Efficacy Evaluation of a Compound Plant Extract Used in Makeup Base
Guangzhou Cadilan Cosmetics Technology Co.,Ltd.,China
Feng Di
Beijing Technology and Business University,China
Wu Jinhao
Beijing Yiweishi Testing Technology Co.,China
Abstract Cytological test and human skin test were used to study the antioxidant,anti-aging and moisturizing effects of a compound plant extract,and to evaluate its safety.The cytological test results showed that the compound extractcouldsignificantlyscavengethe reactiveoxygenspecies (ROS)generatedbyH2O2stimulation and protectcellsfromoxidativeda mage.Itcan significantly inhibit the expression of matrix metalloproteinase -1(MMP-1).The results of human skin test showed that the moisture content of cheek stratum corneum,R2 value and Q1 value of skin elasticity were significantly improved after 28 days of continuous use of the Makeup Base product added with the compound plant extract.The Ra value,Rz value and Rt value of skin texture around eyes have been significantly improved,which shows that the product has the effects of improving the moisture content of stratum corneum,improving skin elasticity and diminishing the fine lines around the eyes; the product has good safety.
Key words makeup base; natural plants; compound extract; efficacy study
The driving force to promote the development of the cosmetic market comes mainly from the two branches: skin care and color cosmetics.Nowadays,the color cosmetic consumers not only value the effect of makeup products,but also hope that the use of the makeup products will not adversely affect their skin,and even optimize their skin status.“Color and care in one” concept emerged accordingly.
As an essential color cosmetic product,the main function of a makeup base is to correct the uneven skin tone and the dullness of skin,and to prepare the skin ready for the use of other cosmetic products,making them look more fit and natural.At the same time,consumers expect the makeup base products to have various functions,such as moisturizing,antiaging,sunscreen etc.
The study results show that Centella asiatica,[1]Ginkgo biloba,[2-4]Peony flower,[5]Rice polypeptide[6,7]and Oat polypeptide[8,9]have their own unique active ingredients and beauty effects,and have been used in cosmetics.In the early stage of this study,a natural plant compound extract was developed,which was composed of Centella asiatica extract,Ginkgo biloba extract,Peony flower extract,rice polypeptide and Oat polypeptide.In this study,the anti-oxidation,moisturizing and anti-aging effects of the compound extract were explored viain vitrocytological test(HaCaT cell ROS scavenging test and HDF cell MMP-1 gene expression test) and human skin test(cheek stratum corneum,skin elasticity R2 and Q1 values,skin texture around the eyes Ra,RZ and Rt value).In the mean time,the safety of the makeup base product with the compound plant extracts was studied.This study can provide reference for efficacy evaluation of cosmetics and raw materials.
1 Experiment
1.1 Reagents and instruments
Cytological evaluation experiment: the compound extract of natural plants is composed of Centella asiatica extract,Ginkgo biloba extract,Peony flower extract,rice polypeptide and oat peptide,and was made in the lab.
Human skin efficacy evaluation experiment: the control sample is the makeup base and was made in the lab.The test sample is the makeup base added with 15% compound plant extract.Preparation of the makeup base: add water (proper amount),Glycerin(2%),Butylene Glycol (2%) and Sodium Chloride(0.6%) into a water pot,heat to 85℃,keep for 10 min,cool to 65℃,add Pentylene Glycol (1.5%)and stir for 5 min.Add Cyclopentasiloxane and PEG-10 dimethicone (3.5%),Cyclopentasiloxane/Acrylates/dimethicone Copolymer (3%),Phenyl Trimethicone (8%),Ethylhexyl Methoxycinnamate(5%),Triethylhexanoin(4%),Caprylyl Methicone(3%),Polyglyceryl-2 Isostearate (1.5%),and Hydrogenated Lecithin (appropriate amount) into the reaction pot,homogenize and disperse evenly.Raise the temperature to 65℃,slowly add the water pot material into the reaction pot by a three-step addition.Homogenize for 3 min,maintaining the temperature and stir for 10 min; Stir and cool to 45℃,add phenoxyethanol (0.5%) and fragrance (appropriate amount),stir well,and discharge after inspection.
HDF cells and their culture medium (ScienCell,USA); HaCaT cells (Kunming cell bank,Chinese Academy of Sciences); DMEM,Fetal Bovine Serum,Trizol (Thermo Fisher Scientific,USA); Penicillin-Streptomycin,Thiazol Blue (Sigma,USA); CCK-8 detection kit (Dojindo Laboratories,Japan Vitamin E (VE) (TCI Japan); H2O2(Shanghai Aladdin Biochemical Technology Co.,Ltd.); ROS detection kit (Shanghai Biyuntian Biotechnol ogy Co.,Ltd.);Reverse Transcription and Fluorescence Quantitative PCR kit (Takara,Japan); plastic consumables such as cell culture plate (Corning,USA).HaCaT cell complete culture medium: 89% DMEM,10% fetal bovine serum,1% Penicillin-streptomycin.During the test,the sample to be tested was diluted to each test concentration with complete medium and applied directly to the experiment.The contents of the samples to be tested are expressed by volume fraction.
CO2cell incubator (Binder,Germany); ultra clean bench (Airtech,USA); AE2000 inverted microscope (MOTIC CHINA Group Co.,Ltd.);ME104E electronic analytical balance (Mettler Toledo,Switzerland); M1000 Microplate reader(TECAN,Switzerland); Real time fluorescence quantitative PCR instrument ViiA 7 (ThermoFisher Scientific,USA); The moisture content measurement of stratum corneum-Corneometer (Courage &Khazaka,Germany); The Skin elasticity measurement instrument - Cutimeter (Courage & Khazaka,Germany); Facial image analyzer- VISIA-CR(Canfield,USA); Rapid optical skin imaging system Derma TOP (Breuckmann,Germany).
1.2 Experimental methods
1.2.1 In Vitro Cytological experiment
1.2.1.1 HaCaT Cytotoxicity and ROS scavenging test[10-12]
HaCaT cells were cultured in the complete Medium.When the density of HaCaT cells reached more than 80%,the cells were seeded on 96 well plate at the density of 1×105cells /mL,and cultured overnight in the environment of 37 ℃ and 5% CO2.After removing the culture medium,the HaCaT cells were treated with the sample of compound extract at 0.156%,0.313%,0.625%,1.25%,2.5% and 5%respectively.Three multiple wells were made for each concentration.Another 6 wells of blank control were set up and incubated in 5% CO2at 37 ℃ for 24 h; 10 μL of 5 mg/mL Thiazolyl Blue (MTT) solution was added to each well and incubated in 5% CO2at 37 ℃for 4 h; After removing the culture medium,150μL DMSO was added into each well and incubated in dark for 10 min.,mix well.The absorbance value was determined at 570 nm and 680 nm with microplate reader (i.e.ELISA instrument).The formula of cell survival rate was: cell survival rate equals the value of each well (A570-A680) / the average value of control group (A570-A680)× 100%.
