Anticancer activity of Gloriosa superba Linn in EAC tumor-bearing Balb/c mice
Priyanka Patil,Shalavadi Mallappa,and VM Chandrashekhar
Abstract—Cancer is a pathogenic condition due to abnormal growth of cells,which is one of the leading reasons for the death of majority of the population throughout the world.Pharmacotherapy associated with the majority of the adverse effects such as cardiotoxicity,hepatotoxicity,nephrotoxicity that suggests identifying new drug which would be safer than well-established chemotherapy.There is a need to get a promising line for research over various diseases including cancer.Gloriosa superba Linn has a long history of use in folk medicine including wounds,hemorrhoids,kidney problems,and tumors.The present study explores the ethanolic extract of Gloriosa superba Linn as the anti-cancer agent using in vitro MTT assay and in vivo Ehrlich’s ascites carcinoma(EAC)bearing Balb/c mice models.Further,the study reports the IC50 value of the DPPH was 1.21 μg/mL,and the IC50 of MTT assay against Hela and MCF7 was found to be 148.50 μg/mL and 147.23 μg/mL respectively.For,in vivo study,animals were divided into five groups(n = 6)experimented for 14 days.The tumor was induced by intraperitoneal transplantation of Ehrlich’s ascites carcinoma cells in Balb/c mice.The normal and control group received normal saline.After 24 hours of Ehrlich’s Ascites Carcinoma transplantation,treatment was started by injecting Cyclophosphamide(50 mg/kg)intraperitoneal whereas Gloriosa superba Linn extract(5 mg/kg & 10 mg/kg)was administrated orally.On the 15th day,mice were sacrificed to estimate hematology,biochemical estimation,and histopathology.The extract showed a significant decrease(P < 0.001)in body weight in the treatment group compare to the control group further there was amelioration in hematology,biochemical estimation,and histopathology.From the result,it was concluded that the extract has a potent antitumor activity.
Key words—Anticancer,Cytotoxicity,Ehrlich ascites carcinoma,Gloriosa superba
INTRODUCTION
Cancer is a multifaceted disease characterized by irregular and uncontrolled development of cells that can invade surrounding tissues and distant organs[1].The development of cancer depends on many factors related to the environment,diet,personal habits,and family history.Chemotherapy,radiotherapy and surgery are the most often used cancer treatment.However,the main concern of chemotherapy is the occurrence of serious side effects during treatment[2].The most common adverse effects of anticancer effects are mutagenic,teratogenic,and carcinogenic.Moreover,it is assumed that malignancy is associated with multiple therapeutic therapies[3,4].Among antineoplastic agents,cyclophosphamide is used as adjuncts to radiation therapy and surgery alone or in combination with chemotherapy regimens for the malignant disease[5].
Natural substances and secondary metabolites isolated from plant sources are considered less toxic to normal cells.Most of the anticancer drugs used in cancer treatment scenarios today come from plant sources.Secondary metabolites,including alkaloids,flavonoids,sesquiterpenes,polyphenols,etc.,are widely used as anticancer agents[6].Further multiple medicinal plants have been reported anticancer activity[7,8].
Gloriosa superbaLinn is a perennial creeper in the Liliaceae family.Being native from Indian especially Southern India and is widely used as a medicinal herb containing toxic alkaloids,namely colchicine and glulisine.All parts ofGloriosa superbaLinn are used for healing purposes in the Sida,Ayurvedic,and Greek systems.In the Chhattisgarh region of India,many traditional healers and herbalists have been treating cancer patients usingGloriosa superbaLinn for many years[8].Gloriosa superbaLinn Methanol extract has shown its antioxidant and cytotoxic effects on Hep-G2 cells[9].In vitro studies have also been shown that purified colchicine can be used in lung cancer,breast cancer,colon cancer,pancreatic cancer,and cervical cancer[10].Therefore,based on its traditional anticancer claims the present study was carried out to evaluate the in vitro and in vivo anticancer activity of the ethanol extract ofGloriosa superbaLinn in Balb/c mice.The workflow of the study is represented in Figure 1.
