Description of a new species of the genus Uropsilus(Eulipotyphla: Talpidae: Uropsilinae) from the Dabie Mountains, Anhui, Eastern China
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium,provided the original work is properly cited.
Copyright ?2021 Editorial Office of Zoological Research, Kunming Institute of Zoology, Chinese Academy of Sciences
Received: 14 September 2020; Accepted: 15 April 2021; Online: 28 April 2021
Foundation items: This work was supported by the National Science &Technology Fundamental Resources Investigation Program of China(2019FY101800), Biodiversity Survey, Observation and Assessment Program of Ministry of Ecology and Environment, China (2019HB 2096001006), Anhui Province Higher Education Revitalization Plan,2014 Colleges and Universities Outstanding Youth Talent Support Program
During a terrestrial vertebrate survey of the Dabie Mountains in Anhui Province, eastern China, we collected four Asian shrew mole specimens (hereafter, shrew moles). Based on published literature and comparison with previously collected materials, the four specimens were similar to shrew moles from the mountains of Southwest China; however, no species in this group has been previously recorded from the Dabie Mountains. The genetic and morphological characteristics of the specimens were analyzed, based upon which a new species of shrew mole is described, namedUropsilus dabieshanensissp. nov.
Shrew moles in the genusUropsilus(Eulipotyphla, Talpidae,and Uropsilinae) inhabit montane forests and shrub grasslands at an altitude of 1 500 to 2 700 m a.s.l. (Hoffmann& Lunde, 2008; IUCN, 2017) in southwestern China, Bhutan,and Myanmar (Jiang et al., 2016, 2017; Smith & Xie, 2008).Uropsilusretains the morphological characteristics and terrestrial living habits of primitive moles, including small forefeet not broadened for burrowing and external ears (Allen,1938; Smith & Xie, 2008). Furthermore,Uropsilusforms the basal branch in the phylogenetic tree of Talpidae (Douady &Douzery, 2003; He et al., 2017; Motokawa, 2004; Sánchez-Villagra et al., 2006).
The classification of shrew moles has undergone several major adjustments over more than a century. Based on collected type specimens, Milne-Edwards (1871) and Thomas(1912) successively established three genera (Uropsilus,Rhynchonax, andNasillus), four species (Uropsilus soricipes,Rhynchonax andersoni,Nasillus gracilis,N. investigator), and two subspecies (R. a. atronates,R. a. nivatus) (Allen, 1923;Milne-Edwards, 1871; Thomas, 1911, 1922), although these classifications were later widely disputed (Ellerman &Morrison-Scott, 1951; Howell, 1929; Osgood, 1937). In 1937,Osgood divided Uropsilinae into two genera (Uropsilus,Nasillus), and incorporatedRhynchonaxintoUropsilusas an intermediate transition type. However, Ellerman & Morrison-Scott (1951) assigned all genera and species into one species with five subspecies (U. s. soricipes,U. s. gracilis,U. s.andersoni,U. s. investigator,U. s. nivatus), while later scholars recognized four effective species within the genus,i.e.,U. soricipes,U. gracilis,U. andersoni, andU. investigator(Hutterer, 2005; Smith & Xie, 2008; Wang & Yang, 1989). With the discovery ofU. aequodoneniain 2013, Uropsilinae is now considered to contain five species (Liu et al., 2013). In this study, for the first time, shrew mole specimens were collected in the Dabie Mountains of eastern China, far from its original distribution range in the montane areas of southwestern China, Bhutan, and Myanmar (Jiang et al., 2017). Based on morphological comparisons and molecular phylogenetic and species delimitation analyses, we report that the newly collected specimens represent a new species withinUropsilus(Figure 1A).
