In vitro Pharmacological Activity of Chromenes in Disk of Helianthus annuus
Zhiying WEI, Li LI, Yaohua LI, Zhenzhen PAN, Fangchan LI, Yupin CAO, Jiagang DENG, Xiaojiao PAN*
1. Teaching Center of Experiment and Practical Training, Guangxi University of Chinese Medicine, Nanning 530200, China; 2. College of Pharmacy, Guangxi University of Chinese Medicine, Nanning 530200, China; 3. Guangxi Collaborative Innovation Center of Study on Functional Ingredients of Agricultural Residues, Nanning 530200, China
Abstract [Objectives] The anti-tumor, anti-bacterial, anti-acetylcholinesterase and anti-α-glucosidase activity in vitro of five chromenes isolated from Helianthus annuus disk was studied, in order to provide reference for the development and utilization of H. annuus disk resources. [Methods] The effect of different concentrations of chromenes on the survival rate of leukemia HL-60 cells, lung cancer A549 cells, liver cancer SMMC-7721 cells, breast cancer MCF-7 cells and colon cancer SW480 cells was detected by MTS method, and the IC50 was calculated. The inhibitory activity of chromenes against Escherichia coli, Staphylococcus aureus subsp. aureus, Salmonella enterica subsp. enterica, Pseudomonas aeruginosa and Candida albicans was detected by microdilution method. The DTNB substrate method was used to detect the inhibitory activity of chromenes on acetylcholine. The PNPG substrate method was used to detect the inhibitory activity of chromenes on α-glucosidase. [Results] The five chromenes had no obvious in vitro inhibitory activity on the five kinds of tumor cells, with IC50 greater than 40 μM. The five chromenes had no obvious in vitro inhibitory activity against the four kinds of bacteria and C. albicans. The five chromenes had certain inhibitory activity on acetylcholinesterase, and among them, 6-acetyl-2,2-dimethylchromene and 6-acetyl-7-hydroxy-2,3-dimethylchromene showed strong inhibitory activity on acetylcholinesterase, with IC50 of 28.253 and 16.945 μM, respectively, both smaller than that (0.275 μM) of the positive control tacrine (P<0.01). The five chromenes showed good inhibitory effect on α-glucosidase, and among them, 7-hydroxy-6-hydroxyacetyl-2,2-dimethylchromene and 6-acetyl-7-hydroxy-2,3-dimethylchromene had stronger inhibitory activity, with IC50 of 20.240 and 21.052 μM, respectively, significantly better than that (169.780 μM) of the positive control acarbose (P<0.01). [Conclusions] The five chromenes in H. annuus disk have certain in vitro inhibitory activity against acetylcholinesterase and α-glucosidase and certain potential in fighting neurodegenerative diseases and diabetes.
Key words Helianthus annuus disk, Chromene, Pharmacological activity, Acetylcholinesterase, α-glucosidase
1 Introduction
HelianthusannuusL. is a plant of the genusHelianthusin the family Compositae, also known as Xiangyanghua, Kuihua, Kuihua,etal.It is an annual herbaceous oil crop. According to the record ofDictionaryofTraditionalChineseMedicine, "H.annuusseeds can penetrate thick blood and cure blood dysentery and chronic osteomyelitis;H.annuusshells can cure tinnitus;H.annuusleaves contain an antimalarial drug similar to quinine, which can lower blood pressure and detoxify;H.annuuspetals can dispel wind, improve eyesight, and cure dizziness and toothache;H.annuusroot system has the effects of clearing heat and dampness, promoting qi and relieving pain;H.annuusdisks can clear dampness and heat, promote urination, reduce inflammation, and lower blood pressure;H.annuuspith has a good effect on the treatment of urethral stones; and the juice inH.annuusstalks can heal wounds"[1]. Therefore, the disks, seeds, leaves, petals and stalks ofH.annuusall have unique pharmacological effects, and they have a positive effect on the prevention and treatment of human diseases and high medicinal value.