METHODS
Plant material and extraction
The tubers were collected from the Western Ghats of Karnataka,India(15.9053°N,73.8213°E),authenticated at the Department of Botany,Basaveshwar Science College,Bagalkot,Karnataka.Specimen number:01 dated 22/8/2015.The tuber was washed in 10% KMnO4and dried under shade and then pulverized to get uniform and moderately coarse powder(# 44).Extraction was carried out in a soxhlet extractor using 99.5 % ethanol.
In vitro studies
DPPH free radical scavenging assay
The experiment was performed as explained by the Brand-Williams W 1995[11].With minor modifications.Briefly,the 0.1 mM DPPH was prepared in methanol and 0.1 mL of this was added to different concentration extract(100-2000μg/mL)in different test tubes,after 30min the absorbance was measured at 517 nm.
The % inhibition was calculated using the Formula:Where A0is the absorbance of the control and A1is the absorbance in the presence of the test.
DPPH(%)=[A0-A1/ A0]×100
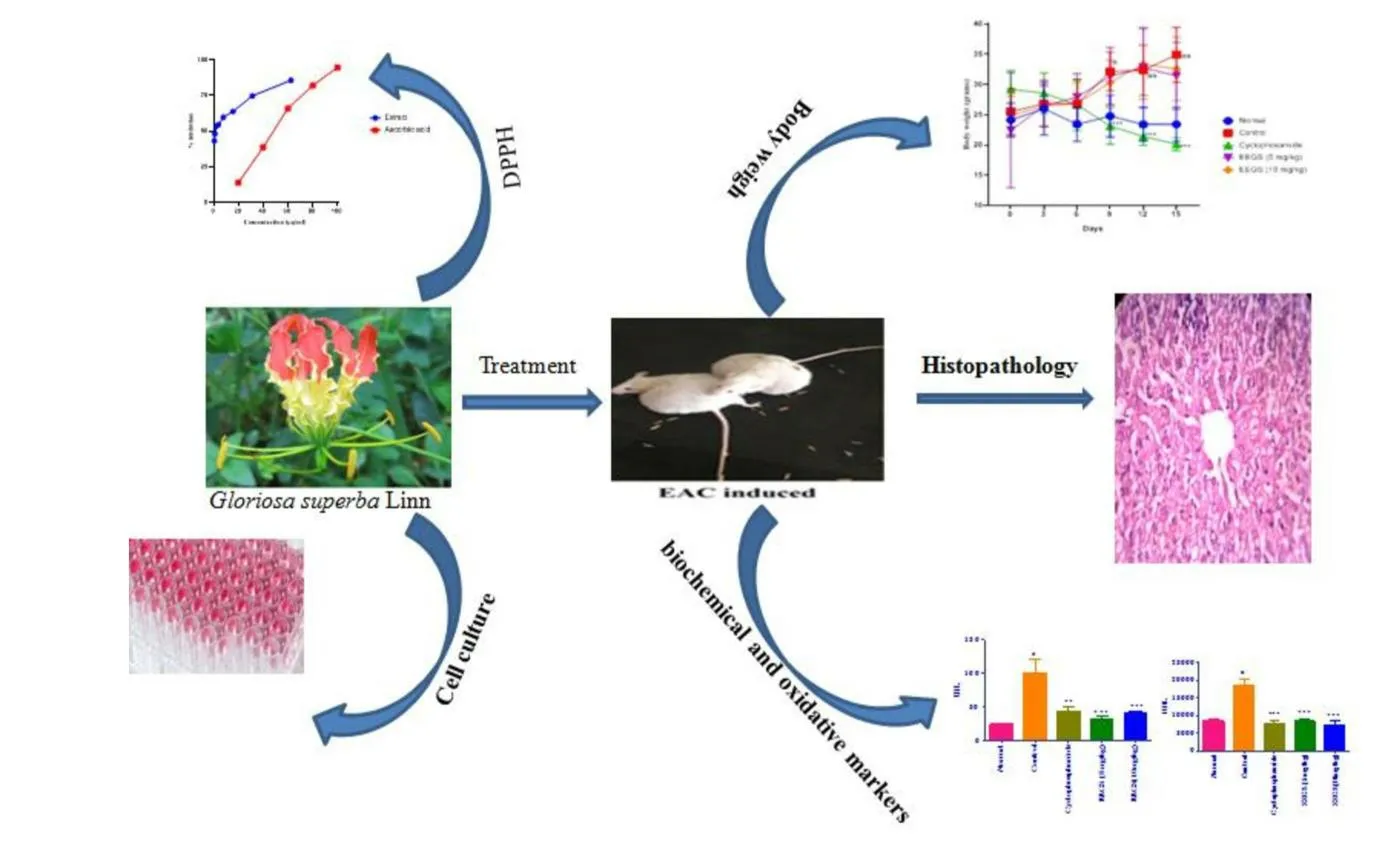
Fig.1.The workflow
IC50values and concentration of extract at which maximum percentage inhibition of DPPH radical occurred were determined.
