Apolipoprotein A1, the neglected relative of Apolipoprotein E and its potential role in Alzheimer’s disease
Kristina Endres
Abstract Lipoproteins are multi-molecule assemblies with the primary function of transportation and processing of lipophilic substances within aqueous bodily fluids (blood, cerebrospinal fluid). Nevertheless, they also exert other physiological functions such as immune regulation. In particular, neurons are both sensitive to uncontrolled responses of the immune system and highly dependent on a controlled and sufficient supply of lipids. For this reason, the role of certain lipoproteins and their protein-component (apolipoproteins,Apo’s) in neurological diseases is perceivable. ApoE, for example, is well-accepted as one of the major risk factors for sporadic Alzheimer’s disease with a protective allele variant (ε2)and a risk-causing allele variant (ε4). ApoA1, the major protein component of high-density lipoproteins, is responsible for transportation of excess cholesterol from peripheral tissues to the liver. The protein is synthesized in the liver and intestine but also can enter the brain via the choroid plexus and thereby might have an impact on brain lipid homeostasis. This review focuses on the role of ApoA1 in Alzheimer’s disease and discusses whether its role within this neurodegenerative disorder is specific or represents a general neuroprotective mechanism.
Key Words: Aβ; ApoA1; cholesterol; high-density lipoproteins; lipids; lipoprotein;neurodegeneration; senile plaque
Introduction
Alzheimer’s disease (AD) is a devastating disease that is characterized by progressive loss of cognitive abilities and with it a loss of ability to cope with every day activities as well as maintaining social relationships. This places a heavy burden on patients, caregivers and relatives, requiring excessive financial, social and emotional support. The seldom-occurring familial form of the disease is clearly linked to mutations in the amyloid precursor protein and/or the presenilins (Cacace et al., 2016). However, the vast majority of cases are designated as sporadic as the underlying cause has not been resolved yet. An effort to reveal risk factors has been attempted via genome wide association studies, which clearly pointed at apolipoprotein E (ApoE) as an important risk factor (Andrews et al., 2020; Squillario et al., 2020). Additionally, various epidemiological reports indicate that diet, and especially lipid homeostasis are closely connected to pathology (Yassine and Finch, 2020). While ApoE’s role in AD is undisputed, the effect of ApoA1, responsible for cholesterol efflux, is still under debate. Several investigations led to the conclusion that reduced ApoA1 CSF levels are associated with this disorder(Castano et al., 2006) but it is not clear if this observation is specific to AD or is a general effect of neurodegeneration.
ApoA1 is the core structural protein of high-density lipoproteins (HDL) that may also contain further additional proteins (ApoA-II, A-IV, C-I, C-II, C-III, and E). Besides properties in stabilizing HDL structures, ApoA1 also mediates interaction of HDL with ATP-binding cassette protein A1 (ABCA1), ABCG1,and class B, type I scavenger receptor (SR-B1). Moreover, it is an activator of lecithin cholesterol acyltransferase. Human ApoA1 consists of 243 amino acids and is synthesized in the liver and intestine. Nascent HDL contains two anti-parallel oriented ApoA1 molecules, giving the aggregate a discoidal shape with binding capability for phospholipids (Figure 1).Upon maturation within blood plasma, the HDL particle adopts a more spherical appearance, which is driven by conversion of cholesterol to cholesterol esters via lecithin cholesterol acyltransferase, integration of triglycerides, and translocation of phospholipids to the surface. The mature plasma HDL particle varies in size (8.8-11 nm) and also in the amount and composition of incorporated lipids as well as in ApoA1 molecules. This might explain the immense difficulties and uncertainties in elucidation of its structure [for an elaborate comparison of different structure models see Gogonea (2016)].
Furthermore, a multitude of different proteins with a putative interaction with ApoA1 can also be found in HDL particles:a recent mass-spectrometric analysis of HDL particles from mouse plasma identified 51 different proteins, representing the four functional categories namely lipid metabolism,immune response, coagulation, and others (Zhang et al.,2019). Only 11 of these proteins were common to all HDL subclasses (small, medium and large), strongly suggesting functional subclasses. This was demonstrated for example for endothelial lipase, where the antioxidative capacity of enzyme-containing HDL was mainly determined by the resulting lipolytic products of the lipase, and not by the resulting size of the enzyme-carrying particles (Schilcher et al., 2019). The interactome and functions of the respective components (Asztalos et al., 2019) might be greatly altered in diseased states while for example transcription and protein expression may remain unaffected. The finding that small RNAs such as miRNA, tRNA, and snRNA can also be found within HDL particles (Michell and Vickers, 2016; Ben-Aicha et al., 2020) offers further intriguing functional considerations that will have to be explored in future studies.
