Larvicidal activity and microencapsulation of tobacco (Nicotiana tabacum) extract on Malacosoma neustria testacea larvae
Yunze Chen·Jing Yang·Guocai Zhang·Bowen Zhang·Jianyong Zeng·Haifeng Zou·Tao Li
Abstract To understand and improve the stability of the insecticidal activity of tobacco extract,the 3rd instar larvae of Malacosoma neustria testacea was determined by the leaf film method.Spectrophotometry identified extract effects on activities of several enzymes.In addition,to improve the stability of the extract,microcapsules were prepared by complex coacervation and phase separation with the extract as core material,and gelatin and gum arabic as wall material.With the embedding rate as the evaluation index,the response surface method was used to optimize the preparation process of the microcapsules.The results show that the extract had a strong insecticidal activity on the larvae,with inhibitory effects on several enzymes examined of carboxylesterase,acetylcholinesterase,glutathione-S transferase,catalase,and superoxide dismutase.The inhibition rate increased with time.The best preparation process of tobacco extract microcapsules was 25% mass fraction of emulsifier,2.05% mass fraction of gelatin,3% mass fraction of gum arabic,1.34 wall core ratio,36 min of complex coacervation time.The embedding rate was 58.4% which is approximately the theoretical embedding rate (58.9%).The microcapsules prepared by this method have a smooth surface,good combining form and particle size distribution,and a median diameter of 8.6 μm.Infrared characteristic peaks of the extracts were preserved at 877.55 cm ?1 and 2922.13 cm ?1 .Microencapsulation can improve the thermal stability of the tobacco extract.Indoor toxicity tests showed that LC 50 of extract microcapsules was 20.2 mg·mL ?1,equivalent to the toxicity level of the tobacco extract itself,indicating that microencapsulation did not reduce extract insecticidal effects.This research may provide a reference for the optimization of the tobacco extract microcapsule preparation process.
Keyword Tobacco extract·Malacosoma neustria testacea·Insecticidal activity·Microcapsule·Optimization
Introduction
Tobacco (Nicotiana tabacumL.) is a crop with high economic value.During its cultivation and processing,residual leaves and stems cannot be used to make flue-cured tobacco,resulting in large amounts of waste,which are often incinerated,not only causing a serious loss of resources but also polluting the environment (Chen et al.2016).By extracting the insecticidal active substances from tobacco waste,the extract may be used to control of agricultural and forestry insect pests.The main bioactive components of tobacco extract are nicotinic acid and solanesol,among which nicotinic acid has good insecticidal properties (Duan et al.2016).It has been reported that tobacco extract has good insecticidal effects on agroforestry pests such as the mealworm beetleAlphitobius diaperinusPanzer (Jacomini et al.2016),the diamondback moth (Plutella xylostellaL.),cabbage aphid (Brevicoryne brassicaeL.) and the green peach aphid(Myzus persicaeSulzer) (Arnoabeng et al.2018).However,plant extracts are susceptible to the external environment,losing their potency and efficacy over time (Paulraj et al.2017).
Malacosoma neustria testaceaMotschulsky (MNT),a subspecies of the lackey moth,is a widely distributed,major defoliator of broad-leaved species.The larvae are active in early May in clusters on twigs around the egg mass.In early June,the larvae are in a stagnation or rest period.When infestation is serious,the tree or shrub may be totally defoliated,often resulting in death.In China,there are several chemical agents to prevent or to control infestations of the moth (Wang et al.2003;Fu and Hu 2014) Although insecticides are often effective,environmental pollution and pest resistance are problematic.Therefore,it has become a priority to develop a pollution-free and environmentally friendly agent for the control of MNT.
Microencapsulation is a technique in which a core material is coated in a wall material by physical,chemical or physicochemical methods,thereby releasing the core material slowly through the wall of the capsule,and extending its efficacy without destroying itself (Kwon et al.2013).Microencapsulation technology has been widely used in the development of plant-derived pesticides such as thyme oil(Chung et al.2013),cinnamon oil (Kim et al.2013) and neem oil (Liu and Xu 2019).Gelatin and gum arabic are natural polymers often used as wall material in the preparation of microcapsules due to their good biocompatibility(Habibi et al.2017).The commonly used microencapsulation technology includes the spray drying method (Yang et al.2018),the complex coacervation method (CCM) (Xiao et al.2014b) and the in situ polymerization method (Nguon et al.2018).The coacervation method causes electrostatic interaction between the two wall materials by changing reaction conditions,thereby embedding the core material.In the reaction process of this method,a reversible reaction may be carried out by changing conditions and,compared with other methods,it does not use an organic solvent.It is a simple,convenient operation for industrial production(Garcia-Saldana et al.2016).However,there have been no reports on the preparation of tobacco extract microcapsules by the coacervation method.
Therefore,in this study,the active substances in tobacco waste were first extracted with absolute ethanol to evaluate its insecticidal effects on MNT.The tobacco ethanol extracted microcapsules were then prepared by the coacervation method.In addition,on the basis of a single factor,the microcapsule preparation process was optimized by the response surface method,and then by surface morphology,particle size,infrared spectrum analysis,and thermal stability analysis.The insecticidal activity of the microcapsules was then determined,with the aim at providing a basis for tobacco waste utilization.
Materials and methods
Tobacco (N.tabacumL.) plants were harvested from Dongning County Heping Forest Farm (44o 0? 36.21?? N,130o 44? 32.23?? E) dried at room temperature (21 °C),crushed into powder and screened through a 140 mesh sieve.M.neustria testacealarvae were collected from the Harbin Experimental Forest Farm of the Northeast Forestry University,Harbin.Healthy,uniform 3-year-old larvae were selected and starved 12 h for the experiment.
