Determination of Enantiomers in ET-26-HCl by Ultra Performance Convergence Chromatography
Yaru MA, Ting KANG, Zhihua CAO, Jianrui MU, Xianghan YE, Hanlin GONG, Liying QIU*
1. School of Pharmacy, Southwest Minzu University, Chengdu 610041, China; 2. Laboratory of Anesthesia & Critical Care Medicine, Translational Neuroscience Center, West China Hospital of Sichuan University, Chengdu 610041, China; 3. Department of Integrated Traditional Chinese and Western Medicine of West China Hospital, Sichuan University, Chengdu 610041, China
Abstract [Objectives] To establish an ultra performance convergence chromatography (UPC2) method to determine the content of enantiomers in (R)-2-methoxyethyl1-(1-phenylethyl)-1H-imidazole-5-carboxylate hydrochloride (ET-26 HCl). [Methods] Chiralpak? AD-3 chromatographic column (4.6 mm×100 mm, 3 μm) was used, with 0.1% diethylamine methanol solution-supercritical carbon dioxide (1∶9) as mobile phase; flow rate 2.0 mL/min; back pressure 2 000 psi, detection wavelength 240 nm, and column temperature 35 ℃. [Results] ET-26-HCl was completely separated from the enantiomers, and the linear relationship was good; the detection limit was 1.5 μg/mL. [Conclusions] This method is suitable for the determination of the content of enantiomers in ET-26-HCl.
Key words Ultra performance convergence chromatography (UPC2), ET-26 HCl, Enantiomers
1 Introduction
The (R)-2-methoxyethyl1-(1-phenylethyl)-1H-imidazole-5-carboxylate hydrochloride (ET-26 HCl) is a novel imidazole-based short-acting intravenous general anesthetic. It is a derivative obtained by structural modification of etomidate[1]. It has been approved for clinical trials by the China National Medical Products Administration Since 2019. Compared with etomidate and other derivatives, ET-26-HCl has more prominent advantages. ET-26-HCl is metabolized in the peripheral liver and its metabolic rate is slow, and its metabolites will not accumulate in the center; besides, its cortical inhibitory activity slowly produced in the periphery is only 1/120 000 of etomidate[2-5], so it can be considered that it has no cortical inhibitory activity. After stopping the drug, it will neither cause the accumulation of metabolites in the nerve center to affect the quality of recovery, nor will it cause continuous corticosteroid inhibition due to the accumulation of metabolites in the periphery. ET-26-HCl has good application prospects. However, there are few reports on methods for determination of its impurity[6]. ET-26-HCl is a chiral compound (in R configuration). In the synthesis process, enantiomeric (in S configuration) impurities may be produced. At present, there is no report about the method for determining the enantiomer of ET-26-HCl.
Ultra performance convergence chromatography (UPC2) takes CO2as the main mobile phase. It has the advantages of high separation efficiency, good mass transfer performance, and environmental protection. It is suitable for the separation and analysis of structural analogs and chiral compounds[7]. In view of this, we established an ultra performance convergence chromatography method to determine the content of enantiomers in ET-26-HCl. The method is fast, simple, reproducible, and sensitive, and is suitable for the quality control of ET-26-HCl. This method is fast, simple, reproducible, and sensitive, and is suitable for the quality control of ET-26-HCl.
2 Materials and methods
2.1 Materials
2.1.1Instrument. Waters Acquity Ultra Performance Convergence Chromatograph (Waters, USA); Satorius-CAP225D semi-micro analytical balance (Satorius, Germany); Milli-Q Advantage A10 water purification system (Millipore, the United States of America).
2.1.2Reagents. ET-26-HCl and ET-26-HCl enantiomers (Tran-slational Neuroscience Center, West China Hospital of Sichuan University, purity>99%), the structure is shown in Fig.1; chromatography grade acetonitrile (Sigma, the United States of America), chromatography grade methanol (CINC High Purity Solvents (Shanghai) Co., Ltd., China) and chromatographic grade diethanolamine (Acros, Belgium).