HaCaT cells were incubated with 0.9 mmol/L H2O2at 37℃ and 5% CO2for 24 h.After removing the culture medium,HaCaT cells were treated with 0.156%,0.313%,0.625%,1.25% and 2.5% H2O2respectively.Three multiple wells were made for each concentration.
In addition,3 wells of control and 3 wells of positive control (2 mg/mL VE) were cultured in the environment of 37℃ and 5% CO2for 24 h; after removing the culture medium,100 μmol/L of DCFHDA was added into each well,and cultured in 37 ℃5% CO2environment for 20 min; after removing the culture medium,DMEM was added to wash for 3 times.The fluorescence values were measured at 488 nm and 525 nm with enzyme label instrument; The relative ROS level was calculated as follows: relative ROS level = fluorescence value of each well / mean fluorescence value of control group × 100%.
1.2.1.2 HDF cytotoxicity and MMP-1 gene expression experiment.[13,14]
HDF cytotoxicity test: HDF cells in logarithmic growth phase were taken and added with 0.01%,0.05%,0.1%,0.5%,1% and 5% test solution.Another 6 wells of control group were set up and incubated in 37℃ and 5% CO2for 24 h; 10 μL of CCK-8 solution was added into each well,and incubated at 5% CO2and 37 ℃ for 2 h.The absorbance value was measured by enzyme-linked immunosorbent analyzer(ELISA) at 450 nm wavelength to calculate the cell survival rate.
Methods: HDF cells in logarithmic growth phase were taken,and 0.5% testing samples were added.The Blank and positive control (0.1% hyaluronic acid (HA)) were set up.Each group was incubated for 24 hours in 5% CO2and 37℃ .After the culture medium was removed and washed with PBS once,the mRNA was extracted by Trizol method; the mRNA was reverse transcribed into cDNA according to the instructions of reverse transcription kit,and then the gene expression was detected according to the instructions of fluorescent quantitative PCR kit,and the gene expression was quantified by 2 - ΔΔCT method.The results were expressed as corrected data based on GAPDH Gene expression.
MMP-1 gene expression experiment: HDF cells in logarithmic growth phase were taken,and 0.5% testing sample was added.The blank and positive control (0.1% hyaluronic acid (HA)) were set up.Each group was incubated for 24 hours in 5%CO2at 37 ℃.After removing the culture medium,the mRNA was extracted by Trizol method after cleaning with PBS once; the mRNA was reverse transcribed into cDNA according to the instructions of the reverse transcription kit,and then the gene expression was detected according to the instructions of the fluorescent quantitative PCR kit,and the gene expression was quantified by 2 - ΔΔ CT method.The results were expressed as corrected data based on GAPDH Gene expression.
The forward primer sequence of the target gene MMP-1 was 5’-AAAATTACACGCCAGATTTGCC-3’-and the reverse primer sequence was 5’-GGTGTGACATTACTCCAGAGTTG-3’; the forward primer sequence of the target gene GAPDH wa s 5’-A AGA AG GTG GTGA AG CAG G-3’;and the reverse pr imer sequence was 5’-AGGTGGAGGAGTGGGTGTCG-3’.
1.2.2 Human skin efficacy evaluation experiment [15-19]
1.2.2.1 The participants selection
Thirty (30) healthy Chinese subjects,male or female,aged from 35 to 55 years old.
Inclusion criteria: dry skin,and skin with imperfections such as loose or with wrinkles; follow instructions and keep a regular habit during the study; able to read and understand all contents of the informed consent form and sign it voluntarily; no participation in clinical trials of any other research center during this study,and agree not to use any cosmetics,drugs and health care products that have an impact on the results.Exclusion criteria: patients with skin diseases at the test area that may affect the judgment of test results; highly allergic-susceptible individuals; women who are pregnant,breastfeeding or intend to be pregnant during the test;patients with severe heart,liver and kidney damage and severe immune dysfunction; patients with mental diseases,serious endocrine diseases and oral contraceptives; participants in drug clinical trials or other trials within 30 days; patients with oral and topical drugs or beauty products that may affect the test results within 2 weeks; those who cannot follow the instructions; and those who are considered not eligible by the researchers.
1.2.2.2 Test arrangement and basic process
Test arrangement: in the half face test,the subjects were randomly selected to use the test product,the makeup base with the natural compound plant extract on one cheek,which was recorded as the experimental group,while the test product,the makeup base (blank) was used on the other cheek as the control group.The test participants used the product quantitatively,and applied evenly once in the morning and once in the evening for 4 weeks.The test measurement was carried out on day 0,and day 14 as well as 28 after using the product.
Basic test process: subject information registration,verification of identity information;facial cleaning,waiting for 30 min in a constant temperature and humidity environment; tracking and inquiring about discomfort symptoms and sample use; image acquisition and data measurement(facial image taking and parameter analysis,stratum corneum moisture content measurement,skin elasticity measurement).
1.2.2.3 Test method
Cheek stratum corneum measurement: the Corneometer was used to detect the left and right cheek,and the skin capacitance value was measured to analyze the moisture content of the skin surface.The measured value was relative.Measure 5 times and take the average value.The higher value represents the higher moisture content of the cheek stratum corneum.
Skin elasticity measurement: cutometer was used to measure left and right cheek.R2 and Q1 values of skin elasticity were measured,and the average value was taken after 3 times of measurement.Skin elasticity Q1 refers to the ratio of skin rebound area to maximum stretch in the test cycle; skin elasticity R2 refers to the ratio of skin resilience to maximum stretch in the test cycle.The closer the ratio of R2 to Q1 is to 1,the better the elasticity is.
Canthus (Eye corner) skin texture measurement:VISIA-CR was used to capture the image of front face,and image from left 45° and right 45° to improve the visibility of skin analysis; Derma Top was used to take photos of the outer side of the left and right eye corners (i.e.canthus) and detect the Ra,RZ and Rt value.Each test is performed once.Ra is the arithmetic average roughness,which refers to the arithmetic average of the absolute value of the distance from each point on the skin contour to the mid-line; Rz is the average roughness,refers to the average of the skin roughness of 5 measurement sections; Rt is the skin roughness,refers to the height between the highest peak and the lowest valley in the measurement sections.The lower the Ra,RZ and Rt value,the better the effect of improving the eye corner wrinkle roughness.