At present, there are not many reports on the chemical composition ofH.annuusdisk at home and abroad. According to existing literature,H.annuusdisk contains diterpenoids[2-3], flavonoids[4-6], polysaccharides[7-8], chromenes[9]and other chemical components. There are few reports on the efficacy ofH.annuusdisk, focusing on the antioxidant activity of flavonoids and polysaccharides and anti-tumor, bactericidal and insecticidal effect of diterpenoids and chromenes[10]. In this study, five monomer compounds were extracted and isolated fromH.annuusdisk and confirmed by MS,1HNMR and13CNMR as chromenes: 6-hydroxyacetyl-2,2-dimethylchromene (chromene 1), 7-hydroxy-6-hydroxyacetyl-2,2-dimethylchromene (desacetylripariochromene B) (chromene 2), 5-acetyl-2-(propan-2-ylidene) benzofuran-3-one (chromene 3), 6-acetyl-2,2-dimethylchromone (demethoxyencecalin) (chromene 4), and 6-acetyl-7-hydroxy-2,3-dimethylchromone (chromene 5). The chemical structure is shown in Fig.1. They are all compounds isolated fromH.annuusdisk for the first time, and there is currently no report on their pharmacological effect in any literature. To this end, the anti-tumor and antibacterial activity of the above-mentioned chromenesinvitro, as well as their inhibitory activity against acetylcholinesterase and α-glucosidase was investigated, in order to provide scientific data for elucidating the pharmacological activity of these chromene compounds and reference for the development and utilization ofH.annuusdisk resources.

Note: (i) 6-Hydroxyacetyl-2,2-dimethylchromene; (ii) 7-Hydroxy-6-hydroxyacetyl-2,2-dimethylchromene; (iii) 5-Acetyl-2-(propan-2-ylidene) benzofuran-3-one; (iv) 6-Acetyl-2,2-dimethylchromone; (v) 6-Acetyl-7-hydroxy-2,3-dimethylchromone.
2 Materials and methods
2.1 Instruments and equipmentSECURA125-1CN precision balance (Sartorius, Germany); TS100-F inverted microscope (Nikon, Japan); Multiskan FC microplate reader (Thermo Fisher Scientific, USA); 3111 carbon dioxide incubator (Thermo Fisher Scientific, USA); constant temperature incubator (Shaoguan Taihong Medical Equipment Co., Ltd., PRC); HH-1 water bath (Guohua Electric Co., Ltd., PRC); TDZ4A-WS low-speed desktop centrifuge (Hunan Xiangyi Centrifuge Instrument Co., Ltd., PRC); SW-CJ-2F clean bench (Suzhou Antai Air Tech Co., Ltd., PRC).
2.2 Drugs and reagentsThe five chromene monomer compounds were all extracted and purified fromH.annuusdisk, and their purity was examined to be greater than 90% by HPLC-PDAD. RPMI-1640 medium (lot No.20190701), fetus bovine serum (batch No.20190827), penicillin-streptomycin double antibody (batch No.20190403), cisplatin (batch No.416B023, purity≥98.0%), paclitaxel (batch No.520D015, purity≥98.0%), amphotericin B (batch No.1026B028, purity≥98.0%), 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB, batch No.756E016), and p-nitrophenyl-α-glucopyranoside (PNPG, lot No.1205E022) were purchased from Beijing Solarbio Technology Co., Ltd. Tacrine (lot No.073L5023D), acetylcholinesterase (lot No.085M2076B), and α-glucosidase (batch No.089M4087V) were purchased from Sigma Corporation. 3-(4,5-Dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTS) reagent was purchased from Abcam Pharmatech Inc. Dimethyl sulfoxide (DMSO) and sodium dodecyl sulfate (SDS) were both analytically pure, and purchased from Sinopharm Chemical Reagent Co., Ltd. The water used was pure water.