Preparation of tobacco ethanol extract
The tobacco powder was placed into an Erlenmeyer flask and anhydrous ethanol added for leaching.The extract was suction filtered,concentrated by rotary evaporation at 40 °C to obtain the extract,and the extraction rate calculated.The extract was stored at 4 °C in the dark.
Extract insecticidal activity and growth of MNT
The extract from 6.25 to 100.0 mg mL?1was prepared with distilled water.The toxicity of the tobacco ethanol extract to 3rd instar MNT larvae was tested by the leaf film method(Zou et al.2017).Fresh,sterilized Mongolian oak leaves were soaked with different concentrations of extract and after the leaves were dried,the petioles were wrapped in moist cotton and placed in an insect bottle together with the starved MNT larvae.Twenty larvae were placed in each bottle and each concentration had three replicates.Bottles were placed in a disease-free constant temperature incubator and deaths recorded every 12 h after feeding up to 72 h.
Antifeedant and growth inhibition rates were determined as described by Zou et al.(2019),with some modifications.Thirty 3rd instar larvae,starved for 12 h,were placed in sterile containers.Fresh leaves of Mongolian oak were treated with sublethal concentration (LC20) and dried in shade.The weight of the leaves was measured at 24,48,and 72 h,and the antifeedant rate was determined based on the mass of the leaves before feeding.Because the leaves can lose weight naturally,the leaves were dried to determine the ratio of dry weight to wet weight so as to accurately determine the feed rejection rate.The method of measuring the growth inhibition rate was measured according to Zeng et al.(2020).The determination method of the TEEM insecticidal activity and growth of MNT is the same as TEE.
Enzyme activity assay
The tobacco ethanol extract,at a sub-lethal dose of LC20(determined by toxicity of the extract for 72 h),was used in the enzyme activity assay.The extract treatment on Mongolian oak leaves and MNT larvae was the same as the preceding toxicity test.The healthy larvae after the extract treatment for 12,24,48 and 72 h were frozen in liquid nitrogen to determine five detoxifying enzyme activities.Carboxylesterase (CarE),glutathione S-transferase (GsTs),acetylcholinesterase (AchE),catalase (CAT),superoxide dismutase (SOD) activities were determined according to Zou et al.(2017).
Preparation of extract microcapsules
A suspension of 20 mL of 3% tobacco extract,1.5% gelatin solution,and 1.5% gum arabic solution were added along with 2 mL of 5% Tween-80 and 1 mL of n-amyl alcohol.The solution was emulsified at 10,000 r min?1for 3 min,placed on a magnetic stirrer and heated to 45 °C.With continuous stirring,a 10% (v/v) glacial acetic acid solution was added dropwise to adjust the pH to 4.0.After 550 r min?1reaction for 30 min,the solution was cooled to 10 °C in an ice water bath,and 10% (v/v) sodium hydroxide solution added to adjust the pH to 8.0.A 2 mL solution of 25% glutaraldehyde was then added dropwise and TEEM obtained after magnetic stirring and cross-linking curing for 60 min at room temperature.The suspension was centrifuged at 5000 r min?1for 3 min,and an extract powder was obtained by drying the filter residue at 45 °C after washing with anhydrous ethanol.
Response surface optimization of extract microencapsulation process
The effects of the emulsifier,gelatin,and gum arabic mass fractions,wall-core ratio and complex coacervation time on the embedding rate of microcapsules were investigated by the single factor method.According to the results,using Design-Expert 8.0 software,three significant factors were selected:gelatin mass fraction (A),wall-core ratio (B) and complex coacervation time (C).The Box-Behnken response surface design was then carried out with the embedding rate(Y) as a response value (Cao et al.2017).The test factors and horizontal coding design were as follows (Table 1):
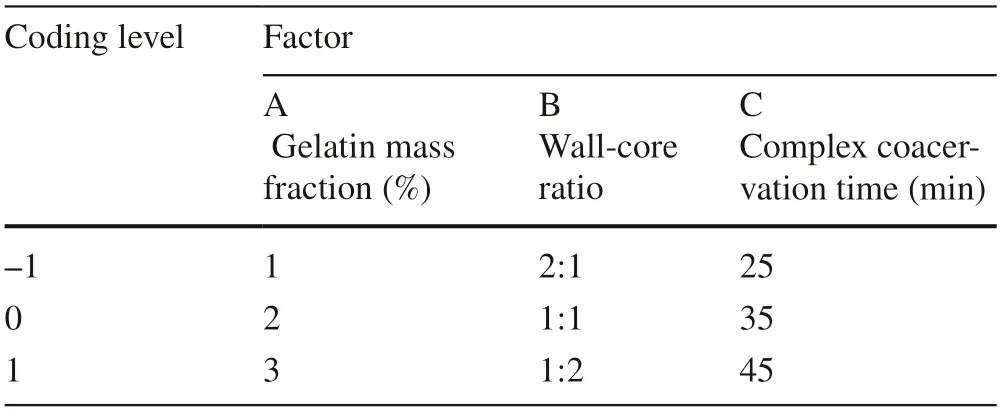
Table 1 Test design factors and coding level
Characterization of the extract microcapsules
Surface morphology was observed by a FEI Quanta 200 scanning electron microscope (FEI,Eindhoven,
Netherlands).Dried microcapsules were place on a stage coated with double-sided conductive adhesive,gold sprayed,and the acceleration voltage set to 12.5 kV.The particle size range and median diameter of the microcapsules were measured by Bt-9300 laser particle size distributor (Dandong Baxter Instrument Co.Ltd.,Dandong,China).The tobacco ethanol extract,gelatin,gum arabic and the extract microcapsules were analyzed by MAGNA-IR56 Fourier transform infrared spectrum (Thermo Nicolet Corporation,Wisconsin,United States).The thermal stability of the extract and the microcapsules were compared by STA449-F3 Thermogravimetric Differential Thermal Analyzer (NETZSCH Instrument Manufacturing Co.Ltd.,Selb,Germany).