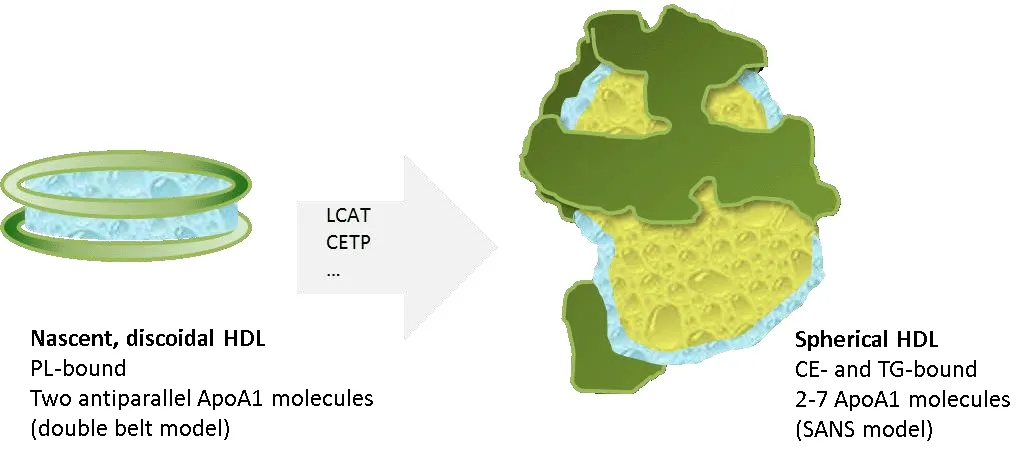
Figure 1 |ApoA1 exerts a highly flexible structure.
Almost all apolipoproteins can be synthesized in the liver,while ApoA4 and ApoB48 are solely generated in the intestine.ApoA1 is derived from both liver and intestine. The intestine presents as a rather exotic milieu considering its close contact to millions of colonizing microorganisms within its lumen. Therefore, the implication of proteins generated in close proximity to this microbiota to the immune response seems plausible. For ApoA1 an immunological function has been demonstrated: HDL has been shown to decrease the amount of monocyte chemoattractant protein-1 that recruits monocytes and reactive oxygen species in vascular smooth muscle cells (Tolle et al., 2008). The lipopolysaccharide sequestering properties of ApoA1 have already been described decades ago (Levine et al., 1993). Moreover, paroxonases(PONs) that are also found to bind HDL and to correlate with HDL concentration are able to hydrolyze and thereby inactivate bacterial quorum-sensing molecules (Furlong et al., 2016). These factors produced by Gram-negative bacteria are regulators of biofilm formation and might thereby directly contribute to pro-amyloidgenic events caused by bacteria(Endres, 2020). This and many more anti-inflammatory and protective characteristics of ApoA1/HDL may allow them to be classified as ‘bug scavengers’ as has been done in a recent review by Meilhac et al. (2020).
With its important role in the immune response and its major role in cholesterol efflux, ApoA1 represents a molecule that might be of high relevance in AD (for a recent meta-analysis on cholesterol and AD see Saiz-Vazquez et al. (2020)). Not least because ApoA1 is produced in the gut: the gut, its enteric nervous system, and its inhabitants, the microbiota,are currently being intensely investigated regarding their role in AD onset and disease progression (Endres and Schafer,2018). Therefore, reviewing what is known about ApoA1s function in AD including this newly identified mechanism is important.
Search Strategy and Selection Criteria
The articles used in this review were retrieved by an electronic search of PubMed database using the following terms:“ApoA1” OR “apolipoprotein A1” AND “Alzheimer”, “Parkinson”AND “ALS” AND “FTD”. The search was conducted on July 24,2020. The retrieved publications were subsequently screened by title and abstract to exclude non-relevant articles and reviews. For the introduction, additional literatures (retrieved from PubMed database) on structure and function of ApoA1 were used. While literature searches for ApoE and AD result in thousands of publications, ApoA1 only gained limited interest so far (Figure 2). Altogether, some hundreds of investigations on ApoA1 have been conducted and interestingly most of them focus on AD and not on other neurodegenerative diseases such as Parkinson’s disease (PD).