2.3 Cell linesThe primary cells of leukemia cell line HL-60, human breast cancer cell line MCF-7, human liver cancer cell line SMMC-7721, human lung cancer cell line A549 and colon cancer cell line SW480 were purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
2.4 Bacteria and fungiEscherichiacoliATCC25922,Staphylococcusaureussubsp.aureusATCC29213,Salmonellaentericasubsp.entericaATCC14028 andPseudomonasaeruginosaATCC27853 were all purchased from China General Microbiological Culture Collection Center; andCandidaalbicansATCC10231 was purchased from Microbiologics, USA.
2.5Invitroanti-tumor activity ofH.annuuschromenesAfter thawing and passage for two generations, each cell line was prepared into suspension with a culture medium containing 10% fetal bovine serum and inoculated into 96-well plate with 3 000-15 000 cells per well, with a volume of 100 μL per well. The cells were cultured in an incubator containing 5% CO2at 37 ℃ for 24 h. The sample to be tested was dissolved with DMSO, and added to a 96-well culture plate at an appropriate amount. The final concentration of each compound was set as 40, 8, 1.6, 0.32 and 0.064 μM, respectively, and the final volume of each well was 200 μL. Three replicates were arranged for each concentration. Cisplatin and paclitaxel were used as positive control compounds, and subjected to the same operation. At the same time, blank control and negative control with drug concentration of zero were set. After administration, the culture of the cells was continued for another 48 h. After removing the original culture medium, 20 μL of MTS solution and 100 μL of culture medium were added to each well, followed by another 4 h of incubation. TheODvalue of each well at a wavelength of 492 nm was determined using a microplate reader, and the results were recorded. Cell survival rate was calculated according to the following formula:
Survival rate = (ODvalue of administration group-ODvalue of blank control)/(ODvalue of negative control-ODvalue of blank control)×100%
Taking concentration as the abscissa and cell survival rate as the ordinate, the cell growth curve was drawn. TheIC50value of each test compound was calculated with Reed and Muench method.
2.6Invitroanti-bacterial activity ofH.annuuschromenes
The sample to be tested was dissolved with DMSO, added to a 96-well plate at an appropriate amount, and added with bacterial liquid ofE.coli,S.aureussubsp.aureus,S.entericasubsp.enterica,P.aeruginosaandC.albicans, respectively. The final concentration of the sample to be tested was 200 μM, and the final concentration of each bacteria and fungus was 5×105CFU/mL. After administration, the cells were cultured at 37 ℃ for 24 h. TheODvalue of each well at 625 nm was determined with a microplate reader. Three replicates were arranged for each treatment. The results were recorded. The inhibition rate on bacteria and fungi was calculated according to the following formula:
Inhibition rate=[1-(ODvalue of administration group-ODvalue of blank control)/(ODvalue of bacterial or fungal control-ODvalue of blank control]×100%
2.7 Inhibitory activity ofH.annuuschromenes on acetylcholinesterase (AChE)
2.7.1Preparation of working fluid. AChE was dissolved in phosphate buffer (pH 8.0) and diluted to 0.1 U/mL. Acetylthiocholine iodide and DTNB were mixed into a 6.25 mM solution for use.
2.7.2Inhibition test of a single concentration of sample on AChE. The sample was diluted with DMSO and water into 1 mM working solution. The concentration of DMSO in sample solutions of different concentrations was all 2%. The total volume of the final reaction system was 200 μL. The final concentration of DMSO in the final reaction system was 0.1%. The final concentration of the chromene compounds was 50 μM. Tacrine was used as the positive control, and its final concentration was 0.333 μM. The negative control was 2% DMSO. At the same time, a blank control group without enzyme was set up. The reaction was carried out in a 96-well plate. The reagents were added to the plate in sequence according to Table 1, and three replicates were arranged for each sample. Within 1 h after adding the developer and the substrate, the absorbance at 405 nm was measured every 30 s. The sample absorbance value when the average absorbance value of the negative control group was about 1 was selected. The inhibition rate on AChE was calculated according to the following formula:
Inhibition rate=[1- (B-b)/(A-a)]×100%
whereAis theODvalue of negative control;ais theODvalue of negative blank;Bis theODvalue of sample; andbis theODvalue of sample blank.