Statistical analysis
The toxicity regression was estimated by SPSS software(Version 19.0,SPSS Inc.,Chicago,IL) according to the probit analysis.An ANOVA (P< 0.05) was performed and the mean differences established via Turkey’s or Duncan’s multiple range tests as needed.
Results
Insecticidal activity of tobacco extract
The insecticidal properties of the tobacco ethanol extract on 3rd instar MNT larvae are shown in Table 2.Sublethal concentrations (LC20) of extract treatment for 24 h were 17.3 mg mL?1,the LC50for 24 h 46.6 mg mL?1,and the LC95for 24 h 324.3 mg mL?1.At 48 h,the LC20was 15.4 mg mL?1,the LC5037.3 mg mL?1,and the LC95210.1 mg mL?1.At 72 h,the LC20was 13.5 mg mL?1,the LC5036.5 mg mL?1,and the LC95148.9 mg mL?1.
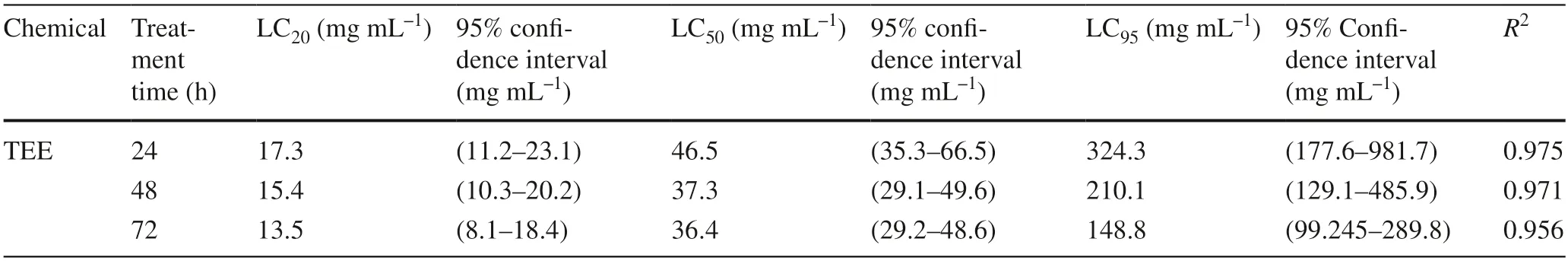
Table 2 Toxicity of tobacco ethanol extract to Malacosoma neustria testacea
Table 3 Response surface test design and results
Inhibitory effects of tobacco ethanol extract on enzymes
To understand the role of the tobacco ethanol extract on MNT larvae,the effects of the extract on enzyme activities was examined.The dynamic changes carboxylesterase,glutathione S-transferase,acetylcholinesterase,catalase,and superoxide dismutase activities were measured after 12,24,48 and 72 h (Fig.1).Carboxylesterase activity was significantly lower than the controls (Fig.1 a),and with time,the inhibition rate increased,reaching 63.5% after 72 h.Similarly,activities of acetylcholinesterase and glutathione S-transferase after treatment were also significantly lower than the controls (Fig.1 b,c).Inhibition increased with treatment time,reaching 37.2% and 68.0% after 72 h,respectively,(Fig.2).Catalase activity was significantly lower than that of the controls (Fig.1 d),with an inhibition rate of 28.9%after 72 h (Fig.2).Superoxide dismutase activity was not significantly different after 12 h but with time (Fig.1 e),the rate of inhibition increased gradually,reaching 34.2% after 48 h.
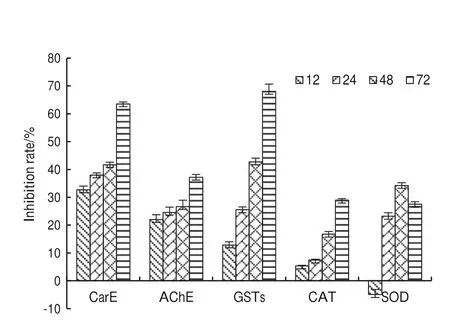
Fig.1 Inhibitory effects of tobacco extract on Malacosoma neustria testacea 3rd instar larvae enzyme activity.Each treatment was repeated in triplicate.Error bars represented the standard error,and data are presented as the mean ± S.E.Statistical significance between samples was evaluated using ANOVA.Different letters on the same list mean significant difference (P < 0.05)