Genetic Linkage of Apolipoprotein A1 and ApoA1 Interacting Proteins with Alzheimer’s Disease
In humans, ApoA1 is encoded on the long arm of chromosome 11, closely linked to the APOC3 and APO4 gene locus. A Han Chinese population based study on 160 healthy controls and 147 AD patients, quantified? mRNA and protein levels of all three apolipoproteins in serum (Lin et al., 2015): all mRNA levels decreased with Clinical Dementia Rating (CDR)score (CDR0 = healthy controls) and similar observation was made for the amount of protein detected. Comparably, HDLcholesterol levels were also reduced. Correlation analysis with the Montgomery-?sberg Depression Rating Scale score that reports on behavioral symptoms and with the RAND-36 score both revealed that a higher ApoA1 concentration seems to inhibit development of AD while strengthening the general health status. Several scenarios might explain decreased ApoA1 levels, ranging from a role in transcription, on epigenetic regulation or on increased rates of degradation of the resulting protein. First investigations regarding the ApoA1 gene locus had already been conducted in 2005: a cohort of English and German citizens, including 427 AD patients and 500 controls, were subjected to genotyping in regard to three ApoA1 SNPS (Vollbach et al., 2005). The two SNPs - C/T at position +83 (rs5069) and G/A at position +84 bp (rs1799837)- did not show any association with AD. The third SNP G/A at-75 bp (rs670), while also located within the promoter region,indicated an association of the A allele with an increased risk in early onset patients (aged 66 years or younger).Homozygous AA carriers had an even earlier onset (8 years)of disease compared to persons with at least one G allele. A meta-analysis with 3000 healthy persons characterized the effect of the A allele of the SNP as a statistically significant but rather mild increasing-risk factor (about 5 mg/dl; Juo et al., 1999). Furthermore, the observed impact of the allele was sex-biased with an even weaker association in females.Parameters such as gender, lifestyle or geographic location of a population might contribute to the inconsistent outcome of SNP evaluations for ApoA1. A study making use of a Tunisian population (173 AD cases and 150 healthy controls (Smach et al., 2011)), concluded that the SNP at -75 bp had no association with AD and serum protein levels were nearly unaffected. Interestingly, this study, however, revealed an association of the A allele with lower levels of CSF amyloidbeta (Aβ peptides and HDL cholesterol in serum of AD patients as well as in healthy controls. Moreover, serum ApoA1 and Mini Mental State Examination (MMSE) score correlated significantly (r= 0.58), confirming a potential, protective role of ApoA1. The analysis of three further European populations came to a similar conclusion: ApoA1 polymorphism (rs670)was not associated with the risk of AD, while the A allele was weakly associated with a lower MMSE score (Helbecque et al., 2008). A study mainly focused on cerebral amyloid angiopathy-associated intracerebral hemorrhage (CAA ICH),also including 73 AD patients, indicated a strong correlation of ApoA1 with plasma Aβ1-40in CAA ICH patients but not in controls (Montanola et al., 2016). Unfortunately, no subgroup analysis of AD patients was included. No significant association could be delineated for the two SNPs of ApoA1 - rs 5069 and 670 - for any of the groups investigated and no significant association between genetic variants and plasma levels. In a Japanese population, two intronic SNPs (rs5070 and 5072)were tested for a potential association with early onset (EO) or late onset (LO) AD (154 controls, 170 EOAD, 162 LOAD (Shibata et al., 2013): no significant difference was found between frequency of alleles within controls and EOAD nor between EOAD and LOAD.
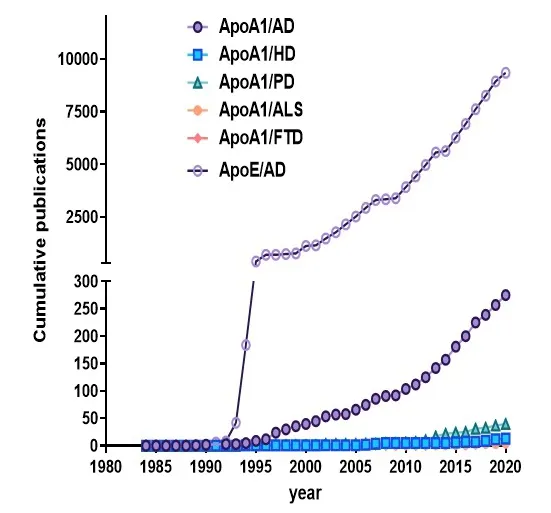
Figure 2 |Research activity on ApoA1 in different neurodegenerative diseases and compared to ApoE.