The evaluation results before and after use of the product were compared by statistical test method to determine whether there was statistical difference.The temperature and humidity are (0.50 ± 1.50)%.
1.2.3 Statistical methods
Cell efficacy evaluation experiment: the statistical analysis software GraphPad Prism 8 was used.The data was described via mean±SEM,and the data was analyzed using one-way ANOVA.Whenp<0.05,it indicates that the difference is significant.
Skin efficacy evaluation experiment: The statistical analysis software SPSS was used for the statistical analysis of measurement data ( including quantity,mean,standard deviation,minimum and maximum value).The measurement value at different time points were compared with the basic value,and the significance of the normal distribution of the data improvement value was tested using software Shapiro-Wilk Test.Sig.(Two-Tailed)> 0.01,the normal distribution; then the paired t test was carried out,and the significant difference level α is taken as 0.05.If Sig.(Two-Tailed) <0.01,the distribution is non-normal,then the Wilcoxon test is performed,and the significance level α is taken as 0.05.
Improvement or change rate after using the product = (post use data - pre use data) / pre use data ×100%.
1.2.4 Skin safety evaluation
The safety of the samples to human skin was observed by human trial experiment.During the follow-up,the subjects should carefully inquire,check and record any adverse events occurred during the use of the samples,including the performance,occurrence time,treatment measures and outcomes of the adverse events,and judge the relationship between the adverse events and the samples used.Thirty subjects were tested for 28 days,and the skin safety was evaluated according to the grading standard of skin adverse reactions in human trial test specified in theSafety and Technical Standards for Cosmetics(2015 edition).After 14 days and 28 days of use,the subjects were asked and observed whether there were erythema,edema,papules,blisters and other adverse reactions in the test process,and graded: no reaction: grade 0; weak Erythema: grade 1;Erythema,Infiltration and Papule: grade 2; Erythema,Edema,Papule and Blister:grade 3; Erythema,Edema and Bullae: grade 4.
2 Results and discussion
2.1 Effect of sample on survival rate of HaCat cells and ROS scavenging capacity
MTT method was used to determine the cytotoxicity of the sample to HaCaT cells in 24 h.The non-toxic dose (cell survival rate ≥ 90%) was selected as the antioxidant effect test dose.The experimental results are shown in Figure 1.It can be seen from Figure 1 that the effect of the sample on the survival rate of HaCaT cells in a dose-dependent manner at 24 h,5% of the samples have obvious toxicity to the cells,and the concentration of 2.5%and below has no effect on the cell survival rate.Five nontoxic concentrations (0.156%,0.313%,0.625%,1.25% and 2.5%) were selected to test the scavenging ability of ROS produced by HaCaT cells stimulated by 0.9 mmol/ L H2O2.The experimental results showed that (Figure 2,compared with the control group,*P< 0.05,***P< 0.001),the relative ROS level of 2.5% sample was significantly reduced to 77.96%,while samples with other concentrations did not significantly scavenge the ROS in cells.The relative ROS level was also significantly reduced to 57.48% by the positive control (2 mg/mL VE).
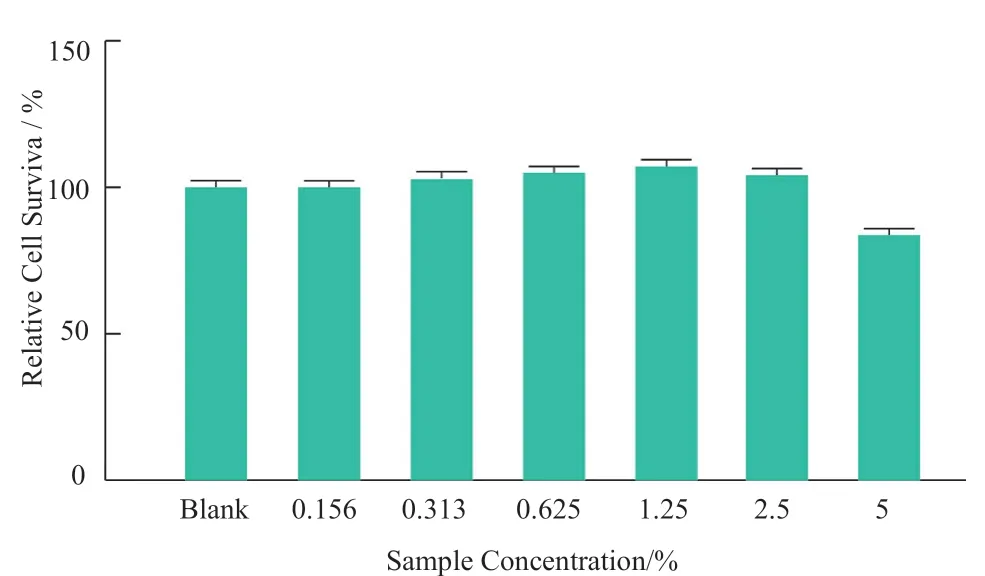
Figure1.Cytotoxicity of the samples to HaCaT cells (24 h)
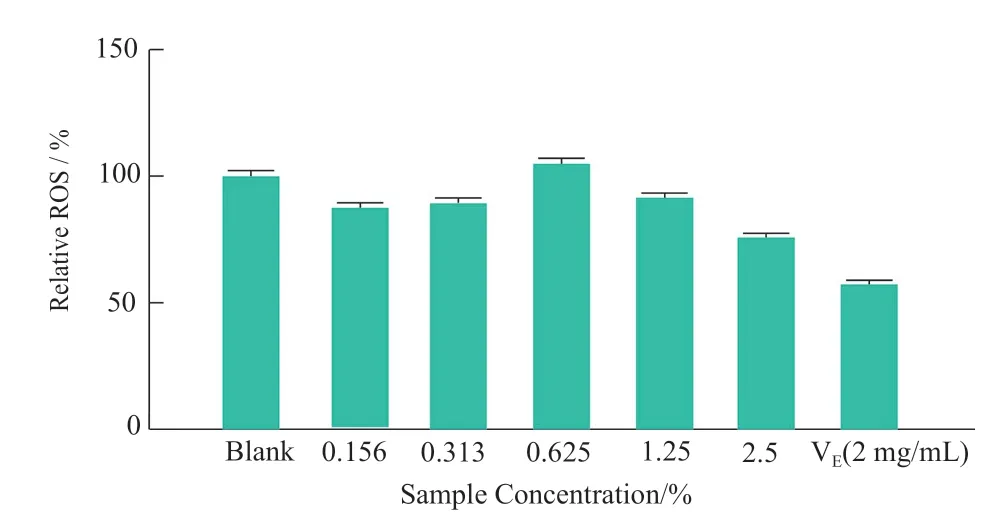
Figure 2.Effects of the samples on the relative ROS level of HaCaT cells (24 h)
2.2 Effect of sample on the survival rate of hdf cells and expression of MMP-1 gene
CCK-8 method was used to determine the cytotoxicity of the sample to HDF cells in 24 h.The non-toxic dose (cell survival rate ≥90%)was selected as the anti-aging effect test dose.The experimental results showed that (Figure 3),more than 1% of the samples has obvious toxicity to the cells,and the concentration of 0.5% or less has no effect on the cell survival rate.The maximum safe concentration of 0.5% was selected to test the effect of samples on the expression of anti-aging related genes in HDF cells.The experimental results showed that (Figure 4,*P< 0.05),0.5% of the sample and 0.1% of the positive control (HA) has similar inhibitory effect in the expression of matrix metalloproteinase-1 gene ( MMP-1),indicating that the sample can effectively protect collagen and proteoglycan and reduce aging damage.