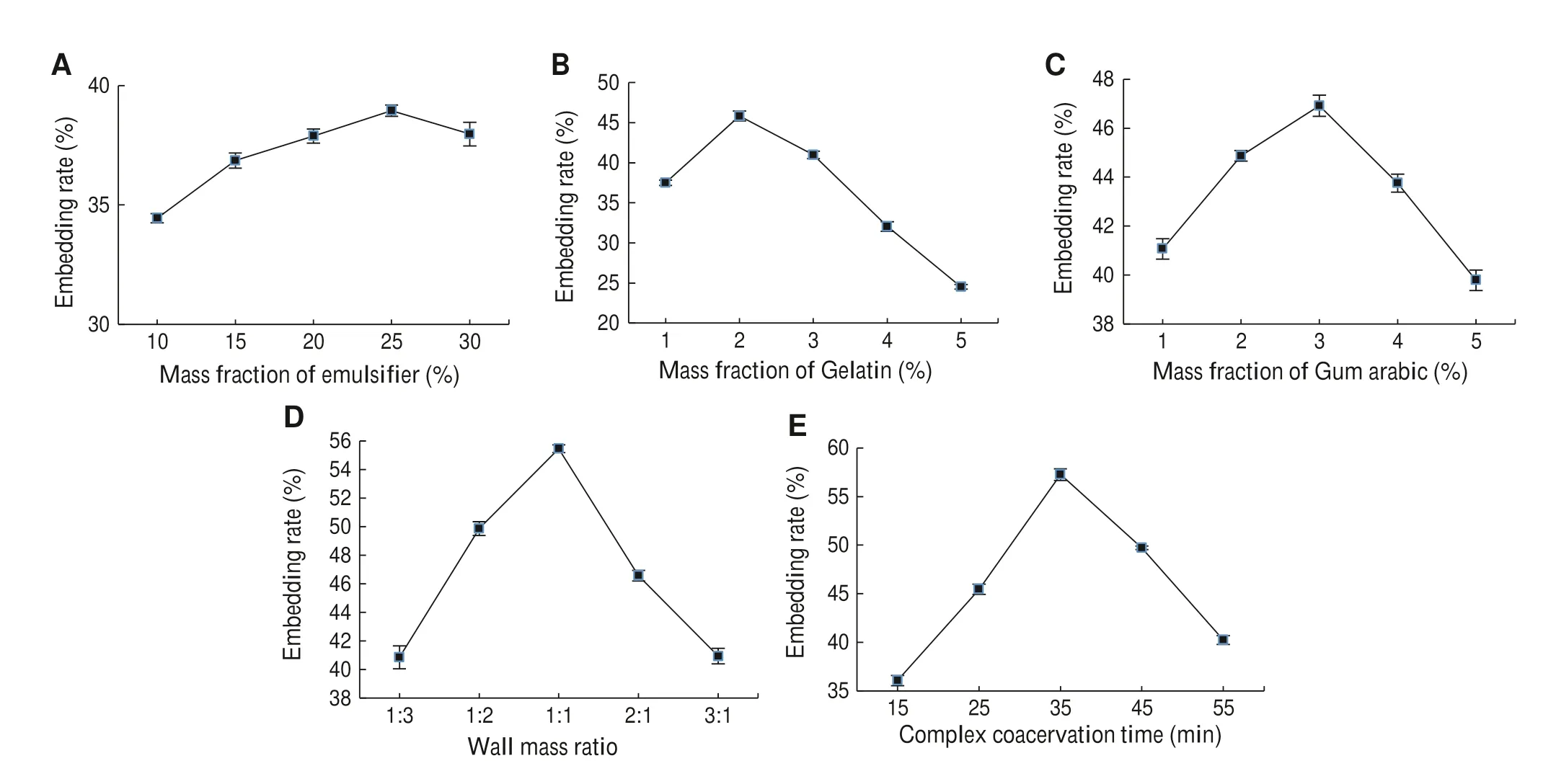
Fig.2 Effects of different factors on the embedding rate of microcapsules;Error bars represented the standard error and data presented as mean ± S.E.The different letters on the same list mean significant difference (P < 0.05)
Optimization of extract microcapsule preparation
The embedding rate of extract microencapsulation first increased and then decreased with the increase of the five factors.When the mass fraction of the emulsifier was 25%,the embedding rate (38.9%) was significantly higher than under other conditions and changed slowly with the change of emulsifier mass fraction (Fig.2 a).When the gelatin massfraction was 2%,the embedding rate (45.8%) was significantly higher than under other conditions and increased rapidly and then slowly declined (Fig.2 b).When gum arabic mass fraction was 3%,the embedding rate (46.9%)was significantly higher and flattened out (Fig.2 c).The embedding rate for the microcapsules increased rapidly and then slowly declined with an increase in the wall-core ratio(Fig.2 d).When the wall-core ratio was 1 :1,the embedding rate (55.5%) was highest.Figure 2 e shows that the embedding rate varies considerably with time.When the complex coacervation time was 35 min,the embedding rate (57.3%)was highest.
Response surface methodology
Three factors,(gelatin mass fraction,wall-core ratio and complex coacervation time),were selected based on the single factor test results.The scheme and results of the Box-Behnken design are shown in Table 3.
Design-Expert 8.0 software was used for a polynomial regression analysis and the quadratic regression equation model fitting all variables is:Y=58.75+0.2A+1.99B +0.79C–0.25AB+1.38AC ? 2.12BC ? 3.30A2? 5.85B2? 2.28C2.
The regression and partial regression coefficient of variance analysis are shown in Table 4 .
Table 4 shows the modelP< 0.0001,meaning that the regression model is highly significant.Lack of fit itemPis 0.3137 (P> 0.05),and the model of fitting is not significant and the test error is small.The model correlation coefficientR2is 0.9964 and the correction coefficient of determinationR2adj is 0.9918,and the coefficient of variation CV is 1.02%,indicating that the model showed a good fit and theregression equation could be used to show the relationship between the chosen factors and embedding rate.