DNA was extracted from the livers and muscles of the four specimens (Sambrook et al., 1989) and polymerase chain reaction (PCR) was performed, targeting sequences of mitochondrial DNA marker cytb(Irwin et al., 1991) and nuclear markersRAG1,RAG2, andPLCB4(Wan et al., 2013)(Supplementary Methods). Uncorrected pairwise genetic distances of the cytbgene were computed (Supplementary Methods). Bayesian inference (BI) and maximum parsimony(MP) approaches were used for phylogenetic analysis of the four specimens and 95 individuals of other species obtained from GenBank (Supplementary Table S2). BEAST v1.10.4(Suchard et al., 2018) was used to construct Bayesian trees and estimate the time of species differentiation(Supplementary Methods). In addition, the Bayesian Poisson tree processes (bPTP) (Zhang et al., 2013a), Bayesian Phylogenetics and Phylogeography (BPP v3.4) (Yang &Rannala, 2010), and Automatic barcode gap discovery(ABGD) (Puillandre et al., 2012) methods were used to perform tree-based multi-site merging and species delimitation to determine the unknown species (Supplementary Methods).According to phylogenetic analysis, morphometric measurements were obtained from the skulls of the four collected specimens and 23 otherUropsilusspecimens with Vernier calipers to the nearest 0.01 mm (Table 1). Principal component analysis (PCA) and discriminant function analysis(DFA) were used to compare samples (Supplementary Methods).
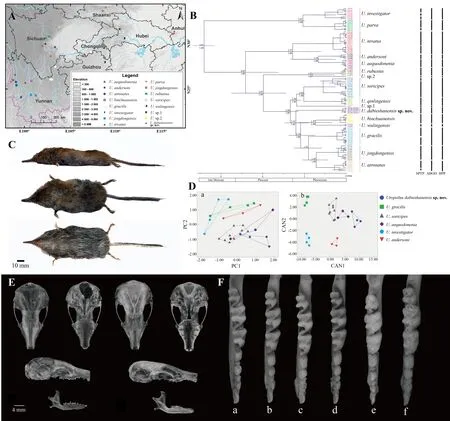
Figure 1 Distribution, molecular, and morphological comparisons ofUropsilus,with views and crania ofUropsilus dabieshanensissp. nov.
Supplementary Figure S1 shows the BI and MP analysis results, which were consistent with each other. In the phylogenetic tree, the four new specimens were clustered on one branch with high nodal support (Bayesian posterior probability=1.00; MP bootstrap values=97). Within the genusUropsilus, the smallest pairwise uncorrected distance was betweenU. soricipesandU. qinlingensis(4.1%), and the largest was betweenU. atronatesandU. investigator(19.8%)(Supplementary Table S3). The genetic distance between the four specimens showed low variation (≤0.62%), and the genetic distances between the four new specimens and other species in the genus ranged from 10.5% (vsU. gracilis) to 17.8% (vsU. investigator).
In total, 95 sequences ofUropsilusobtained from GenBank and the four newly collected samples were used for analysis(Supplementary Table S2). Species delimitation analysis using bPTP, BPP, and ABGD produced different results, suggesting 15-23 potential species in the genusUropsilus(Figure 1B).However, all three analyses supported the clade of the new specimens as an independent species. Time to the most recent common ancestor (MRCA) ofUropsiluswas estimated to be 6.81 million years ago (Ma) (95% confidence interval(CI)=4.79-9.16), and the unknown species clade diverged from the other species clades in the early Pleistocene ~2.41 Ma (95% CI=1.83-2.95) (Figure 1B). Thus, the species delimitation results supported the specimens as a new species, consistent with the phylogenetic and genetic distance results.
Based on PCA of the morphological data of the skull, two major components were extracted, which together accounted for 69.02% of total variation (Supplementary Table S4). The first principal component (PC1) accounted for 51.41% of the variation and was positively correlated with all variables(eigenvalue=5.655), thus reflecting differences in the whole skull. The second principal component (PC2) accounted for 17.61% of the variation and was dominated by the height of the braincase (HB) and length of the mandibular tooth row(LMTR) (eigenvalue=1.937). As seen in the PCA plot, the four specimens occupied the positive region of PC1, indicating that their skulls were larger than those ofU. soricipes, and occupied the negative region of PC2, indicating that their mandibular toothrows and HBs were smaller than those ofU.gracilis,U. investigator, andU. andersoni(Figure 1D). DFA correctly classified 100% (n=27) of the six groups of samples.Canonical axes 1-4 explained 77.8%, 14.1%, 6.3%, and 1.5%of the total change, respectively (Figure 1D; Supplementary Table S4). Thus, based on PCA and DFA, which indicated that our specimens and five other species occupy different regions in the morphological space of skull features, we considered the four specimens to be a new species.