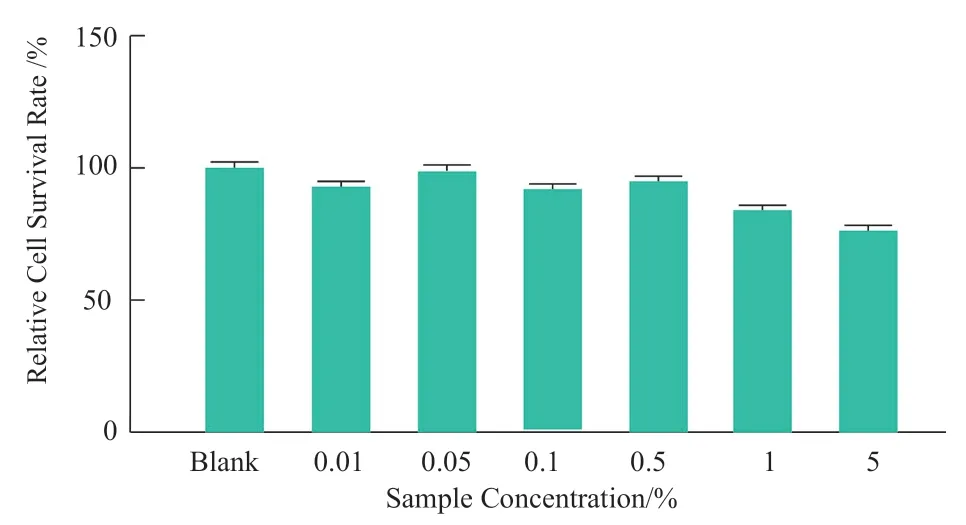
Figure 3.Cytotoxicity of the Samples to HDF cells (24 h)
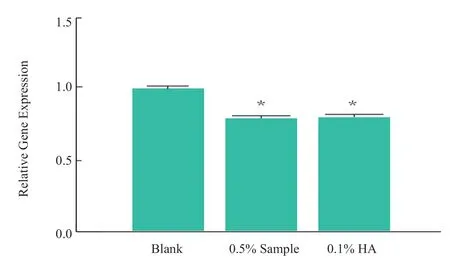
Figure 4.Effects of the samples on the expression of MMP-1 gene in HDF cells
2.3 Skin efficacy test
2.3.1 Moisture content of cheeck stratum corneum
Before using the product,there was no significant difference between the experimental group and the control group (P> 0.05); after 14 days of continuous use of the product,the moisture content of the cheek stratum corneum increased by 12.82%,significantly better than that before use (P< 0.001),and significantly better than that of the control group (P>0.05); After 28 days of continuous use of the product in the experimental group,the moisture content of the cheek stratum corneum increased by 15.57%,which was significantly better than that before use(P< 0.001),and significantly better than that of the control group (P< 0.001).The results showed that after 28 days of continuous use of the products in the experimental group,the moisture content of the cheek stratum corneum can be significantly increased,and the effect was significantly better than that of the control group.The statistical results are shown in Table 1,in which “N.S.” means no statistical difference,P> 0.05; “*” indicates significant difference,0.01 ≤P< 0.05; “* *”,0.001 ≤P< 0.01;“***”,P< 0.001.
2.3.2 Skin elasticity R2 value
Before using the product,the basic value of cheek skin elasticity R2 value of the experimental group (day 0) was significantly higher than that of the control group (P< 0.05); after 14 days of continuous use of the product,the cheek skin elasticity R2 value of the experimental group increased by 5.48%,which was significantly better than that before use(P< 0.001),and was significantly better than that of the control group (P< 0.001).After 28 days of continuous use of the product in the experimental group,the elasticity R2 value of the cheek skin of the subjects increased by 12.39%,which was significantly better than that before use (P< 0.001),and significantly better than that of the control group(P< 0.001).The results showed that,after 28 days of continuous use,the R2 value of cheek skin elasticity in the experimental group was significantly improved,and was significantly better than that of the control group,and the experimental group tested product has the effect of improving skin elasticity.The statistical results of R2 value of skin elasticity are shown in Table 2,in which “N.S.” means no statistical difference,P> 0.05; “*” means significant difference,0.01 ≤P<0.05,“* *”,0.001 ≤P< 0.01; “***”,P< 0.001.