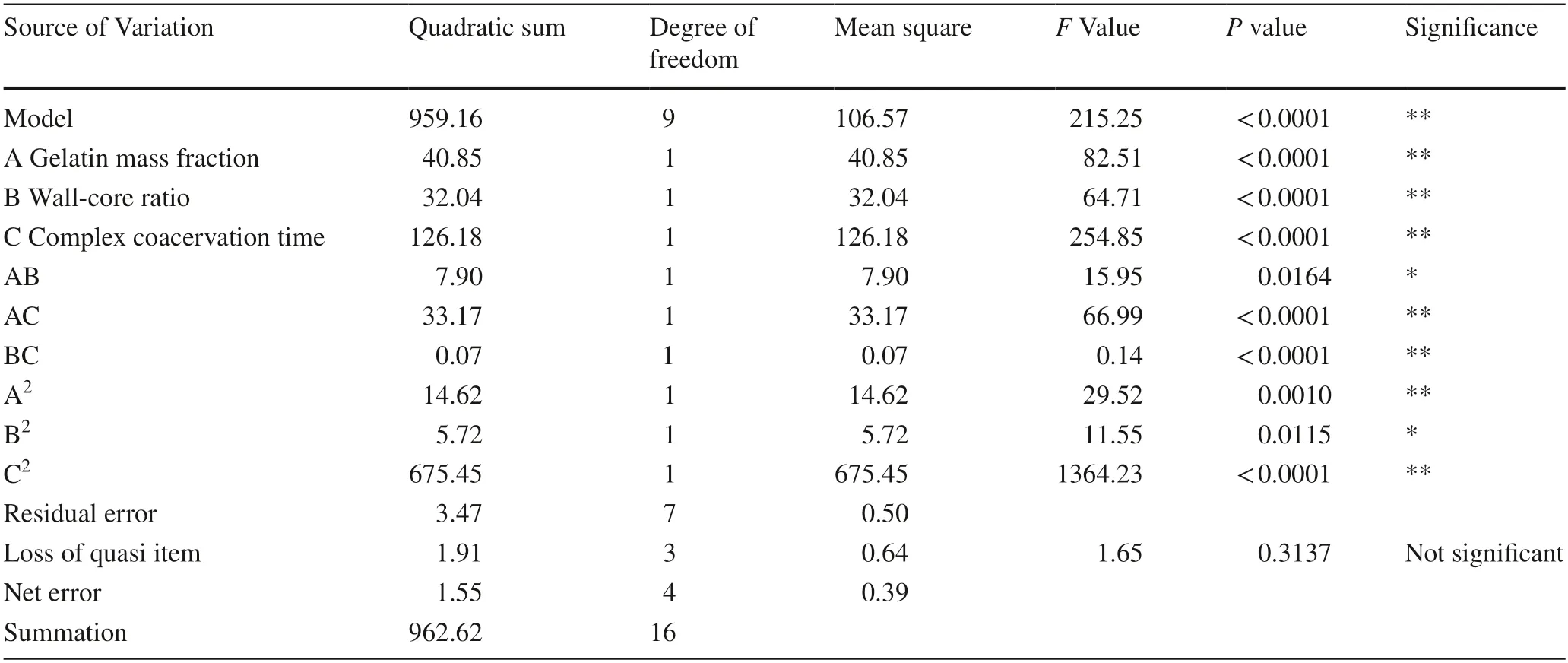
Table 4 Response surface variance analysis table
Space
From the perspective of embedding rate,the primary terms A,B and C have a highly significant influence with C > A > B.Quadratic terms A2and C2highly are highly significant on the embedding rate,while B2has a significant effect.The interaction term BC has a significant impact on the embedding rate,and the impact of AB and AC is highly significant.
Figure 3 shows steep AC and BC response surface curves,indicating that AC and AB have highly significant interaction,while the AB response surface curve is relatively steep with a significant interaction,which is consistent with the results of ANOVA.
The regression model response surface was analyzed and the optimum conditions identified were a mass fraction of gelatin of 2.1%,a wall-core ratio of 1.3 (g:g),a complex coacervation time of 36.3 min.Under these conditions,the predictive value of embedding rate will be 58.9%.When the actual operation is taken into account,the complex coacervation time was approximately 36.0 min,and the average embedding rate reached 58.4% which was close to the theoretical value.
Extract microcapsule insecticidal activity and growth of MNT larvae
An indoor toxicity test of the extract microcapsule was carried out and the results are shown in Table 5.At 24 h,LC20of the microcapsule extract was 25.6 mg mL?1,LC50was 69.2 mg mL?1,and LC95was 481.2 mg mL?1.At 48 h,the LC20was 12.7 mg mL?1,LC5033.3 mg mL?1,and LC95221.2 mg mL?1.At 72 h,the LC20was 9.3 mg mL?1,LC50was 20.2 mg mL?1,and LC95was 80.2 mg mL?1.
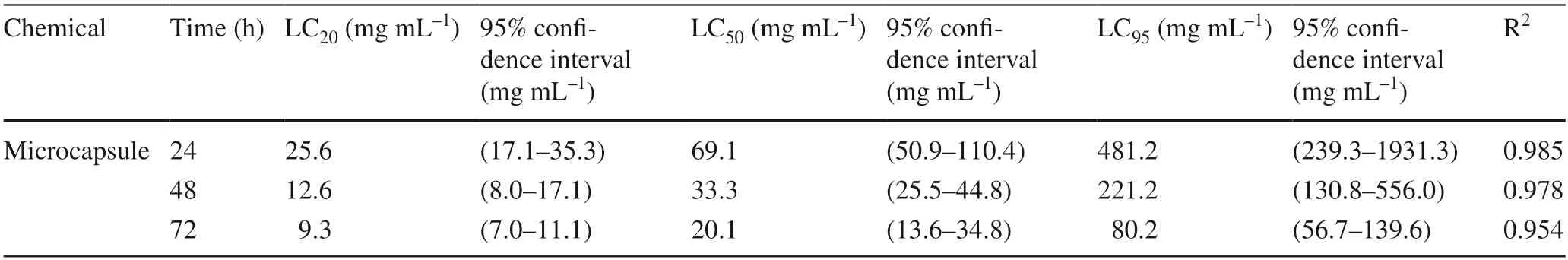
Table 5 Toxicity of microcapsules to Malacosoma neustria testacea
Compared to the insecticidal activity of the tobacco extract,it may be concluded that the confidence intervals of the two overlap and their toxicity levels are similar,indicating that microencapsulation did not reduce the toxicity of tobacco extract.