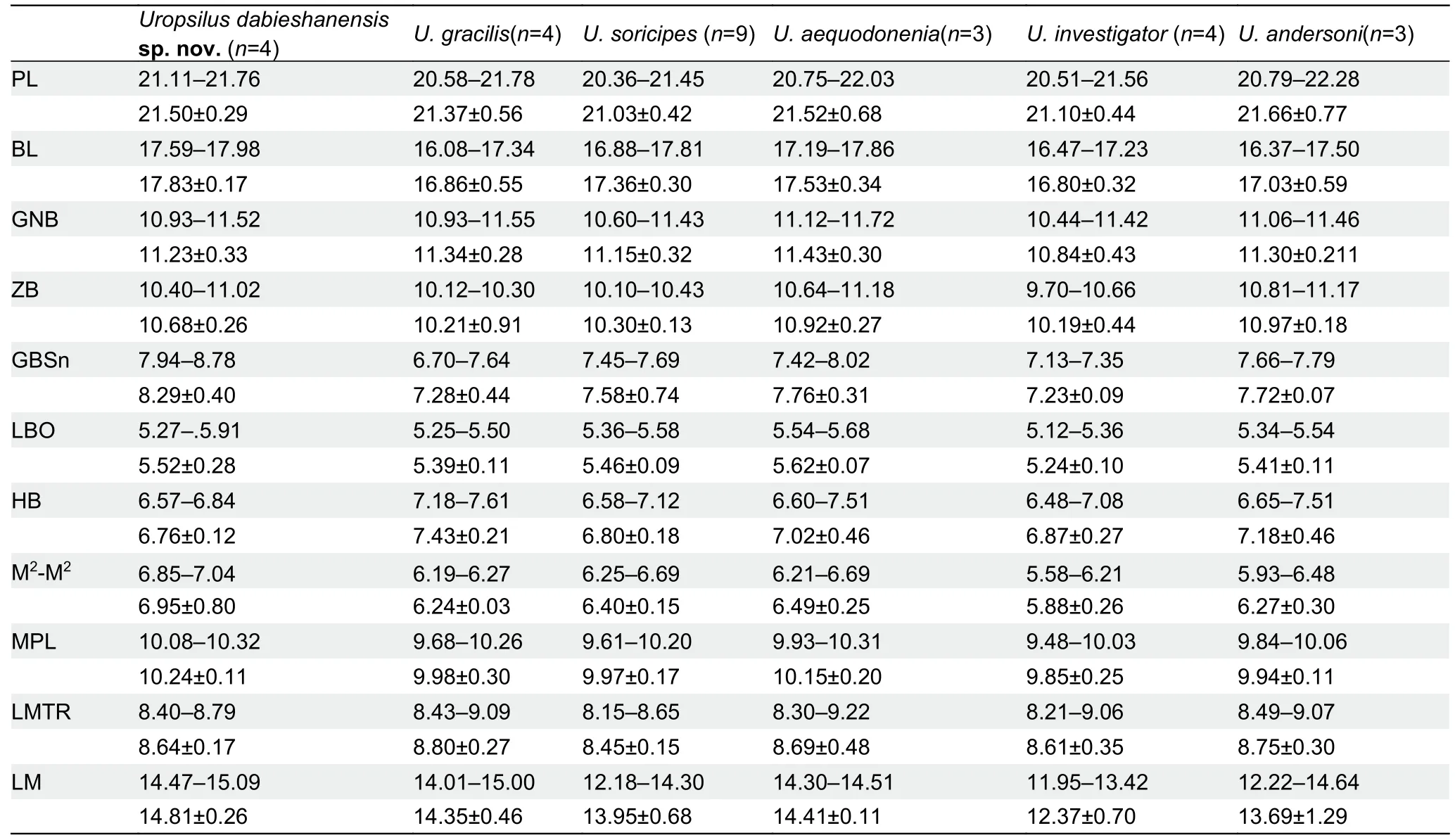
Table 1 Means (±SD) and ranges of skull measurements (mm) in morphometric analyses of genus Uropsilus
Taxonomic account
Uropsilus dabieshanensis sp. nov.