Table 1 Dosage and sequence of reagents for acetylcholinesterase inhibition test
2.7.3Determination ofIC50against AChE. The sample to be tested was diluted with DMSO into a certain concentration gradient, and the inhibition rate of each sample on AChE was calculated according to the method under Section2.7.2. The final concentrations of chromene compounds were 100, 50, 30, 10, 3, 1 and 0.2 μM, respectively. The final concentrations of tacrine were 2, 1, 0.5, 0.2, 0.04, 0.008 and 0.001 6 μM. The fitting curve was drawn using origin 8 software to analyze theIC50of each sample to be tested against AChE.
2.8 Inhibitory activity ofH.annuuschromenes on α-glucosidase
2.8.1Preparation of working fluid. A-glucosidase was dissolved and diluted to 0.25 U/mL with phosphate buffer (pH 6.8). A 5.0 mM solution was prepared with PNPG. A 3% SDS solution was prepared with pure water.
2.8.2Inhibition test of a single concentration of sample on α-glucosidase. The sample was diluted with DMSO and water into 1 mM working solution. The concentration of DMSO in sample solutions of different concentrations was all 2%. The total volume of the final reaction system was 200 μL. The final concentration of DMSO in the final reaction system was 0.1%. The final concentration of each chromene compound was 50 μM. The positive control was acarbose with a final concentration of 400 μM; and the negative control was 2% DMSO. At the same time, a blank control group without enzyme was set. The reaction was carried out in a 96-well plate, and the following reagents were added to the 96-well plate according to Table 2, with three replicates for each sample. After the reaction was ended, theODvalue of each well at 405 nm was measured with a microplate reader. The inhibition rate on α-glucosidase was calculated according to the formula in Section2.7.2.
2.8.3Determination ofIC50against α-glucosidase. The sample to be tested was diluted with DMSO into a certain concentration gradient. The inhibition rate of each sample onα-glucosidase was measured and calculated according to the method under section2.8.2. The final concentrations of chromene compounds were 50, 30, 10, 3, 1, and 0.2 μM, respectively. The final concentrations of acarbose were 400, 200, 100, 50, 25, and 10 μM. The fitting curve was drawn with origin8 software to analyze theIC50of each sample to be tested for α-glucosidase.
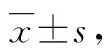
3 Results and analysis
3.1Invitroanti-tumor activity ofH.annuuschromenesUnder different concentrations (0.064-40 μM) of the five chromenes, the survival rate of the five kinds of tumor cells was all greater than 85%. It was impossible to calculate the accurateIC50, but it could be inferred that theIC50of these chromenes was greater than 40 μM, significantly greater than those of the positive control drugs cisplatin and paclitaxel. It can be seen that these five chromenes have no obvious inhibitory activity on the five kinds of tumor cells used in the test (Table 3).
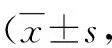
Table 3 In vitro antitumor activity of chromenes n=3)
3.2Invitroanti-bacterial activity ofH.annuuschromenes
When the final concentration was 200 μM, the inhibition rates of the five chromenes against the four common bacteria andC.albicanswere negative, indicating that none of the five chromenes has obviousinvitroantibacterial and antifungal activity (Table 4).