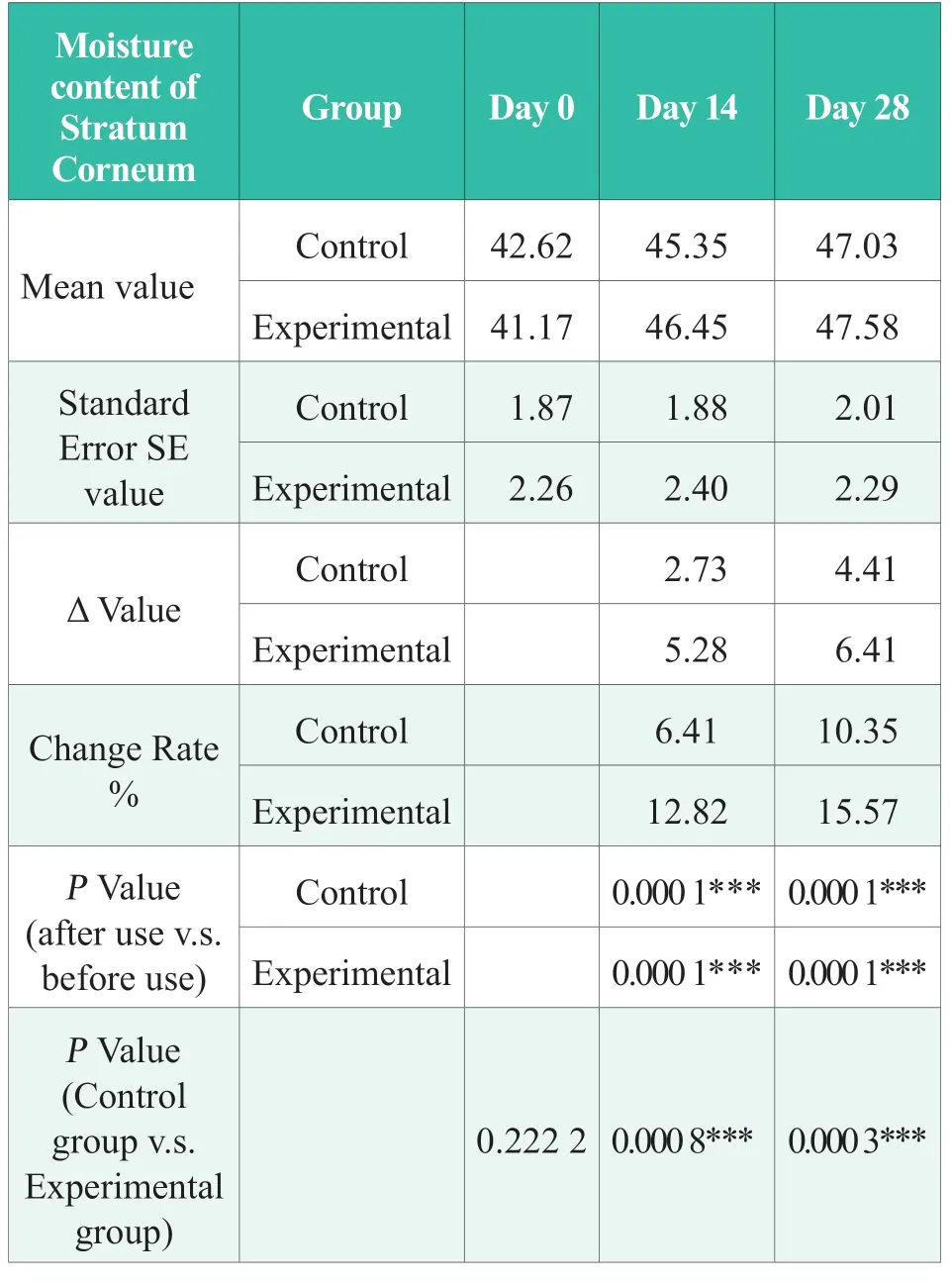
Table 1.Statistical Results of the moisture content of cheek stratum corneum
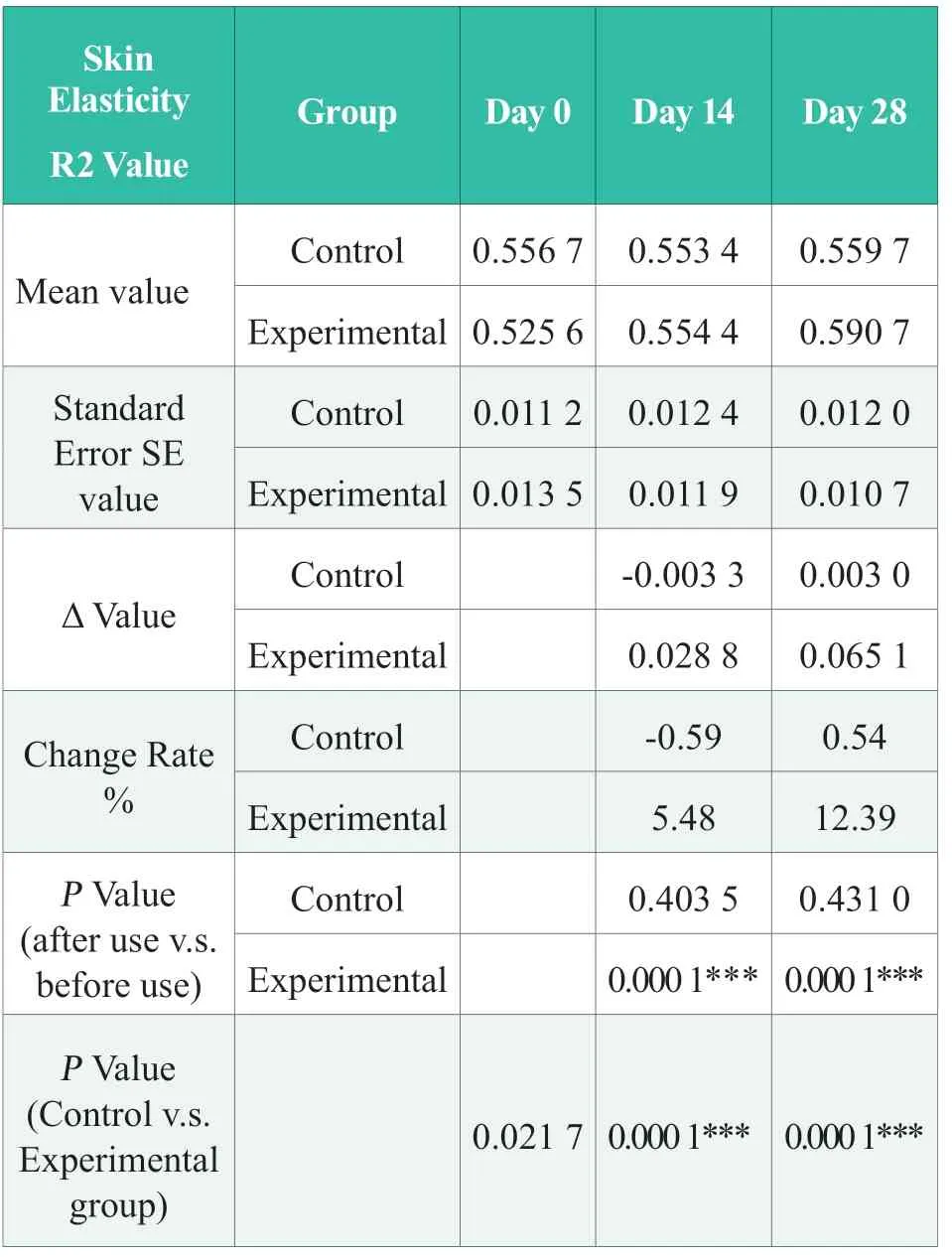
Table 2.Statistical results of skin elasticity R2 value
2.3.3 Skin elasticity Q1 value
Before using the product,there was no significant difference in the basic value of cheek skin elasticity Q1 between the experimental group and the control group (day 0) (P> 0.05); after 14 days of continuous use of the product in the experimental group,the cheek skin elasticity Q1 increased by 4.41%,which was significantly better than that before use (P< 0.01),and was significantly better than that in the control group (P< 0.05); After 28 days of continuous use of the product in the experimental group,the elasticity Q1 value of the cheek skin increased by 7.60%,significantly better than that before use (P< 0.001),and significantly better than that of the control group (P< 0.05 < 001).The results showed that the Q1 value of cheek skin elasticity in the experimental group was significantly improved after 28 days of continuous use of the product,and the improvement effect was significantly better than that in the control group,and the experimental group tested product has the effect of improving skin elasticity.The statistical results of Q1 value of skin elasticity are shown in Table 3.In the table,“N.S.”means no statistical difference,P> 0.05; “*” indicates significant difference,0.01 ≤P< 0.05,“* *”,0.001≤P< 0.01; “***”,P< 0.001.