Holotype:Specimen AE1807FZL007, adult male, collected in July 2018 by Zhen Xu and Ruo-Lei Sun from Foziling Provincial Nature Reserve, Anhui Province, China (N31.119°,E116.245°, altitude 1 064 m a.s.l.). The specimen was deposited in the Biological Museum of Anhui University,Anhui, China.
Paratypes:Three females; AE1612YLP017 collected in December 2016 by Heng Zhang and Lei Zhou from Yaoluoping National Nature Reserve, Anqing, Anhui Province,China (N30.964°, E116.075°, altitude 1 109 m a.s.l.);AE1907FZL001 collected in July 2019 by Zhen Xu and Ruo-Lei Sun from Huoshan County, Foziling Provincial Nature Reserve, Anhui Province, China (N31.112°, E116.227°,altitude 1 093 m a.s.l.); AE2005YLP001 collected in May 2020 by Jie Shi from Yaoluoping National Nature Reserve, Anqing,Anhui Province, China (N30.982°, E116.127°, altitude 1 273 m a.s.l.).
Distribution: Uropsilus dabieshanensis sp. nov.is currently known from the Yaoluoping National Nature Reserve and Huoshan Foziling Provincial Nature Reserve, Anhui Province,East China. The known elevational range is 1 064-1 273 m. It may occur in other areas in the Dabie Mountains.
Etymology:The specific name refers to the type locality of the new species, i.e., Dabie Mountains, Anhui Province,China. We suggest ‘Dabie Mountains shrew mole’ as the English common name and ‘大別山鼩鼴’ as the common Chinese name for the new species.
Diagnosis:Ten teeth in upper jaw, nine teeth in lower jaw.Dental formula i2/1, c1/1, pm4/4, m3/3=38. Hair on back dark gray and dark brown, hair on abdomen dark gray, color difference between back and abdomen not obvious. Front and back feet with black speckled scales, feet smaller than those of other species in genus. Two-colored tail, upper part black,lower part light pink (Figure 1C).
Description:Medium-sized Asian shrew mole. Long lip protruding from front of head, with light gray beard of varying length on end of rhynchodaenm (Figure 1C). Triangular ears covered by hair with gray tips. Forefoot short, hindfoot slender and well-developed with five fingers. Thin tail, with annular scales and short hairs growing between each scale. Total length 118.6-136.4 mm, tail length 52.4-54.1 mm, ear height 8.1-8.9 mm, hindfoot length 12.8-12.9 mm, and weight 6.2-8.9 g.
Wide skull at anterior cusp, round brain, and semicircular occipital bone (Figure 1E). Parietal suture composed of herringbone and arrow shapes, arch complete. Dental formula i2/1, c1/1, pm4/4, m3/3=38; first upper incisor of upper jaw slightly larger than second upper incisor. Canine teeth visibly distinct, smaller than second upper incisor, but approximately same size as first premolar p1; second premolar p2 larger than first premolar p1 and third premolar p3, and fourth premolar p4 larger than first three premolars; second mandibular m2 developed, with “W” shape, larger than first mandibular m1 and third mandibular m3. First mandibular m1 and third mandibular m3 relatively degraded, and “W” teeth tips incomplete. Lower mandibular first incisor i1 larger than lower canine teeth, first lower premolar pm1 slightly smaller than lower canine teeth. First lower premolar pm1 and third lower premolar pm3 same size. Second lower premolar pm2 and fourth lower premolar pm4 larger than pm3 and pm4, pm4 largest. Mandible first molar m1 slightly larger than or equal to third molar m3; mandible second molar m2 with lateral tooth tip and “W” shape visibly larger than first molar m1 and third molar m3 (Figure 1F).
Morphological comparison:Multivariate analyses (PCA and DFA, Figure 1D) revealed obvious differences betweenUropsilus dabieshanensissp. nov. and recognizedUropsilusspecies, which could be easily distinguished based on teeth and crania (Figure 1E-F).