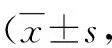
Table 4 In vitro antibacterial and anti-fungal activity of chromones n=3)
3.3 Inhibitory activity ofH.annuuschromenes on acetylcholinesterase (AChE)When the final concentration was 50 μM, the inhibition rates of the five chromenes against AChE was all above 50%. Among them, chromene 4 and chromene 5 showed the strongest inhibitory activity, with inhibition rates of 74.91% and 84.81%, respectively. TheIC50values of these two chromenes were calculated to be 28.253 and 16.945 μM, respectively, significantly greater than that (0.275 μM) of the positive control drug tacrine (P<0.01). The results showed that the five chromenes had a certain inhibitory effect on AChE. Chromene 4 and chromene 5 showed the strongest inhibitory activity, but their inhibitory activity against AChE was weaker than that of tacrine (Table 5).
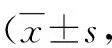
Table 5 Inhibitory activity of chromenes on acetylcholinesterase in vitro n=3)
3.4 Inhibitory activity ofH.annuuschromenes on α-glucosidaseAt the final concentration of 50 μM, the inhibition rates of the five chromenes on α-glucosidase were all above 40%. Among them, chromene 2 and chromene 5 had the strongest inhibitory activity, with the inhibitory rates being 69.23% and 69.73%,respectively. TheIC50values of the two most active chromenes were calculated to be 20.240 and 21.052 μM, respectively, significantly lower than that (169.078 0 μM) of the positive control drug acarbose (P<0.01). It suggested that the five chromenes had a certain inhibitory effect on α-glucosidase, and chromene 2 and chromene 5 showed the strongest inhibitory activity, both significantly stronger than that of acarbose (Table 6).
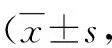
Table 6 Inhibitory activity of chromenes on α-glucosidase in vitro n=3)
4 Discussion
Chromene is an oxygen-containing heterocyclic compound. The chemical name is benzo-γ-pyrone. Chromene compounds are chemical structural analogues derived from benzo-γ-pyrone as the core. They are widely found in plants, and have anti-tumor[11-13], anti-bacterial[14-16], anti-hyperglycemia[17-18], anti-inflammatory[19-20], anti-oxidant[21-22]and other pharmacological effect.
Based on existing literature on the efficacy ofH.annuusdisks and chromene compounds, this study detected the inhibitory activity of five chromenes against five types of tumor cells (leukemia HL-60 cells, lung cancer A549 cells, liver cancer SMMC-7721 cells, breast cancer MCF-7 cells, and colon cancer SW480 cells), four common bacteria (E.coli,S.aureussubsp.aureus,S.entericasubsp.EntericaandP.aeruginosa) and a common fungus (C.albicans) using the MTS method and the micro-dilution method, and their inhibitory activity against acetylcholinesterase and α-glucosidase with DTNB method and PNPG method. The results showed that these five chromenes had no obvious inhibitory activity on the above-mentioned common tumor cells, bacteria and fungi, but they had a certain inhibitory effect on acetylcholinesterase and a very significant inhibitory activity on α-glucosidase. Among the five chromenes, 6-acetyl-2,2-dimethylchromene and 6-acetyl-7-hydroxy-2,3-dimethylchromene were the most active, and theirIC50were better than that of acarbose by about 8 times. The inhibitory activity ofH.annuuschromenes on α-glucosidaseinvivowill be studied in later research.
In summary, the chromene compounds contained inH.annuusdiscs have certain anti-neurodegenerative diseases and anti-diabetic potential.
- Medicinal Plant的其它文章
- Potential Preventive and Therapeutic Effects of Traditional Chinese Medicine Compound Prescription and Traditional Chinese Medicine Products on COVID-19
- Review of Modern Research on Toxicity of Traditional Chinese Medicine Aconm Lateralis Radix Praeparaia
- Advances in Research of Pharmacological Action and Mechanism of Tibetan Medicine Ranasampel
- Research Progress of External Treatment of Acute Gouty Arthritis with Traditional Chinese Medicine
- Study on Pharmacological Effects of Harmine Hydrochloride
- Research Progress in the Application of Network Pharmacology in Traditional Chinese Medicine and Compound Prescriptions