Epigenetic investigations targeting ApoA1 are scarce and only one could be identified with the applied search strategy (see legend of Figure 2). Analysis of two cohorts of cognitively normal older individuals from the OATS study(Older Australian Twin study, Lazarus et al., 2015) revealed no significant heritability for ApoA1 protein levels; however, a CpG methylation site (cg03010018) associated with memory performance was identified that could be replicated in the Sydney MAS study. This CpG methylation site also correlated with ApoA1 protein levels but no relation with a composite memory domain score could be demonstrated.
Besides direct genetic effects, allelic variation in encoding sequences of interaction partners of ApoA1 are also conceivable: 95-100 protein species have been found in proteomic analyses of HDL (reviewed in Wilkins and Seckler,2019) that might all in principle be able to interact with ApoA1 and thereby determine its function and/or life time. One such example might be PON1, a protein that was shown to have a direct interaction site with ApoA1 in HDL particles (Huang et al., 2013), andin vivoandin vitroprevented accumulation of oxidized lipids in low density lipoproteins (LDL). Erlich et al.(2006) examined 29 SNPs in PON1, 2, and 3 encoding genes in 730 Caucasians and 467 African American cases and nondemented controls. An association was reported for SNP C/T at -161 bp in both ethnic groups and the deleterious effect of the T allele was assumed to be based on the affected nuclear factor-I binding site. Another important interaction partner for ApoA 1 is its receptor ABCA1. Comparing groups of healthy individuals (n= 100), AD (n= 105), and PD (n= 116) patients from northern China regarding the R219K genotype, resulted in the observation of a lower RR and RK allele frequency in both, AD and PD patients (Ya and Lu, 2017). The RR genotype was also positively correlated with MMSE of AD and negatively with the Hoehn-Yahr scale, a read out of PD symptoms.Moreover, ABCA1 as well as ApoA1 were lowered in the serum from AD patients. However, ApoA1 levels were comparable to those of PD patients. An investigation in American caucasians(992 cases and 699 controls) identified a gender-dependency for this allelic variation, since female 219K allele carriers had a 1.75-fold increased risk of developing AD while males showed the opposite trend (Sundar et al., 2007). Importantly, this might explain why within other studies - probably due to lack of gender stratification - no effects were observed: within an Iranian population (154 sporadic AD cases and 162 control subjects) for example, no association could be found for the R219K polymorphism and AD (Khorram Khorshid et al., 2011).This was also the case within another study with an Anglo-American population (Wahrle et al., 2007). Intriguingly, a large cohort including 92,726 participants from the Copenhagen General Population Study delineated a strong association of AD with the loss-of-function N1800H mutation in ABCA1(Nordestgaard et al., 2015), underpinning the immense importance of a functional cholesterol efflux for the disease.
Apolipoprotein A1 as an Amyloid-Promoting Agent
Recent reviews highlight the interaction of the Aβ peptide,one of the hallmarks of AD, with other amyloidogenic proteins and peptides of human or even non-human origin ( Endres,2019; Ciccone et al., 2020). In this regard, it is important to acknowledge that ApoA1 itself can also form protein aggregates (Cottini et al., 2019; Colombat et al., 2020). No ApoA1 reactivity could be found in for example primary and secondary cutaneous amyloidosis (Chang et al., 2001),but43 out of 48 formalin-fixed heart-specimen with different kinds of systemic amyloidosis reacted with an anti-ApoA1 antibody (Sakata et al., 2005). The amyloidogenic property of the apolipoprotein is mediated by mutations such as the Iowa mutation (G26R) and seems to depend on cell surface heparin sulfates (Kuwabara et al., 2015). In addition to this self-aggregation, in 1995, ApoA1 was already found in senile plaques of six AD cases and four Down syndrome cases(Wisniewski et al., 1995), which led the authors to suggest a conformational mimicry of ApoA1 that could augment or even initiate fibril formation of Aβ in AD. Shortly thereafter,a first publication described binding of apolipoproteins to immobilized Aβ40, with hydrophobic forces as a main driver for ApoA1-Aβ interaction (Shuvaev and Siest, 1996).A KD of 25 nM was found; however, in these experimental conditions, ApoA1 must have been predominantly provided as a monomer which does not represent its major form in biological systems. The relevance of the ApoA1 conformation might also be concluded from the fact that ApoA1 alone had no protective effect on MC65 cells, while HDL or ApoA1 in combination with alpha-tocopherol shielded cells against Aβinduced toxicity (Maezawa et al., 2004). These neuronal cells produce the C-terminal part of the amyloid precursor protein(APP) - a prerequisite for Aβ synthesis - after tetracycline removal, and thereby induce autotoxicity if no protective agents are administered. Further investigations followed that confirmed the finding of a saturable, specific and reversible binding between ApoA1 and Aβ with an even higher