Uropsilus dabieshanensissp. nov. can be distinguished from certain congeners based on its dental formula of i2/1,c1/1, pm4/4, m3/3=38 (Figure 1F) (vs i2/1, c1/1, pm3/3,m3/3=34 inU. soricipes, i2/ 2, c1/1, pm4 /3, m3/3=38 inU.andersoni, and i2/2, c1/1, pm3/3, m3/3=36 inU.aequodonenia).Uropsilus dabieshanensissp. nov. can be further distinguished based on cranial measurements(Table 1), with a longer ‘snout’ breadth and mandible length(GBSn=7.94-8.78, LM=14.47-15.09) thanU. soricipes(GBSn=7.45-7.69, LM=12.18-14.30), and longer basal length and median palatal length (BL=17.59-17.98, MPL=10.08-10.32) thanU. andersoni(BL=16.37-17.50, MPL=9.84-10.06). In addition,Uropsilus dabieshanensissp. nov.has a narrower anterior labial margin (M2-M2=6.85-7.04) of the second upper molars thanU. aequodonenia(M2-M2=6.21-6.69).
AlthoughUropsilus dabieshanensissp. nov. shares the same dental formula asU. gracilisandU. investigator, their skulls are very different (Table 1).Uropsilus dabieshanensissp. nov. has a longer and wider skull and shorter height of braincase (BL=17.59-17.98, ZB=10.40-11.02, HB=6.57-6.84)thanU. gracilis(BL=16.08-17.34, ZB=10.12-10.30, HB=7.18-7.61). Overall, the skull ofUropsilus dabieshanensissp. nov.(BL=17.59-17.98, M2-M2=6.85-7.04, MPL=10.08-10.32,LM=14.47-15.09, GBSn=7.94-8.78) is obviously larger than that ofU. investigator(BL=16.47-17.23, M2-M2=5.58-6.21,MPL=9.48-10.03, LM=11.95-13.42, GBSn=7.13-7.35),especially mandible length.
Taxonomic implications and distributional patterns:Phylogenetic analysis showed that theUropsilus dabieshanensissp. nov. branch acted as a monophyletic group in all phylogenetic trees with high support(Supplementary Figure S1). However, the topology of the species tree constructed using BEAST was slightly different from the other phylogenetic trees. A similar phenomenon has been discussed in previous literature (Zhang et al., 2013b)and phylogenetic studies ofUropsilus(Wan et al., 2018),which may be due to limitations of the BEAST tree model.Although there were differences among the phylogenetic trees reconstructed by diverse analysis methods, they consistently harvested the same independent branch for the new specimens, indicating thatUropsilus dabieshanensissp. nov.is an authentic species.
In species delimitation analysis, bPTP revealed additional candidate species withinUropsiluscompared with the BPP and ABGD methods (23, 16, and 15, respectively). However,all methods supported the specific status ofUropsilus dabieshanensissp. nov. and other species inUropsilus.Moreover, these results indicate that species diversity inUropsilusis substantially higher than previously thought(Figure 1B).
The phylogenetic results showed that the lineage forUropsilushad two matrilines, one from the Gongshan Mountains west of the Nujiang River (Salween River),includingU. investigatorandU. parva, and the other from the mountains of Southwest China east of the Nujiang River and the Dabie Mountains (Figure 1A). From the view of spatial distribution,Uropsilus dabieshanensissp. nov. in the Dabie Mountains is distant from other species in the genusUropsilus(Figure 1A). From the perspective of vertical elevation,previously discovered Asian shrew moles mainly inhabit mountain forests at elevations of 1 400-3 600 m a.s.l.(Hoffmann & Lunde, 2008; Smith & Xie, 2008). However, the average habitat ofUropsilus dabieshanensissp. nov. was 1 064-1 273 m a.s.l.. The distribution patterns ofUropsilus dabieshanensissp. nov. are similar to those ofEothenomys colurnusin the mountainous regions of South-Central China,which was recently elevated as a valid species in the genusEothenomys(Liu et al., 2019).