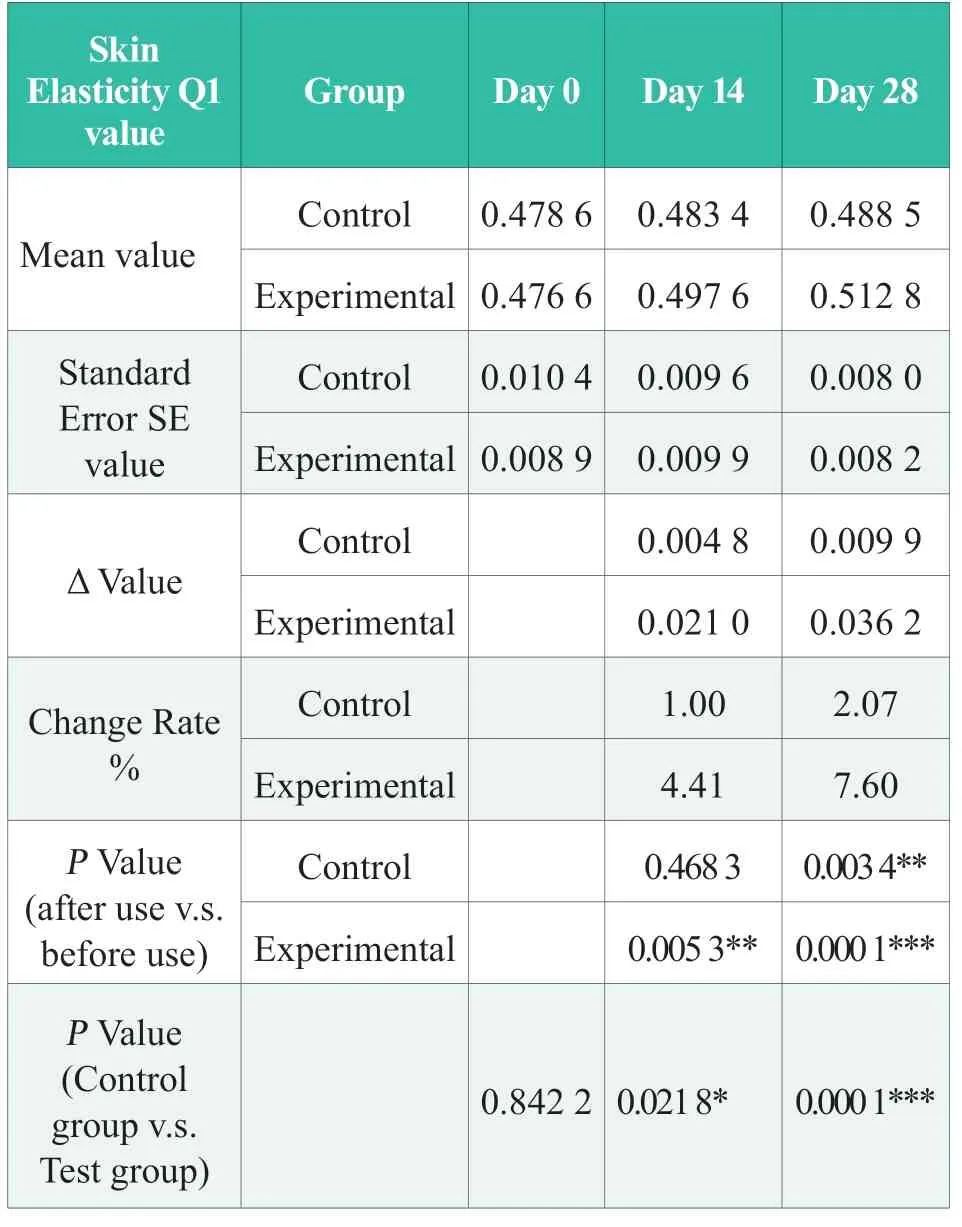
Table 3.Statistical results of skin elasticity Q1 value
2.3.4 Ra value of skin texture
Before using the product,there was no significant difference in the basic value of cheek skin texture RA between the experimental group and the control group (day 0) (P> 0.05); after 14 days of continuous use of the product in the experimental group,the subjects’ Ra value of the eye corner canthus skin texture was improved by 3.15% (absolute value),which was significantly better than that before use (P< 0.001),and better than the control group;after 28 days of continuous use of the product in the experimental group,the subjects’ Ra value of the canthus skin texture was improved by 4.72% (absolute value),which was significantly better than that before use (P< 0.001),and significantly better than the control group (P< 0.01).The results showed that,after 28 days of continuous use of the experimental group,the Ra value of the canthus skin texture can be significantly improved,and the improvement effect is significantly better than that of the control group.The experimental group tested product has the effect of diminishing the fine lines of the canthus.The statistical results of skin texture Ra value are shown in Table 4,in which “N.S.” means no statistical difference,P> 0.05; “*” means significant difference,0.01 ≤P< 0.05; “* *”,0.001 ≤P< 0.01;“***”,P< 0.001.