affinity than ApoE (Koldamova et al., 2001). This binding prevented β-sheet formation and was initially reported to lower Thioflavin T (ThT)-derived fluorescence inin vitroassays respectively. However, Paula-Lima et al. (2019) who once more confirmed the neuro-protective function of ApoA1 against Aβ revealed that this lowering of ThT fluorescence signal only occurred with a low molar ratio of ApoA1 (20:1 Aβ to ApoA1),while higher ratios (2:1) resulted in elevated ThT binding and fluorescence. The ThT signal reduction could be also obtained by a ratio of 10:1 (Stoye et al., 2020a). The binding of ApoA1 to Aβ was accompanied by generation of smaller Aβ aggregates with shorter, curvilinear and thinner structures(Paula-Lima et al., 2009). Interestingly, Aβ/ApoA1 complexes could be also identified in AD CSF. Furthermore, the binding site of Aβ could be narrowed down by biopanning against fibrillar Aβ to the following stretch in the N-terminal domain of ApoA1: namely42LNLKLLD48. This peptide stretch is located directly N-terminal to the first class A amphipathic helix of ApoA1 (Segrest et al., 1992). The 4F peptides, mimics of these structural domains of apolipoproteins that do not necessarily share sequence homology to the naturally occurring proteins, have been analyzed for their potential to bind Aβ:electrostatic interaction with Aβ42has been reported (Sahoo et al., 2020) which subsequently caused peptide unfolding.The resulting heteromers, however, induced neurotoxicity.Nevertheless, these peptide mimetics have been already tested as therapeutics: for instance, they have been found to elevate ApoE secretion and lipidation in murine and human astrocytes by positively influencing protein transport(Chernick et al., 2018). The more stable D-enantiomer has been used in combination with pravastatin in the APP/PS1 AD mouse model and evoked improved learning and memory formation as well as a reduction of Aβ load by exerting anti-inflammatory actions after oral application (Handattu et al.,2009). While these peptides also showed beneficial effects for other inflammatory diseases than AD (Meriwether et al.,2019; McGrath et al., 2020; Oehler et al., 2020), they might not fully substitute the full spectrum of HDL functionality as has been shown for anti-atherogenic effects and protection of endothelial cells (Xu et al., 2019). Presumably, the structure of such applied HDL-derived therapeutics might play a role:discoidal HDL displayed a superior capability to promote efflux of Aβin vitrothrough an artificial blood-brain barrier in comparison to spherical HDL particles, which also led to destabilization of fibrils (Dal Magro et al., 2019).
Apolipoprotein A1’s Role in Alzheimer’s Disease:Observations from Mouse Models
As soon as ApoA1 was identified as part of senile plaques of AD patients’ brains, the question arose whether ApoA1 might contribute to amyloidogenic processes itself, and preclinical attempts to answer this were conducted. In an early attempt, mutant C57BL/6J mice that lacked ApoA1 were analyzed concerning secondary induced amyloidosis. Here, no changes in splenic anti-amyloidogenic immunoreactivity were assessed (Elliott-Bryant and Cathcart, 1997). Subsequently,analyses in AD mouse models were conducted that are based on heterologous APP and presenilin (PS1/2) expression. The first investigation of a direct effect on AD-like pathology in PDAPP mice revealed the expected effects on plasma and brain cholesterol; however, no alteration of Aβ amount or Aβ deposits were found in the hippocampus (Fagan et al.,2002; Fagan et al., 2004). In these publications, no analysis of amyloids in the vasculature was reported. However, two following publications suggested that ApoA1 does not seem to influence parenchymal amyloids but angiopathic events.Knock-out of ApoA1 in APP/PS1D9 mice increased cerebral amyloid angiopathy (Lefterov et al., 2010), while a twofold elevation of ApoA1 by overexpression reduced it and moreover also reduced glial inflammation (Lewis et al., 2010).Interestingly, two recent reports came to opposing results with the same ApoA1 knock-out mouse cross-bred with two AD mouse model lines. Button et al. (2019) described that 12 month old APP/PS1 mice showed increased cortical and vascular plaque burden with reduced ApoA1 amount and increased phospho-Tau in dependency of ApoA1 reduction.These animals revealed no behavioral changes (contextual and cued fear conditioning). The same time, Contu et al. (2019)described that in 12-13 month old Tg2576 mice a knock-out of ApoA1 resulted in diminished parenchymal and vascular amyloid pathology. The contradictory outcome might be based on the fact that AD models differed. Endogenous regulation due to the genotype of the respective models might have affected ApoA1 levels and led to an adaptive response. The Tg2576 mice for example showed reduced ApoA1 levels in the brain as compared to wild type controls (Contu et al., 2019).An investigation of the proteome after intracerebroventricular injection of Aβ in wild type rats described elevated amounts of ApoA1 in hippocampus 40 days after establishing the spontaneous model (Zali et al., 2015). This indicates that precising timing and expression levels of Aβ might lead to differential regulation of endogenous ApoA1.