As shown in Fig.4 a,the antifeedant effect of the tobacco ethanol extract after 24 h was significantly higher than that of the microcapsules (P< 0.05).There is no significant difference in the antifeedant effect between the two after 48 and 72 h (P> 0.05).In Fig.4 b,there is a significant difference between tobacco ethanol extract and microcapsules in inhibiting MNT growth after 24 h and 48 h (P< 0.05).After 72 h,there was no significant difference in growth inhibition between the two (P> 0.05).By comparing tobacco ethanol extract and extract microcapsules on MNT antifeedant rate and growth inhibition rate,tobacco extract inhibits MNT growth and development better than the microcapsules in a shorter period of time.As time increases,there were no significant differences.
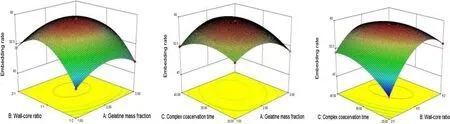
Fig.3 Contour plot and response surface of the effect of factor interaction on the embedding rate
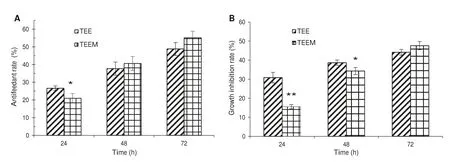
Fig.4 The effect of TEE and TEEM on Antifeedant rate and Growth inhibition rate.Note:*Significant difference (P < 0.05);**difference is extremely significant (P < 0.01)
Surface morphology and particle size
Scanning electron microscopy was used to observe the composition of microcapsules and from Fig.5,they have a good cystic appearance and smooth surface with no adhesion.Figure 6 shows a median particle size of the microcapsule was 8.63 μm and particle size distribution was concentrated between 4.72 and 17.05 μm.

Fig.5 Scanning electron microscope images of tobacco extract microcapsules
Infrared spectrum analysis
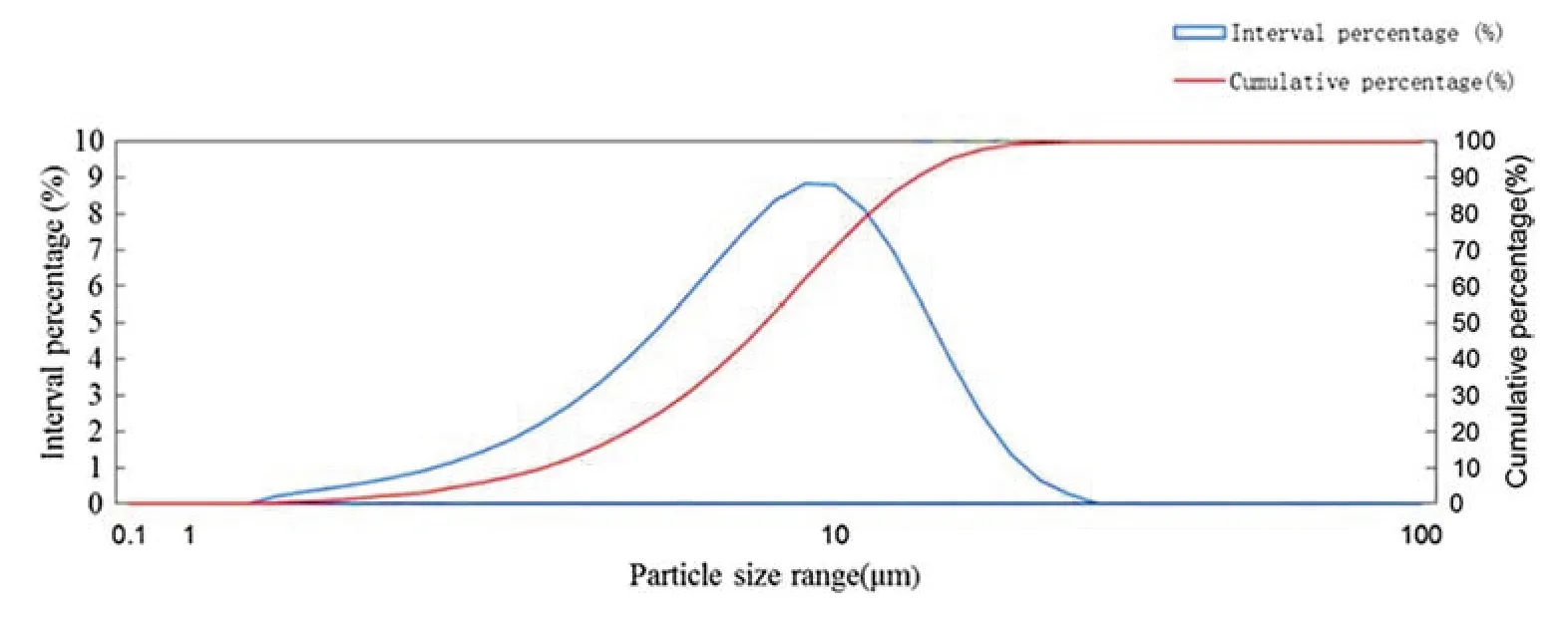
Fig.6 Particle size distribution map of tobacco extract microcapsules
Figure 7 a shows the results of infrared spectrum analysis of gelatin.C-H bending vibration peaking at 1078.97 cm?1,a C-N stretching vibration peak at 1232.29 cm?1.A C-N coupling absorption peak and N–H in-plane bending at 1521.56 cm?1.Amide-carbonyl stretching vibration peak at 1628.59 cm?1,alkyl stretching vibration peak at 2934.16 cm?1,and N–H amino stretching vibration peak at 3273.57 cm?1(Comunian et al.2013).In Fig.7 b,the bending vibration peak at 1015.82 cm?1was a C-H plane,at 1592.43 cm?1it was a -coo-asymmetric stretching vibration peak,at 2893.67 cm?1a carboxylic acid-coo-vibration peak,and at 3273.85 cm?1it was N–H amino stretching vibration peak (Gimenez et al.2011;Xiao et al.2014a) From the infrared spectra of the extract microcapsule (Fig.7 c)and the tobacco ethanol extract (Fig.7 d),the characteristic absorption peaks of TEE were retained at 877.45 cm?1and 2922.11 cm?1.The characteristic absorption peaks disappeared at 1521.56 cm?1,3273.57 cm?1and 3273.57 cm?1,indicating the disappearance of the amino group with a positive charge.The contraction vibration peaks of the amide carbonyl group were preserved at 1634.86 cm?1;the contraction vibration peaks of -coo-asymmetric carboxylic acid retained at 1592.6 cm?1and 2893.6 cm?1,which meant the disappearance of the carboxylic -COO ? with a negative charge.The change of characteristic peaks indicated that the compound condensation reaction of gelatin -NH3+and acacia -COO ? occurred,and the tobacco extract was successfully coated by the compound condensation of gelatin and gum arabic (Shaddel et al.2018).