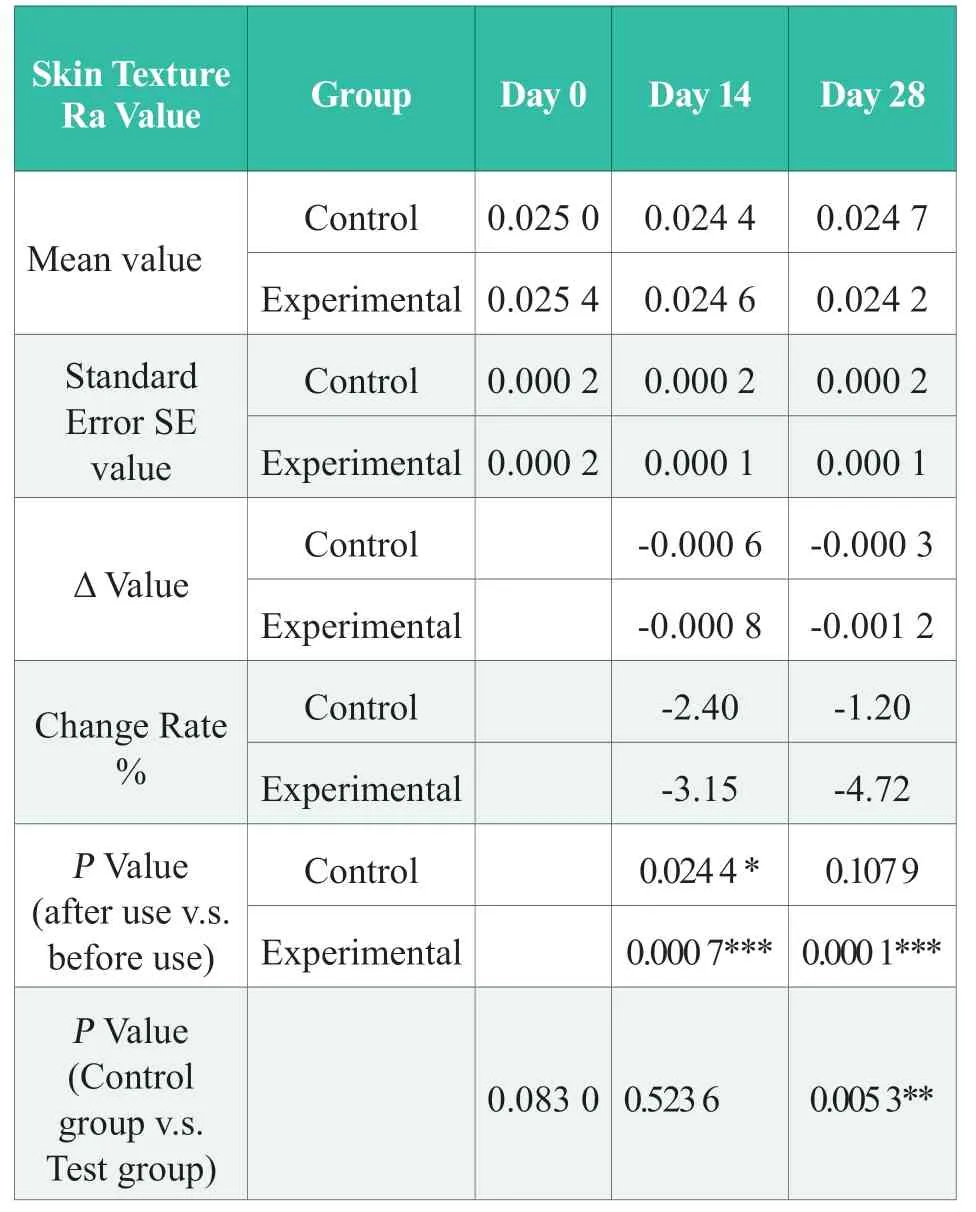
Table 4.Statistical Results of the Ra value for skin texture
2.3.5 Skin texture RZ value
Before using the product (day 0),there was no significant difference in Rz value between the experimental group and the control group (P> 0.05);after 14 days of continuous use of the product in the experimental group,the canthus skin texture RZ value improved by 5.36% (absolute value),significantly better than that before use (P< 0.01),and the control group (P< 0.05); After 28 days of continuous use of the product in the experimental group,the canthus skin texture RZ value improved by 9.08% (absolute value),which was significantly better than that before use (P< 0.001),and the control group (P< 0.05).The results show that after 28 days of continuous use of the product,the subjects in the experimental group,the Rz value of the canthus skin texture can be significantly improved,and the improvement effect is significantly better than that of the control group.The experimental group tested product has the effect of diminishing the fine lines of the canthus.The statistical results of skin texture Rz value are shown in Table 5.In the table,"N.S." indicates no statistical difference,P> 0.05; “*” indicates significant difference,0.01 ≤P< 0.05,“* *”,0.001 ≤P< 0.01;“***”,P< 0.001.

Table 5.Statistical results of the Rz value for skin texture
2.3.6 Skin texture Rt value
Before using the product (day 0),there was no significant difference between the experimental group and the control group in Rt (P> 0.05); after 14 days of continuous use of the product in the experimental group,the panelists’ skin texture around the eyes,the Rt value improved by 8.80% (absolute value),which was significantly better than before use (P< 0.001),and the control group (P< 0.001); After 28 days of continuous use of the product in the experimental group,the panelists’ skin texture around the eyes,the Rt value improved by 7.56% (absolute value),significantly better than before use (P< 0.001),and the control group (P< 0.05).The results showed that after 28 days of continuous use of the products in the experimental group,the Rt value of the skin texture around the eyes could be significantly improved,and the improvement effect was significantly better than that of the control group.The statistical results of skin texture Rt value are shown in Table 6.In the table,“N.S.” means no statistical difference,P> 0.05;“*”means significant difference,0.01 ≤ P< 0.05,“* *”,0.001 ≤P< 0.01; “***”,P< 0.001.