The promoter for neurodegenerative disease-inducing transgenes such as APP or PS1 in rodent models is most often neuron-specific such as a deleted Thy1-promoter variant.In this regard, it is important to know that the intestine provides its own nervous system - the enteric nervous system(ENS) - and heterologous expression of Aβ in AD models -driven by the Thy1 promoter -has been described not only in brain, but also in the neurons of the ENS (Brandscheid et al.,2017). Therefore, the reaction of the intestinal neurons to overexpression of neurotoxic Aβ peptides might be of higher relevance regarding ApoA1 as secondary effects occurring in cortical neurons. Recently, age-dependent regulation of ApoA1 in the colon of 5xFAD model mice was described: at an age of 1 or 2 months ApoA1 was reduced while with a higher age and progression of pathology, a strong increase in its amounts was assessed (Stoye et al., 2020a). Interestingly, ApoA1 also exerted a protective effect against Aβ on enteric neurons as has been shown before in endothelial cells (Fernandez-de Retana et al., 2017) or neurons of different origins (Paula-Lima et al., 2009).
Evidence for significant mRNA levels of ApoA1 has not been described in the human brain and it is assumed that Apoa1 may enter CSF via the choroid plexus (Elliott et al., 2010).Apoa1 mRNA levels were for example found to be at only 0.3-0.7% of the mean levels found in within different gut sections of C57Bl6/J mice (own unpublished results). Entry of peripheral ApoA1 into the brain has been doubted: while a chronic intravenous application of recombinant human ApoA1 was able to decrease cerebral Aβ and inflammatory markers in APP23 mice, it could not be detected within brain tissue(Fernandez-de Retana et al., 2017). However, Alexa Fluor 647-labeled recombinant ApoA1 could be demonstrated in the choroid plexus after intravenous injection in mice (Stukas et al., 2014). More recently, bolus injection of radioiodinated ApoA1 in wild type rats revealed transportation across the blood-brain barrier (permeability-surface area product of hippocampus and cerebellum: 0.36 and 0.76 mL/g per second× 10-6(Zhou et al., 2019)).
It has been suggested that ApoA1 imported into the brain elicits neuroprotection via promoting efflux of the neurotoxic Aβ peptide. In a combinatory knock-out of Abca1 and ApoA1 in AD model mice, a delayed export of Aβ from the brain has been described (Fitz et al., 2015). Therefore, the effect of ApoA1 might affect the soluble pool of Aβ: soluble brain Aβ40and Aβ42levels were significantly reduced via acute administration of reconstituted HDL from human serum in APP/PS1 mice (Robert et al., 2016), while chronic administration over 4 weeks failed to do this. Lack of reduction of soluble brain Aβ40and Aβ42levels with prolonged treatment might rely on the elimination of rHDL, due to the fact that the investigation was performed 7 days after the final dose.
Approaches to regulate endogenous ApoA1 levels have been successfully conducted, as for example theLavendula angustifoliaextract was able to counterbalance an Aβ-evoked increase in ApoA1 in intracerebroventricular injected rats(Zali et al., 2015).Ganoderma lucidumpreparations increased brain ApoA1 protein concentration and also improved learning and memory in APP/PS1 mice (Qin et al., 2017).Moreover, as the intestine presents one of the major sites of ApoA1 production, its regulation by pro- or prebiotics might be assumed. For example, reduced expression of ApoA1 was found to be associated with alterations in Firmicutes specific for Crohn’s disease (Haberman et al., 2014). Rescue of such dysbioses that are also assumed for AD might also result in beneficial effects by reconstituting an optimal ApoA1 level.