Fig.1 Structural formulas of ET-26-HCl and its enantiomers
2.2 Methods
2.2.1Chromatographic conditions. Took 0.1% diethylamine methanol solution-supercritical carbon dioxide (1∶9) as mobile phase; flow rate 2.0 mL/min; back pressure 2 000 psi, detection wavelength 240 nm, and column temperature 35 ℃; injection volume 5 μL.
2.2.2Preparation of solution. (i) Preparation of ET-26-HCl enantiomer reference solution: precisely weighed an appropriate amount of ET-26-HCl enantiomer reference substance and diluted with acetonitrile to make a constant volume to prepare a 4 mg/mL enantiomer stock solution. Then, precisely weighed an appropriate amount of enantiomeric stock solution, diluted it with acetonitrile to a constant volume, and prepare a 20 μg/mL enantiomeric reference solution. (ii) Preparation of ET-26-HCl test solution:precisely weighed an appropriate amount of ET-26-HCl test product, diluted it with acetonitrile to make a 4 mg/mL test solution. (iii) Preparation of resolution solution: precisely weighed an appropriate amount of ET-26-HCl test product, diluted it with the enantiomeric reference solution to a constant volume, that is, the resolution solution.
3 Results and analysis
3.1 System suitability testSeparately measured the blank solution, resolution solution, and ET-26-HCl enantiomer reference solution according to the chromatographic conditions in Section2.2.1. The results show that the blank solution does not interfere with the impurity peaks of the solvents to be tested. In the resolution solution, the theoretical plate number of the main peak of ET-26-HCl enantiomer was greater than 5 000, the resolution between the enantiomeric peak and the main peak was greater than 3.0, and the tailing factor was in the range of 0.8-2.0, indicating that the system suitability is good (Fig.2-3).
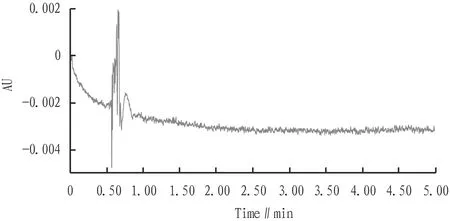
Fig.2 Chromatogram of blank solution
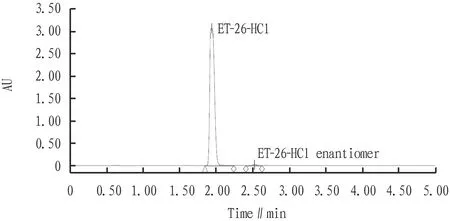
Fig.3 Chromatogram of resolution solution
3.2 Limit of detection and limit of quantificationPrecisely weighed an appropriate amount of the enantiomer reference solution using the method in Section2.2.2, diluted it with acetonitrile to the desired volume, and injected and analyzed in sequence in accordance with chromatographic conditions in Section2.2.1. Taking the signal-to-noise ratio S/N=3 as the indicator, the limit of detection was 1.5 μg/mL. Taking the signal-to-noise ratio S/N=10 as the indicator, the limit of quantification was 5.0 μg/mL, and the peak areaRSDof the three injections of ET-26-HCl enantiomer at this concentration was 5.42%, indicating the sensitivity of the method is good.
3.3 Linearity and rangePrecisely weighed the enantiomer reference solution using the method in Section2.2.2, diluted with acetonitrile to make a linear solution of 5.0, 10.0, 16.0, 20.0, 24.0, 30.0 μg/mL, and injected and analyzed in sequence in accordance with chromatographic conditions in Section2.2.1. Taking the mass concentration (mg/mL) as the abscissa and peak area as the ordinate, used the least square method to conduct the linear regression. The results show that the enantiomer had a good linear relationship with the peak area within 5.0-30.0 μg/mL, and the regression equation isY=4 856.183X+5 219.335,R=0.995.
3.4 Precision
3.4.1Injection precision. Performed 6 consecutive injections of the enantiomer reference solution, the retention timeRSDof the enantiomer of ET-26-HCl was 0.21%, and theRSDof the peak area was 4.34%, indicating that the injection precision is excellent.
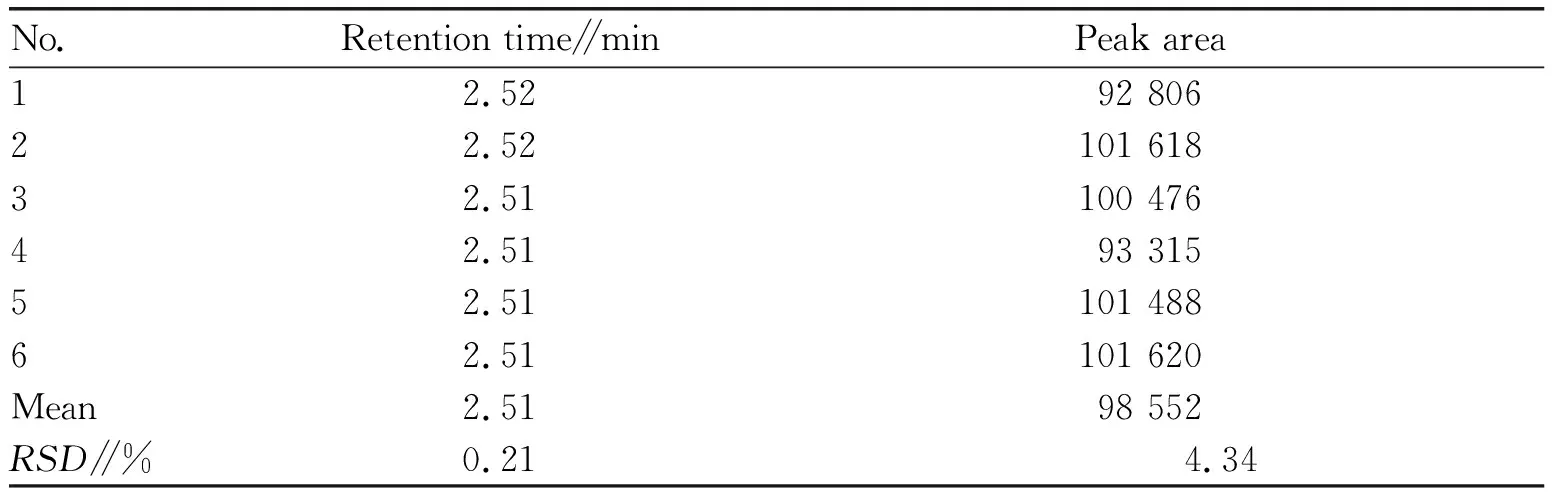
Table 1 Injection precision test results
3.4.2Repeatability. Prepared 6 solutions of spike test solution in parallel in accordance with the resolution solution preparation method in Section2.2.2, and performed the injection analysis in accordance with chromatographic conditions in Section2.2.1. The results showed that theRSDof ET-26-HCl enantiomer content was 5.09%, indicating the repeatability is good (Table 2).