MTT Assay of Gloriosa superba Linn
MTT cytotoxicity assay was performed as explained by Khanal P& Patil BM 2020[12].In the present study,the extract ofGloriosa superbaLinn was tested in a different cancer cell line(MCF7 & HELA cells).Briefly,the monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 × 105cells/mL using Dulbecco’s Modified Eagle Medium containing 10% FBS.To each well of the 96 well microtiter plate,0.1 mL of the diluted cell suspension(approximately 20,000 cells)was added.After 24 h,when an 80% confluence was formed,the supernatant was flicked off,washed the confluence once with PBS and different test concentration(62-1000μg/mL)of test drugs was added on to the microtiter plates.The plates were then incubated at 37oC for 24h in a 5% CO2atmosphere,and microscopic examination was carried out and observation was noted After 24 h,the test agent in the wells was discarded and 50μL of MTT in PBS was added to each cell.The plates were gently shaken and incubated for 3 h at 37oC in a 5% CO2at atmosphere.The supernatant was removed and 100μL of DMSO was added the plates were gently shaken to solubilize the formed formazan.The absorbance was measured using an ELISA plate reader at 540 nm.Where Acis the control and Asis the test.
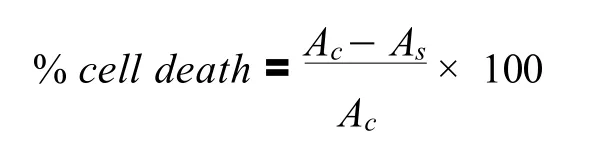
In vivo study
Female Balb/c mice weighing between(20-30 g)were used for the study.All animals were kept under standard husbandry conditions(22-28oC).Relative humidity 65 ±10% for 12 h light and 12 h dark cycle respectively.The animals were fed with standard food pellets(Amruth,Sangli)and water ad libitum throughout the experimental period.The experiments were performed according to the guidelines of the Institutional Animal Ethics Committee(IAEC),H.S.K College of Pharmacy,Bagalkot,Karnataka,India.Further,an acute toxicity study(OECD 420)revealed that 55 mg/kg dose was safe.Hence,1/5thand 1/11thdose 10 mg/kg and 5 mg/kg were selected for anticancer activity.
Induction of Ehrlich’s Ascites Carcinoma
The initial inoculation of Ehrlich’s Ascites Carcinoma(EAC)cells was obtained by Manipal College of pharmacy,Karnataka.The cell line was maintained in female mice by intraperitoneal injection of 1 × 106cells/mouse which was characterized by its moderate rapid growth but could not kill the animal due to accumulation of ascites(14 days)after transplantation.The desired number 1 × 106cells were injected in a volume of 0.1 mL/mouse.
Experimental Design of Ascites Tumor
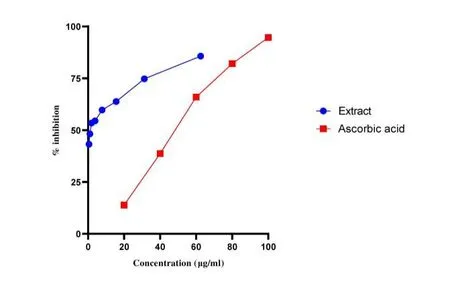
Fig.2.PPH radical scavenging activity of EEGS and Ascorbic acid
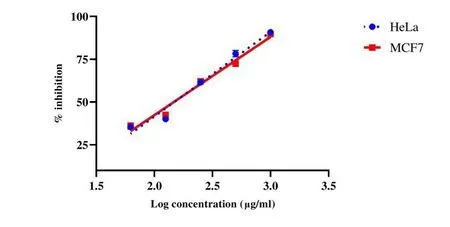
Fig.3.Cytotoxicity effect of Ethanol extracts of Gloriosa superba Linn(EEGS)in(A)Hela cell line and(B)MCF7 cancer cell line.