Human Studies on Peripheral and Central Apolipoprotein A1 Levels in Cognitive Impairment and Alzheimer’s Disease
In human patients, the majority of investigations regarding ApoA1 is based on plasma or serum samples (Table 1). These mainly report a reduction of the protein or related structures and binding partners (e.g. PON-arylesterase; Romani et al.,2020). Besides reduced protein concentrations, single reports also suggest a correlation with other disease hallmarks such as CSF Tau (Romani et al., 2020), brain atrophy (Choi et al.,2016; Liu et al., 2019) or MMSE score (Uchida et al., 2015).This is substantiated by other reports describing significant association of plasma ApoA1 with mild cognitive impairment(MCI) to AD conversion (Westwood et al., 2018). In the Amsterdam dementia cohort, one standard deviation increase of CSF ApoA1 was associated with a 30% increased risk of clinical progression in non-demented persons with subjective cognitive decline (effect attributable to ApoEε4 carriers; Slot et al., 2017). This might point to ApoA1 as being involved in the earliest stages of AD. Moreover, ApoA1 was also shown to decrease from mid-life onwards [1.21 μg/mL per decade(Muenchhoff et al., 2017)] even if a significant correlationwith frailty or cognition could not be identified. As one of the major risk factors for at least sporadic AD is age, this might underline the importance of ApoA1 in pathogenesis.
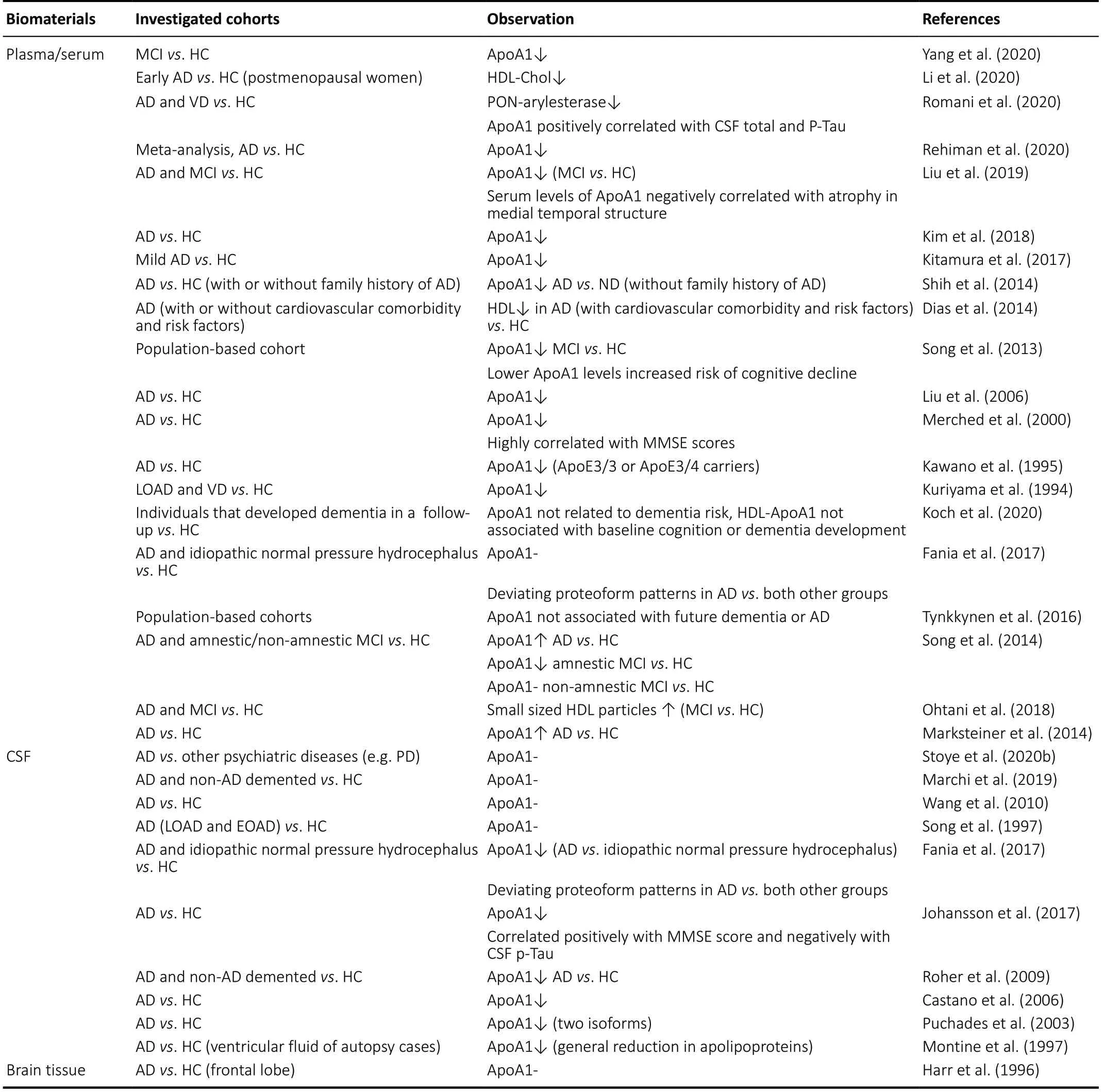
Table 1 |Analysis of ApoA1 levels in patients-derived samples
Nevertheless, there are also individual reports on reduced ApoA1 or HDL particles associated with AD. Besides the mere reduction of the protein in blood-based samples, qualitative differences have also been observed: for instance, Fania and colleagues described that while ApoA1 levels were comparable between AD cases, idiopathic hydrocephalus patients with normal pressure and healthy controls, proteoform patterns of AD cases differed as compared to both other groups (Fania et al., 2017). This might explain why some reports conclude that ApoA1 decreases in AD, whilst others not, depending on the methods used to detect comparable proteoforms. Another investigation in a Taiwanese AD population found elevated Nε(carboxyehtyl)lysine adducts of serum ApoA1, accompanied by increased amounts of autoantibodies against these modifications (Lin et al., 2018) while in CSF carbonylation status was not found to differ between AD cases and healthy controls (Korolainen et al., 2007).
Interestingly, even if the number of publications is still low (Figure 2), there are at least some reports on other neurodegenerative diseases in the context of ApoA1: for example, ApoA1 was also reduced in serum samples from patients with general paresis (late form of neurosyphilis) while syphilitic patients without neurosyphilis were comparable to healthy controls (Jiang et al., 2016). Moreover, there was a significant positive correlation between increased ApoA1 level and MMSE score after treatment with antibiotics. Also a decline in CSF ApoA1 was reported for PD patients (Maarouf et al., 2013) and a reduced plasma level in a behavioral variant of FTD (Kim et al., 2018).