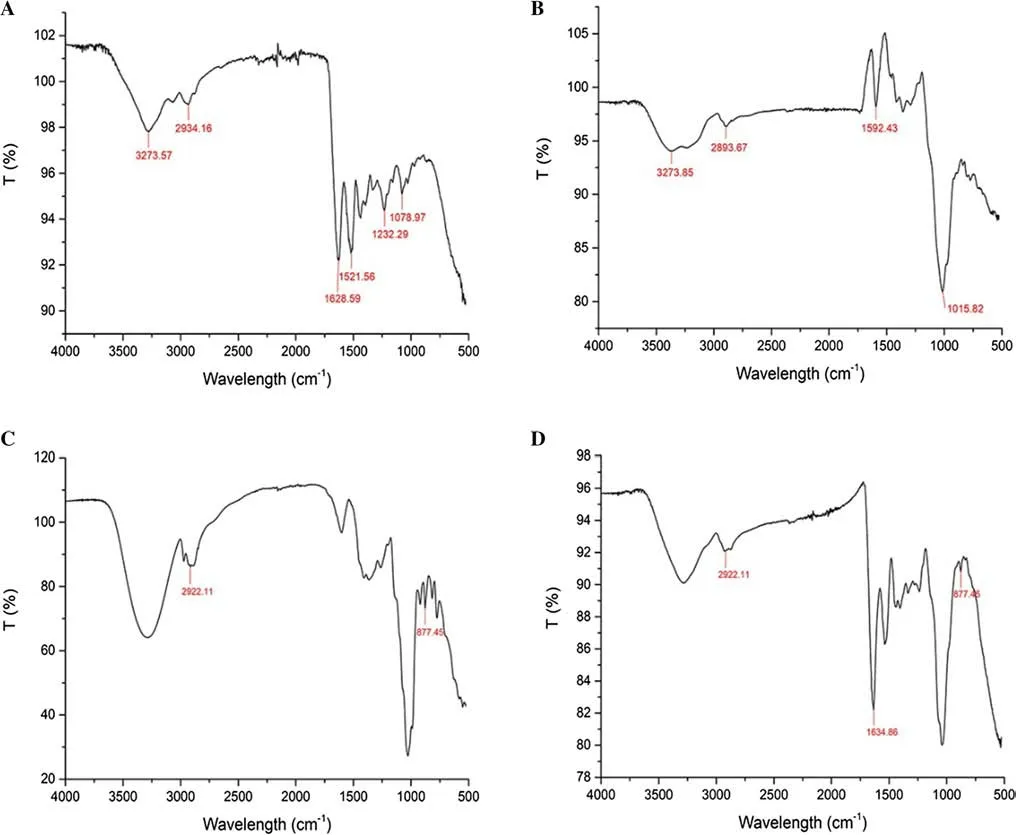
Fig.7 Infrared spectroscopic analysis
Thermal stability analysis
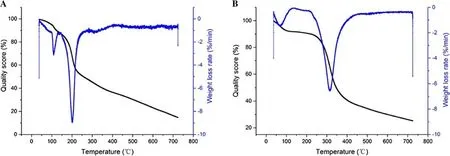
Fig.8 Thermal stability analysis.a The thermal stability of TEE,and b the thermal stability of TEEM
Figure 8 a shows the results of the thermal stability of tobacco extract.The 5.6% drop in total weight at the beginning is due to the decomposition of small molecular substances and the loss of water.When temperatures reached 104 °C,the mass fraction of the tobacco extract decreased rapidly,and weight loss increased rapidly.The weight loss rate in this process was as high as 40.1%,which was due to the start of the decomposition of macromolecular organic compounds.When the temperature reached 221 °C,the decomposition rate of tobacco extract remained constant and there was no obvious peak in weight loss.Tobacco extract begins to decompose at the beginning of the reaction.The rate of weight loss does not stabilize until temperatures reach 221 °C,so the thermal stability is very unstable.Figure 8 b shows the results of the tests of thermal stability of the tobacco extract microcapsules.The decomposition process has four stages.From the beginning of the reaction to 91 °C,the microcapsule mass fraction decreased slowly with a weight loss of 7%.From 91 °C to 259 °C,weight loss was slight and the weight loss rate remained at 0.2%·min?1.When temperatures reach 259 °C to 378 °C,the microcapsule mass fraction decomposition rate was faster and the maximum weight loss rate 6.5%·min?1.Comparing the thermal stability analysis charts of tobacco extract and tobacco extract microcapsules,it can be seen that microencapsulation can significantly improve the thermal stability of tobacco extract.This is because the complex agglomerates formed by gelatin and gum arabic are coated with the material to improve the thermal stability of the tobacco extract.