All groups were transplanted with Ehrlich’s ascites carcinoma(0.1 mL,1 × 106cells/mL,i.p.)except the normal group; considered as 0thday the weight of mice noted.After 24 h of Ehrlich’s ascites carcinoma induction,treatment was started as the normal group received 2%gum acacia vehicle and cancer(control)group received normal saline(0.9% w/v NaCl)i.p.route,cyclophosphamide group received 50 mg/kg orally whereasGloriosa superbaL.group received 5 mg/kg and 10 mg/kg orally treatment continued up to 14 days.
During the 14 days treatment,the weight of each animal was recorded every three-day interval and on the 15th-day animals were sacrificed.The homogenate of the liver was prepared and assays like lipid peroxidation(LPO),reduced glutathione content(GSH)were conducted.Histopathological examination of the liver was done using Hematoxylin.
Statistical analysis
All Data were expressed as Mean ± SEM.The difference between the means of the groups was analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests using Prism 5 software.The means are considered to be significantly different ifP< 0.05.
RESULTS
Effect of Gloriosa superba Linn on DPPH radical scavenging activity
There was an increase in % scavenging capacity of DPPH with the increase in concentration.The highest concentration ofGloriosa superbaLinn extracts 62.5μg/mL had an 85.7% scavenging capacity with an IC50of 1.21μg/mL.The % inhibition of each concentration of both extract and ascorbic acid is represented in Figure 2.
Effect of Gloriosa superba Linn on MTT cytotoxic assay
There was an increase in cell death with the increase in concentration.The % cytotoxicity ofGloriosa SuperbaLinn Extract in HeLa and MCF7 cell lines was found to be 148.50 μg/mL and 147.23 μg/mL respectively same is represented in Figure 3.
Effect of Gloriosa superba Linn on EAC induced animal weight
As shown in Figure 4.Cancer inoculated animals exhibited significant weight gain as(P< 0.001)compared to normal.There was a decrease in body weight of treated groups as compared to the untreated control group.
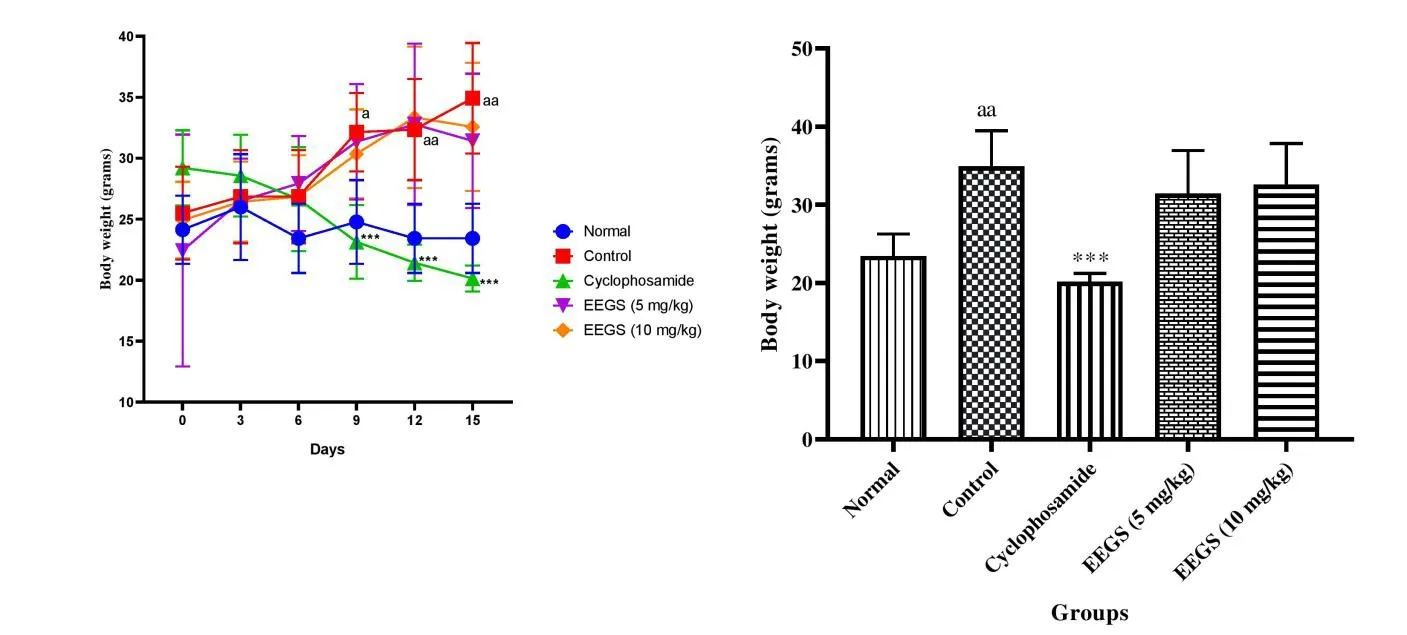
Fig.4.Effect of Ethanol extracts of Gloriosa superba Linn(EEGS)on body weight in Ehrlich Ascites Carcinoma(EAC)bearing mice.