Table 2 Repeatability test results
3.4.3Intermediate precision. Two persons performed sample injection analysis under the repeatability item at different times. The results showed that theRSDof ET-26-HCl enantiomer response factor was 5.74% in the 6 solutions; theRSDof enantiomer content of ET-26-HCl in 12 solutions was 6.20%, indicating the intermediate precision is high.
3.5 AccuracyPrecisely weighed the test solution, diluted it with acetonitrile to an appropriate concentration, and performed sample injection analysis in accordance with Section2.2.1, and measured three samples of test solution in parallel. The results show that the recovery rate of ET-26-HCl enantiomer at each concentration was in the range of 80%-120%; theRSDof the sample recovery rate at each concentration was 1.78%, 0.81%, 0.36% and 5.37%, respectively; theRSDof recovery rate of 12 test samples was 4.57%, which was less than 10.0%, indicating that the accuracy is high.
3.6 Solution stabilityPlaced the enantiomer reference solution and the test solution at room temperature for 0, 2, 4, 6, 8, and 24 h respectively, and injected and analyzed samples to test the solution stability. TheRSDof peak area of the ET-26-HCl enantiomer reference solution in 24 h at room temperature was 1.24%; no impurity of the ET-26-HCl enantiomer was detected in the test solution within 24 h at room temperature, indicating that the ET-26-HCl enantiomer reference solution and the test solution have good solution stability within 24 h at room temperature (Table 3).

Table 3 Stability test results of the reference substance solution
3.7 Method robustnessThrough slightly changing the chromatographic conditions in Section2.2.1, such as flow rate (2.0 ± 0.2) mL/min and column temperature (35±1) ℃, we tested the method robustness. The results indicate that theRSDs of the peak area of the enantiomers of the reference solution in three injections were less than 10.0%, and theRSDs of the retention time were all less than 1.0%, indicating the method robustness is good (Table 4-5).

Table 4 Robustness test results at flow rate of 1.8 and 2.2 mL/min

Table 5 Robustness test results at column temperature of 34 and 36 ℃
4 Conclusions
In this study, we established an ultra performance convergence chromatography (UPC2) method to determine the content of enantiomers in ET-26 HCl (in S configuration). Besides, we tested the method from the system suitability, specificity, limit of detection and limit of quantification, linearity and range, solution stability, precision, and robustness. Finally, we proved that this method is suitable for determination of impurities in ET-26 HCl enantiomer.
- Medicinal Plant的其它文章
- Potential Preventive and Therapeutic Effects of Traditional Chinese Medicine Compound Prescription and Traditional Chinese Medicine Products on COVID-19
- Review of Modern Research on Toxicity of Traditional Chinese Medicine Aconm Lateralis Radix Praeparaia
- Advances in Research of Pharmacological Action and Mechanism of Tibetan Medicine Ranasampel
- Research Progress of External Treatment of Acute Gouty Arthritis with Traditional Chinese Medicine
- Study on Pharmacological Effects of Harmine Hydrochloride
- Research Progress in the Application of Network Pharmacology in Traditional Chinese Medicine and Compound Prescriptions