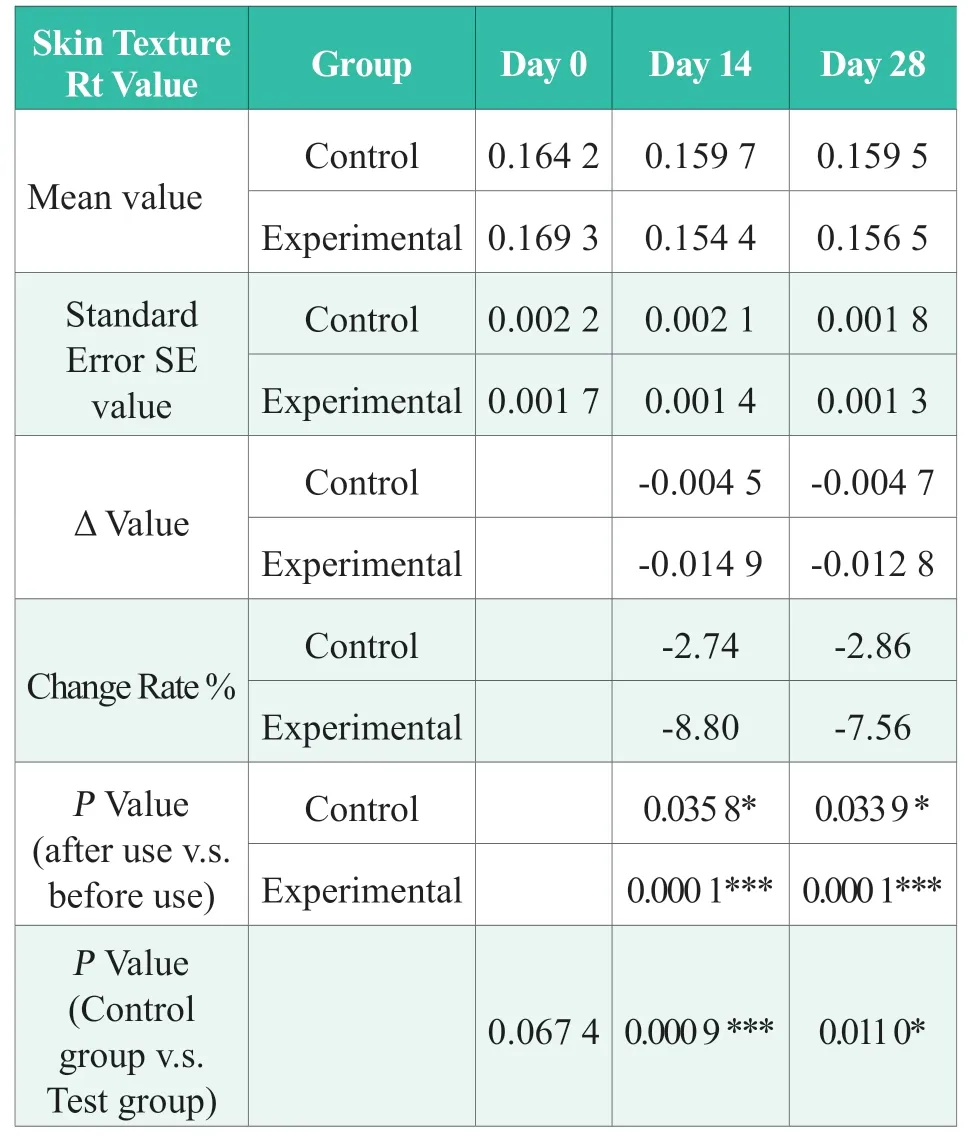
Table 6.Statistical results of the Rt value for skin texture
2.3.7 Effective cases of wrinkle diminishing around the eyes
After 28 days of using the test product in the experimental group,a subject 030 was selected,who showed obvious effect of attenuating fine lines around the eye,as shown in Figure 5A (photo of VISIA - CR,light source mode,standard 2) and 5b(photo of Derma Top: color,brown and inverted image mode).
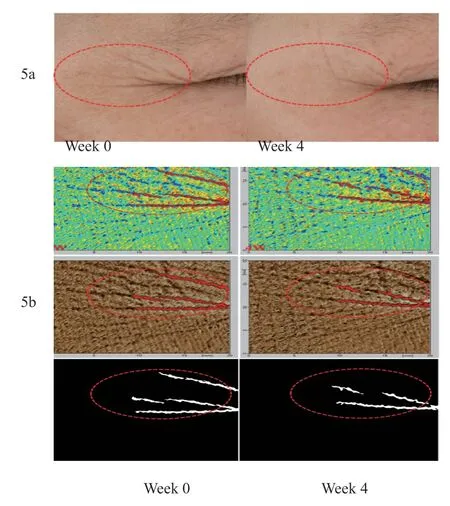
Figure 5.Effective cases of wrinkle diminishing
2.4 Safety evaluation results
No skin adverse reactions such as erythema,edema,papules,blisters and bullae were found in 30 subjects after 28 days of test.According to the grading standard of skin adverse reactions in human trial test specified in the 2015 edition of “Safety and Technical Standards for Cosmetics”,the test samples both in the experimental group and the control group are found safe.
3 Conclusion
The cytotoxicity on HaCaT and on HDF of a natural compound plant extract were tested.Its ROS scavenging ability and aging-related gene expression were also tested.
The results showed that there is no obvious toxicity on HaCaT cells when used at 2.5% or less; it can significantly scavenge the ROS generated by H2O2stimulation,and has protective effect on cell oxidative damage; it has no obvious toxicity on HDF cells when used at 0.5% or less.When used at 0.5%,it has a significant inhibition on the expression of MMP-1,which indicates that the product can protect collagen and proteoglycan and reduce aging-related damage.
A makeup base product containing a natural compound plant extract was tested by 30 Chinese healthy subjects with dry,loose skin and skin with fine lines and wrinkles.The test period was 28 days.The product showed good safety for human skin.After 28 days of continuous use of the product,the moisture content of cheek Stratum Corneum,the elasticity R2 value and Q1 value of subjects were significantly improved,and the Ra value and RZ value of skin texture around the eyes were also improved.The improvement effect of the experimental group was significantly better than that of the control group,indicating that the makeup base product with natural compound plant extract can improve the moisture content of the stratum corneum,enhance the skin elasticity and diminish the fine lines and wrinkles around the eyes.
 China Detergent & Cosmetics2021年1期
China Detergent & Cosmetics2021年1期
- China Detergent & Cosmetics的其它文章
- Specification on cosmetics registration record ready to be operated
- Risk Analysis and Quality Control of Cosmetic Raw Materials
- The Application of Modified Oil Ethoxylates in Laundry Beads Formulation
- Study on the Anti-inflammatory and Repairing Effects of Cannabis Sativa Leaf Extract by Epikutis Model Substitution
- Laboratory Study on the Whitening Ability of A New Type of Toothpaste Against the Tobacco Stains on Teeth
- Research and Application of Nanofiber Technology in Mask Products