Fig.5.Effect of ethanol extracts of Gloriosa superba Linn on antioxidant enzyme level in Ehrlich Ascites Carcinoma(EAC)bearing mice.
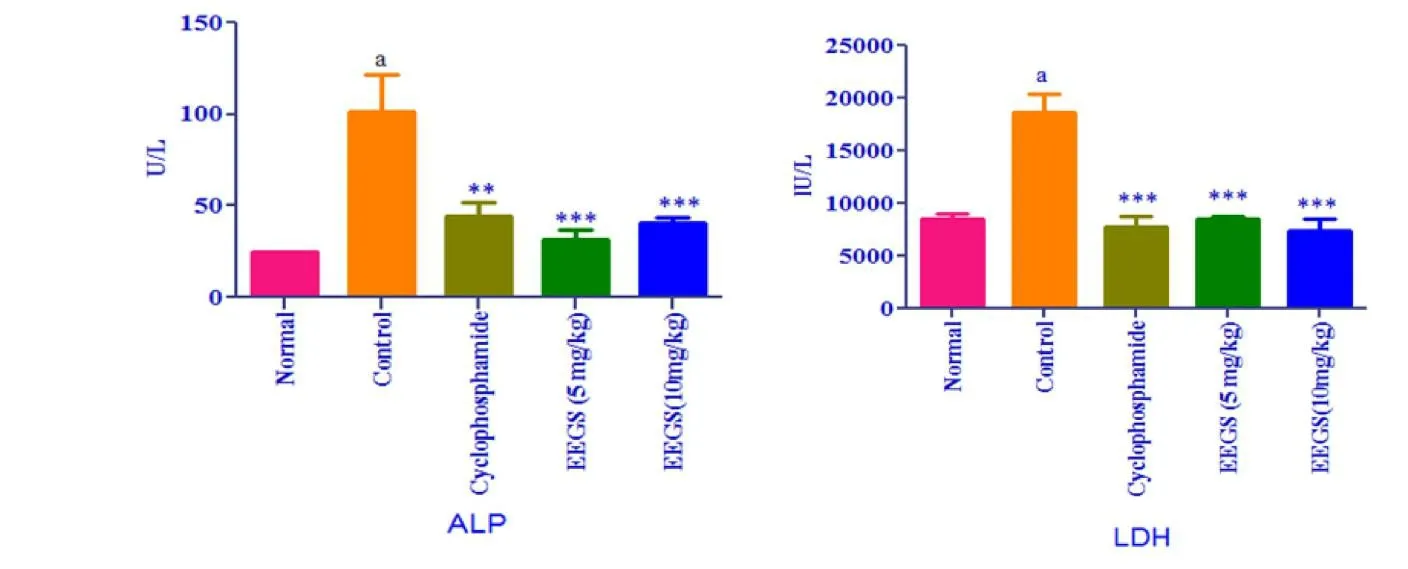
Fig.6.Effect of ethanol extracts of Gloriosa superba Linn on serum ALP & LDH in Ehrlich Ascites Carcinoma(EAC)bearing mice.