Uropsilus dabieshanensissp. nov. formed a single lineage in the early Pleistocene ~2.41 Ma (Figure 1B). Topographical changes in the Dabie Mountains gradually stabilized by the end of the Tertiary (Feng, 1976). Thus, the Dabie Mountains may have providedUropsiluswith continuous suitable habitat,which helped buffer the dramatic climatic changes that occurred in the Pleistocene. Moreover, other species in the same lineage asUropsilus dabieshanensissp. nov. are distributed in the mountains of Southwest China. Therefore,this may have promoted the formation of allopatric speciation within a long evolutionary time frame through physical isolation, resulting in a new species (He et al., 2019).Uropsilus dabieshanensissp. nov. expands the known range ofUropsilusto the Dabie Mountains in eastern China.
Operational key to species of the genus Uropsilus*
1) Number of upper and lower jaw teeth equal, dental formula i2/2, c1/1, pm3/3, m3/3=36 ...........................U. aequodoneniaNumber of upper and lower jaw teeth not equal .............2
2) Ten teeth on one side of upper jaw, two incisors on lower jaw, dental formula i2/2, c1/1, pm4/3, m3/3=38 .................................................................................................U. andersoni
Nine or 10 teeth on one side of upper jaw, one incisor on lower jaw teeth.......................................................................3
3) Nine teeth on one side of upper jaw, eight teeth on one side of lower jaw, dental formula i2/1, c1/1, pm3/3, m3/3=34..................................................................................U.soricipes
Ten teeth on one side of upper jaw, nine teeth on one side of lower jaw, dental formula i2/1, c1/1, pm4/4, m3/3=38.............................................................................................4
4) Medium size, coat color difference between back and abdomen not obvious.............................................................5
Small size, coat color on back and abdomen different......7
5) Tail, back, and abdomen coat color jet black.............................................................................................U. investigator
Coat color on back dark brown............................................6
6) Coat color on abdomen dark gray and dark brown...........................................................................................U. gracilis
Coat color on abdomen dark gray .............................................................................Uropsilus dabieshanensissp. nov.
7) Coat color on back chestnut red......................U. atronates
Coat color on back black gold..................U. nivatus
* Description of some species quoted from Wan (2015).
NOMENCLATURAL ACTS REGlSTRATlON
The electronic version of this article in portable document format represents a published work according to the
International Commission on Zoological Nomenclature (ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5-8.6 of the Code). This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefixhttp://zoobank.org/.
Publication LSID:
urn:lsid:zoobank.org:pub: BA986BF6-4F69-42ED-A9F1-5947 724C6221.
Uropsilus dabieshanensisLSID:
urn:lsid:zoobank.org:act: 968FD322-1D43-4B22-8320-C7FBFB 90F512.
SClENTlFlC FlELD SURVEY PERMlSSlON lNFORMATlON
Permission for field surveys in Anhui Province was granted by the Huoshan Forestry Bureau and Management Office of Yaoluoping National Reserve.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETlNG lNTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRlBUTlONS
B.W.Z. and S.Y. L. conceived and designed the study. Z.X.performed the experiments, analyzed the data, and prepared the manuscript. T.L.H. and H.Z. helped analyze the data.R.L.S. took pictures of the samples. Y.X.L., R.L., G.D.Y., Q.B.,J.S., and C.L.L. collected materials. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Guo-Tao Chen, Lei Zhou, and Zheng Kong for their assistance in collecting specimens and taking pictures of the samples and thank Gui-You Wu for assistance in analyzing the data. In addition, we also thank Xue-Long Jiang and Tao Wan for providing morphological data of the samples.

- Zoological Research的其它文章
- LIN28A inhibits DUSP family phosphatases and activates MAPK signaling pathway to maintain pluripotency in porcine induced pluripotent stem cells
- Molecular mechanisms of intermuscular bone development in fish: a review
- Species bias and spillover effects in scientific research on Carnivora in China
- Northern pig-tailed macaques (Macaca leonina)infected with SARS-CoV-2 show rapid viral clearance and persistent immune response
- Potential aquatic environmental risks of trifloxystrobin:Enhancement of virus susceptibility in zebrafish through initiation of autophagy
- Particulate matter exposure exacerbates susceptibility to SARS-CoV-2 infection in humanized ACE2 mice