Discussion
Many plants have insecticidal properties (Zou et al.2019),such as marigold,Tagetes erectaL.,(Islam and Talukder 2005),tripterygium (Tripterygium wilfordiiHook.f.) (Ma et al.2014) and garlic (Allium sativumL.) (Sadeghi et al.2008).Hu et al.(2008) studied celandine (Chelidonium majus) andArchangelica decurrensextracts and reported stomach toxicity to 3rd instar MNT larvae.This study also showed that tobacco extract caused stomach toxicity to 3rd instar MNT larvae,and the toxicity increased with time.
In response to these insecticidal active substances,insects have developed highly effective antioxidant defense systems,including antioxidant and detoxifying enzymes (Zhao et al.2016).The effects of tobacco ethanol extracts on key enzymatic activities were examined in this study.Carboxylesterase is an important detoxifying enzyme to metabolize a large number of endogenous and exogenous toxins to maintain normal physiological activities (Wang et al.2019).Active substances in plants can inhibit this enzyme in insects.For example,Ginkgo bilobaextracts inhibit carboxylesterase activity ofHyphantria cuneaDrury larvae (Pan et al.2016).This study indicates that the tobacco ethanol extract significantly inhibits carboxylesterase activity ofM.neustria testacea.This may be due to the extract combining with an esterase,resulting in the insect’s inability to convert ester compounds into alcohols and acids,leading to death (Tang et al.2012).Acetylcholinesterase can rapidly hydrolyze acetylcholine at the level of insect choline synapses to stop the transmission of nerve impulses and maintain normal nerve impulses (Felton and Tumlinson 2008).For example,Solanum rostratumDunal extracts have significant inhibitory effects on the acetylcholinesterase enzyme of the cotton boll worm (Liu et al.2020).This research shows that the tobacco ethanol extract significantly inhibits MNT larvae which may be due to the combination of the extract with the nicotinic acetylcholine receptor in the larvae to interfere with neurotransmission (Tong et al.2013).Secondary metabolites fromBrassicaplants inhibit glutathione S-transferase enzyme activity in the green peach aphid,M.persicae(Sulzer)(Francis et al.2005).The results of our study also indicate that the tobacco ethanol extract significantly inhibits glutathione S-transferase activity in MNT larvae,which may be due to the effect of the extract on nucleophilic conjugation reactions between endogenous glutathione and exogenous electrophilic substances.MNT larvae cannot metabolize lipophilic organic substances and this leads to death (Sun et al.2020).In addition,the tobacco ethanol extract has different inhibitory effects on the activities of superoxide dismutase and catalase.These inhibitions cannot clear the MNT hydrogen peroxide and ROS,thereby producing toxic effects on the larvae (Cui et al.2019).
Due to the instability of plant-derived pesticides,the tobacco extract must be prepared in microcapsule form.The particle size distribution of the microcapsules prepared by the CCM (complex coacervation method) described in this study were 4.72 to 17.05 μm.Infrared spectroscopy analysis of the microcapsules revealed that amino groups of the gelatin had caused an electrostatic effect with carboxyl groups of the gum Arabic.The microcapsules retained the characteristic absorption peaks of tobacco at 877.55 cm?1and 2922.13 cm?1,indicating that the formation of microcapsules was a reaction between gelatin and gum arabic,but not with tobacco extract which was consistent with (Comunian et al.2013) By comparing the thermal stabilities of tobacco extract and the microcapsules,it was determined that microencapsulation improves the thermal stability of the tobacco extract.This is because the complex agglomerates formed by gelatin and gum arabic cover the extract and decompose with heat to protect the core material.Comparing indoor toxicity effects of extract and microcapsules on MNT larvae,showed that microencapsulation did not reduce the toxicity of the tobacco extract;this is consistent with Xin et al.(2018).The effects of extracts and microcapsules on larvae growth and development indicated that microencapsulation did not reduce the inhibitory effect of the extract but achieves this by slow release (Yong et al.2006).This is because the tobacco extract does not react but is coated by the capsular material.
In general,studying how tobacco extracts affects antioxidant enzyme activities in MNT larvae helps reveal insecticidal mechanisms of plant-derived pesticides.The microencapsulation process was optimized by the response surface method to significantly improve the stability of the extract.Therefore,the study may provide a possible format for forest pest control.
Conclusions
Tobacco ethanol extract has good insecticidal activity against larvae ofM.neustria testaceand inhibits enzyme activities.To improve the extract insecticidal activity,microcapsules were prepared by the complex coacervation method and results showed that the best preparation process was 25%mass fraction of emulsifier,2.0% mass fraction of gelatin,3.0% mass fraction of acarax,a 1.34 wall-core ratio,36 min of complex coacervation time,and 58.9% theoretical embedding rate.The microcapsules prepared by this method can successfully wrap tobacco extract and significantly improve its stability.
Author contributionsYunze Chen and Jing Yang contributed equally to this work.
 Journal of Forestry Research2021年4期
Journal of Forestry Research2021年4期
- Journal of Forestry Research的其它文章
- Flexible transparent wood enabled by epoxy resin and ethylene glycol diglycidyl ether
- A new species of Exoristobia (Hymenoptera:Encyrtidae)from China
- Resistance genes mediate differential resistance to pine defensive substances α-Pinene and H2 O2 in Bursaphelenchus xylophilus with different levels of virulence
- Investigation of beetle species that carry the pine wood nematode,Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle,in China
- Termite-killing components in Serratia marcescens (SM1)
- Driving force of soil microbial community structure in aburned area of Daxing’anling,China