Effect of Gloriosa superba Linn on hematological parameters
There was a significant decrease in hemoglobin and Red Blood Cell content in the cancer group(P< 0.001)compared to the normal group which is reversed in the treated group and a significant decrease(P< 0.001)in the total white Blood Cell content in the cyclophosphamide group and inGloriosa superbaLinn extract group compared to control and shown in Table 1.In the differential count,the significant(P< 0.01)decrease in platelet count and % packed cell volume in the control group is reversed in theGloriosa superbaLinn group.There is a significant(P< 0.05)increased in mean corpuscular volume polymorphs in the control group compared to normal.
Effect of Gloriosa superba Linn on oxidative markers
As shown in Figure 5,anti-oxidant biochemical markers were significantly(P< 0.001)decreased(such as GSH)and increased(such as LPO)(P< 0.01)in experimental groups compared with the control group.
Effect of Gloriosa superba Linn on Serum biochemical markers
In the control group,the level of ALP and LDH were significantly increased(P< 0.01)compared to normal was shown in Figure 6.Extract and standard treated groups showed a significant(P< 0.001)decrease in serum level of LDH and ALP as compare to the control group.
Effect of Gloriosa superba Linn on Histopathology
In addition,we assessed the physiological status of liver tissue sections to determine histopathological changes in response to cancer control,cyclophosphamide,or individual treatment withGloriosa superbaLinn.H&E-stained liver sections of normal mice showed normal hepatic architectures(Figure 7).The control group showed spotty necrosis,inflammation,venous congestion area is in hepatic cells,andGloriosa superbaLinn treatment significantly reversed these symptoms.
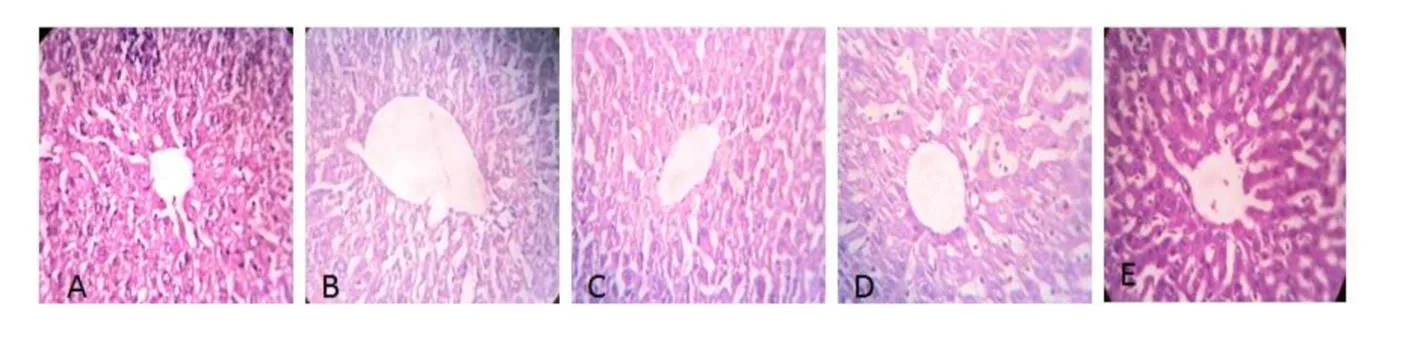
Fig.7.Effect of ethanol extract of Gloriosa superba Linn Photograph of liver section of different treatment groups stained with Haemotoxylin and Eosin.
DISCUSSION
Today,science and pharmaceutical companies are trying to find bioactive phytochemicals that have health benefits in a non-toxic way.It has been reported that excessive levels of ROS in normal cells are involved in various aspects of carcinogenesis by altering cellular signaling and gene expression patterns[13,14].Herbal dietary compounds play an anticancer role either by decreasing ROS levels in cancer cells,leading to induction of programmed cell death or by counterregulation of elevated ROS levels in normal cells through antioxidant mechanisms[15].Antioxidants can balance ROS levels either by neutralizing or producing new,less active,long-lasting,and less harmful molecules or by destroying them by reacting directly with reactive radicals.
In the present study,we have investigatedGloriosa superbaLinn extract at different concentrations exhibited strong scavenging activity against DPPH radical(Figure 2).Gloriosa superbaLinn has been proven previously to possess considerable antioxidant capacity[16].However,our current findings show thatGloriosa superbaextract exhibited comparatively higher antioxidant potential than ascorbic acid.Radicals Inhibition can be caused by lack of a ferryl-preferred complex or by scavenging of·OH radicals Thus,inhibition of lipid peroxidation by extract may be due to the above mechanisms[17].