Conclusion
In summary, the majority of publications involving patient studies demonstrate a reduction of ApoA1 in peripheral biomaterials such as serum or plasma in AD patients or prodromal stages. For CSF and brain material, data are less clear-cut, as half of the publications show reduced levels,while the other half reports no changes. This inconsistency is mirrored in preclinical studies using AD mouse models:even knock-out of ApoA1 led to contradictory results in terms of pathological hallmarks. Interestingly, there are hardly any data on the expression of ApoA1 in its tissues of origin- the intestine or liver - for AD. However, with an increasingly systemic view of the disease, data on this will undoubtedly emerge over the coming years. The inclusion of the gut-brain axis or the gut-liver axis could provide valuable insights into disease pathogenesis. In particular, the importance of the gut microbiome and its possible influence on ApoA1 should be further explored in the future. The fact that ApoA1, in addition to its well-known role in cholesterol regulation, is also an important component of the immune system, allows speculation about the influence of the microbiome on host ApoA1 levels and vice versa. Significantly, ApoA1 is found, for example, in direct contact with myeloperoxidase (Huang et al., 2013), an enzyme that plays a crucial role in antimicrobial defense starting from neutrophil granulocytes. Altered MPO levels have already been described in AD patients and the influence of the enzyme on disease progression has recently been demonstrated in a mouse model (Volkman et al., 2019).Perhaps, therefore, it would be more fruitful to consider ApoA1 not in isolation but in its interplay with other binding partners to elucidate its role in AD and related diseases in the future.
Acknowledgments:The author would like to thank Helen May-Simera(Department of Biology, Johannes Gutenberg-University Mainz) for critically reading and editing the manuscript.
Author contributions:KE designed and wrote this review and approvedthe final version of this work.
Conflicts of interest:None declared.
Financial support:This work was supported by grants from the MWWK,Germany (research consortium NeuroDegX) to KE.
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Mechanisms implicated in the contralateral effect in the central nervous system after unilateral injury: focus on the visual system
- Toward three-dimensional in vitro models to study neurovascular unit functions in health and disease
- Oral frailty and neurodegeneration in Alzheimer’s disease
- Non-coding RNAs and other determinants of neuroinflammation and endothelial dysfunction:regulation of gene expression in the acute phase of ischemic stroke and possible therapeutic applications
- Altered microRNA expression in animal models of Huntington’s disease and potential therapeutic strategies
- Mesenchymal stem cell treatment for peripheral nerve injury: a narrative review

