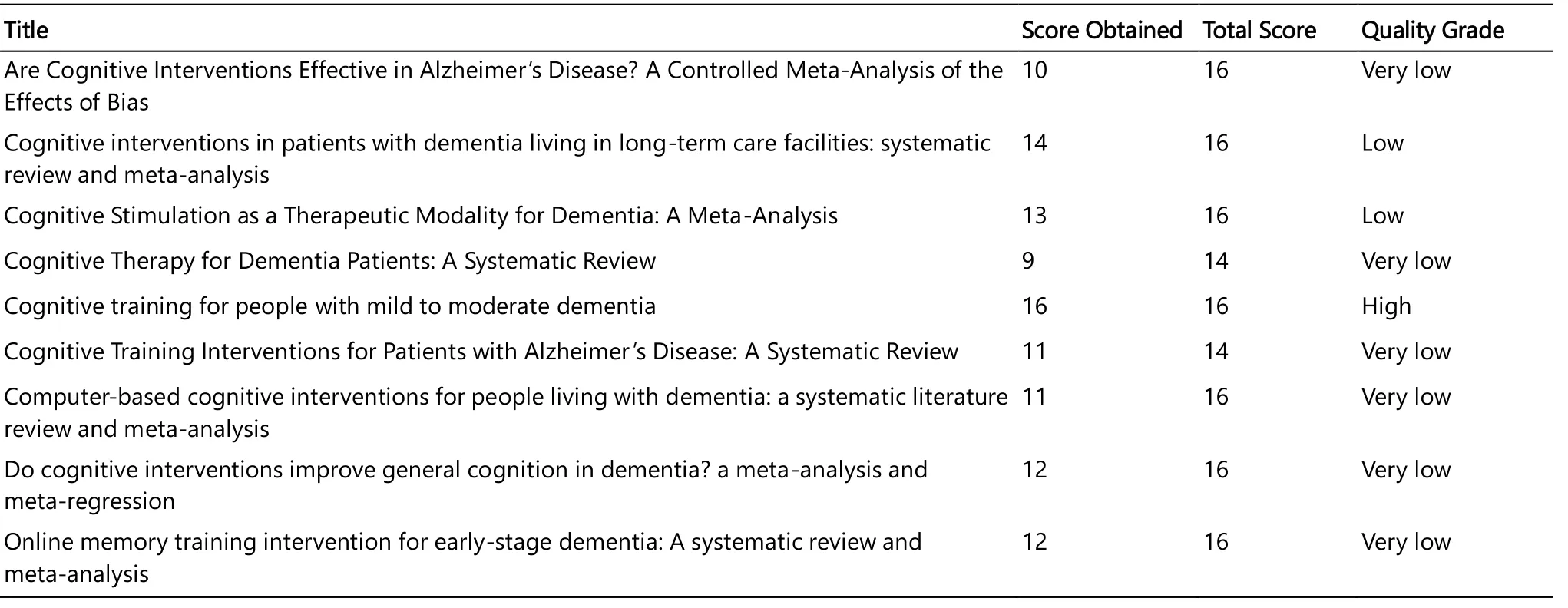
Table 2.The Methodological Quality of the Studies Included
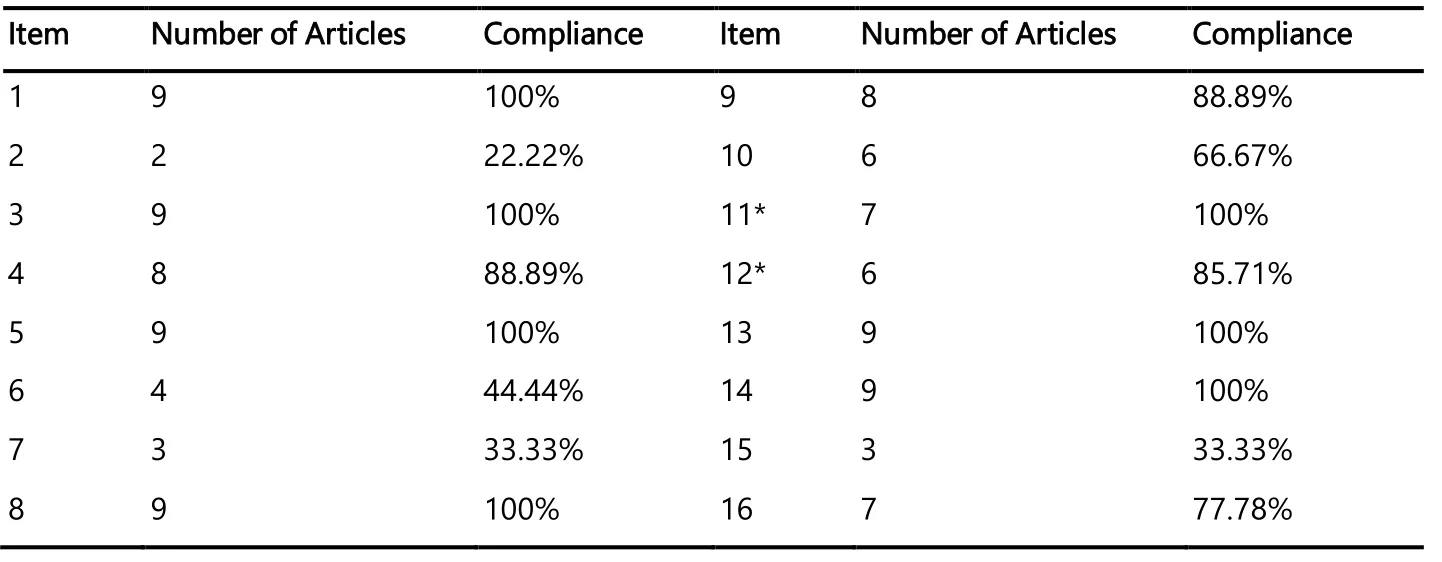
Table 3.Report Rates for 16 AMSTAR Items
Discussion
As essential sources of high-quality evidence in evidence-based medicine, systematic reviews provide important information for medical workers [9].Systematic reviews/meta-analyses provide scientific evidence for the formulation of medical guidelines, and their quality determines the strength of guideline evidence [10].Low-quality meta-analyses are bound to affect the value of research results and mislead clinical decision-makers [11, 12].
Our study conducted a rigorous methodological quality assessment of the systematic reviews of cognitive interventions in Alzheimer'sdisease.It demonstrated that the methodological quality was not ideal and there was still room for improvement.Other research results demonstrated that the methodological quality of existing systematic reviews was generally low, especially in the aspects of search strategy, financial support, potential bias and statistical analysis [13, 14].
Nine studies in our research had compliance of less than 70% for five AMSTAR 2 items.This indicated that the reporting rate of aforementioned five AMSTAR 2 items was not high, which needed to be paid attention to.The report rate for the item 2 was merely 22.22%, indicating that most systematic reviews of cognitive interventions in Alzheimer's disease did not report prior design proposals.The registration and publication of design proposals effectively reduce the bias of systematic reviews, and readers can judge whether a design proposal is rigorous or not [15].The report rate for the item 6 was merely 44.44%, indicating that many studies did not describe how to extract data.This reduces the repeatability of systematic reviews [15].The report rate for the item 7 was merely 33.33%, showing that lots of studies did not give the list of excluded literature and the reasons for it.The report rate for the item 10 was 66.67%, manifesting that some studies did not report the funding they received.The report rate for the item 15 was merely 33.33%, showing that most studies did not carry out rational analysis of publication bias, when carrying out a quantitative analysis.Hence, the aforementioned five AMSTAR 2 items should be paid attention to.
Conclusions
The methodological quality of meta-analyses of cognitive interventions in Alzheimer's disease is not ideal, and there is still room for improvement.Future studies are supposed to explore the relevant factors that possibly incluence the methodological quality.
- Nursing Communications的其它文章
- Application progress of cognitive behavioral therapy in coronary heart disease
- A brief introduction of the pan-Canadian Oncology Symptom Triage and Remote Support (COSTaRS) project
- Effect of lavender aromatherapy on pruritus, anxiety, and sleep quality of patients undergoing hemodialysis: a randomized controlled trial