The anticancer activity in this study would be the result of Colchicine activity present in theGloriosa superbaLinn extract.Colchicine is a class of alkaloids present mainly in plants especially in tuber in abundant quantity and affect cell membrane structure indirectly by inhibiting the synthesis of membrane constituent[18].This antimitotic property disrupts the spindle apparatus that separates chromosomes through metaphase,cells with high metabolic rates are most implicated by the arrest of mitosis[19].Therefore,we have evaluated the cytotoxic activity ofGloriosa superbaextract Linn on HELA and MCF7 cell line which showed the IC50197.06μg/mL and 199.45μg/mL respectively.
Based on the encouraging data obtained in vitro studies,we decided to proceed further in vivo activities towardsGloriosa superbaextract; the current study was highly promising there was an increase in body weight and ascetic fluid in the control group whereas in the treatment group significant reduction of body weight as observed.The ascitic fluid is the direct nutritional source for tumor cells and a rapid increase in the ascitic fluid would help to provide the necessary nutritional requirement of tumor cells[20].
One of the reliable criteria for evaluating anticancer activity is a decrease in WBC count[21].Usually,in cancer chemotherapy,the major problems that are being encountered are myelosuppression and anemia[22].The anemia encountered in tumor-bearing mice is mainly due to a reduction in RBC or hemoglobin percentage,and this may occur either due to iron deficiency or due to hemolytic or myelopathic conditions[23].In the present study,Hemoglobin and Red Blood Cell content increased in treated groups,there was a significant(P< 0.001)reduction in the total white Blood Cell content in(Cycophosphramide)and extract-treated groups.
The liver is a major organ with macrophages,NK cells,NKT cells which are a key component of the innate immune system.EAC is mainly Damages the liver with ROS-produced cell membrane damage cause an increase in serum ALP and LDH levels whereas in the treatment group significant reduction in ALP level LDH level.Malondialdehyde(MDA)is formed during oxidative stress as a product of reactive oxygen species,which acts as an indicator for lipid peroxidation[24].MDA,the end product of lipid peroxidation,was reported to be higher in cancer tissues[25].Glutathione,a potent inhibitor of the neoplastic process,plays an important role in the endogenous antioxidant system.It is found in a particularly high concentration in the liver and is known to have a key function in the protective process.In the present study,anti-oxidant biochemical markers show significantly decreased and increased lipid peroxidation and GSH as compared with the control group.
Similarly,histopathological examination of the liver of Ehrlich’s ascites carcinoma control mice showed significant changes indicating tumor toxic effects.Normalizing this effect observed inGloriosa superbaLinn treated animals also supports the strong hepatoprotective and antioxidant effects of the extract.
CONCLUSION
The present study demonstrates the potent anticancer properties ofGloriosa superba Linn extract.The treatment of ethanol extract ofGloriosa superbaLinn in tumor mice showed significant changes in hematological,biochemical,and histopathology parameters towards normal level as compared with the control mice.Further studies on the elucidation of its mechanism of action and isolation of its active constituents may prove rewarding in cancer treatment.
ACKNOWLEDGMENT
The authors gratefully acknowledge the support of the H.S.K College of Pharmacy,Bagalkot,Karnataka,India for conducting experimental studies.
Author contributions:Priyanka Patil experimented with the work and drafted the manuscript.Mallappa Shalavadi confirmed the findings and revised the manuscript.VM Chandrashekhar initiated the study,supervised and revised the manuscript.
Ethical statements:This work was performed after receiving the ethical clearance by Institutional Animal Ethical committee at H.S.K College of Pharmacy,Bagalkot,Karnataka,India.
Competing interests:The authors declare that they have no conflict of interest.
Citation:Patil P,Mallappa S,Chandrashekhar VM.Anticancer activity ofGloriosa superbaLinn in EAC tumor-bearing Balb/c mice.TMR Cancer.2021;4(3):10.doi:10.53388/TMRC201800101.
Executive editor:Shan-Shan Lin.
Submitted:31 May 2021,Accepted:27 June 2021,
Online:28 June 2021
? 2021 By Authors.Published by TMR Publishing Group Limited.This is an open access article under the CC-BY license(http://creativecommons.org/licenses/BY/4.0/)

