Chronic peripheral inflammation: a possible contributor to neurodegenerative diseases
Patrick Süβ, Addison J. Lana, Johannes C. M. Schlachetzki,
Abstract The contribution of chronic peripheral inflammation to the pathogenesis of neurodegenerative diseases is an outstanding question. Sustained activation of the peripheral innate and adaptive immune systems occurs in the context of a broad array of disorders ranging from chronic infectious diseases to autoimmune and metabolic diseases.In addition, progressive systemic inflammation is increasingly recognized during aging.Peripheral immune cells could potentially modulate the cellular brain environment via the secretion of soluble molecules. There is an ongoing debate whether peripheral immune cells have the potential to migrate into the brain under certain permissive circumstances.In this perspective, we discuss the possible contribution of chronic peripheral inflammation to the pathogenesis of age-related neurodegenerative diseases with a focus on microglia,the resident immune cells of the brain parenchyma.
Key Words: aging; Alzheimer’s disease; inflammation; innate immune system; microglia;neurodegeneration; Parkinson’s disease
Introduction
Microglia, the resident innate immune cells of the brain, are involved in the pathogenesis of neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD), but the contribution of systemic inflammation to disease risk and progression of these diseases is ambiguous.
The innate immune system is shaped and modulated by the interplay between an individual’s genetic background and environmental factors. Genetic variants linked to AD and PD have been shown to influence gene regulation in monocytes,the peripheral blood mononuclear cells of the innate immune system (Raj et al., 2014). Conditions such as diabetes,hypertension and infectious disease potentially cause a sustained, low-level inflammatory activation state. Aging,the number one risk factor for neurodegenerative diseases,has been shown to alter the state of the innate immune system. These changes include alterations in the molecular signature of monocytes and a reduction in monocytes’ ability to phagocytose and respond properly to inflammatory stimuli(Hearps et al., 2012; Bliederhaeuser et al., 2016).
Search Strategy and Selection Criteria
We searched PubMed to find articles on peripheral inflammation and neurodegeneration that were published between 2000 and 2020.
Crosstalk between Chronic PeripheralInflammation and Microglia
Little is known about how chronic peripheral inflammation influences the microglia cell compartment. At steady-state,microglia perform functions including immune surveillance and phagocytosis. Interestingly, microglia express numerous genes implicated in AD (Gosselin et al., 2017). It is thought that microglia may be involved in mediating the activation of astrocytes, neuronal dysfunction, aberrant removal of synapses, and eventually cell death via increased production of cytokines, chemokines and reactive oxygen species when responding to pathophysiological conditions (Figure 1; Salter and Stevens, 2017). The precise mechanisms that reprogram microglia into a dysregulated state are unclear. While studies aiming at deciphering the pathogenesis of AD and PD have been primarily conducted in a very brain centric lens, recent findings suggest an involvement of the peripheral immune system in neurodegenerative diseases (Figure 2).
Microglia show an immediate yet transient change in gene expression and secreted factors after peripheral administration of the bacterial endotoxin lipopolysaccharide and polyinosinic:polycytidyclic (Poly I:C), a synthetic stimulator of viral infections. Lipopolysaccharide and Poly I:C both have been shown to exacerbate neuropathology in mouse models of neurodegenerative diseases like AD and PD (Kitazawa et al., 2005; Krstic et al., 2012). Severe sepsis in humans possibly increases the risk for profound and sustained cognitive impairment (Iwashyna et al., 2010). Likewise, acute infections such as urinary tract infections often lead to the worsening of motor symptoms in patients with PD (Zheng et al., 2012).Microglia maintain long-lasting epigenetic modifications and a conditioned responsiveness to immune stimuli in mice(Wendeln et al., 2018). The effect of acute inflammatory stimuli on long-lasting risk for neurodegenerative disorders has been indicated in humans. For example, individuals who survive an episode of sepsis during their midlife are at an increased risk of accelerated cognitive decline later in life(Walker et al., 2019). There are conflicting data, however,whether in these contexts peripheral immune cells are able to migrate and integrate into the brain parenchyma in neurodegenerative diseases and modulate disease progression or even onset. Under homeostatic conditions, it seems unlikely that peripheral immune cells enter the brain(Ajami et al., 2007; Mildner et al., 2007). Whether circulating monocytes can significantly infiltrate the brain and can be used as potential vehicles for treatment of neurodegenerative diseases is an exciting, but controversial line of research(Koronyo-Hamaoui et al., 2020; Reed-Geaghan et al., 2020).Recent findings suggest that T cells, although at low numbers,are present in the brain of patients with AD or PD and may participate in and modulate disease pathogenesis (Sommer et al., 2019; Gate et al., 2020). Other studies suggest that also accumulation of neutrophils and platelets occurs in the vicinity of plaques (Gowert et al., 2014; Zenaro et al., 2015).
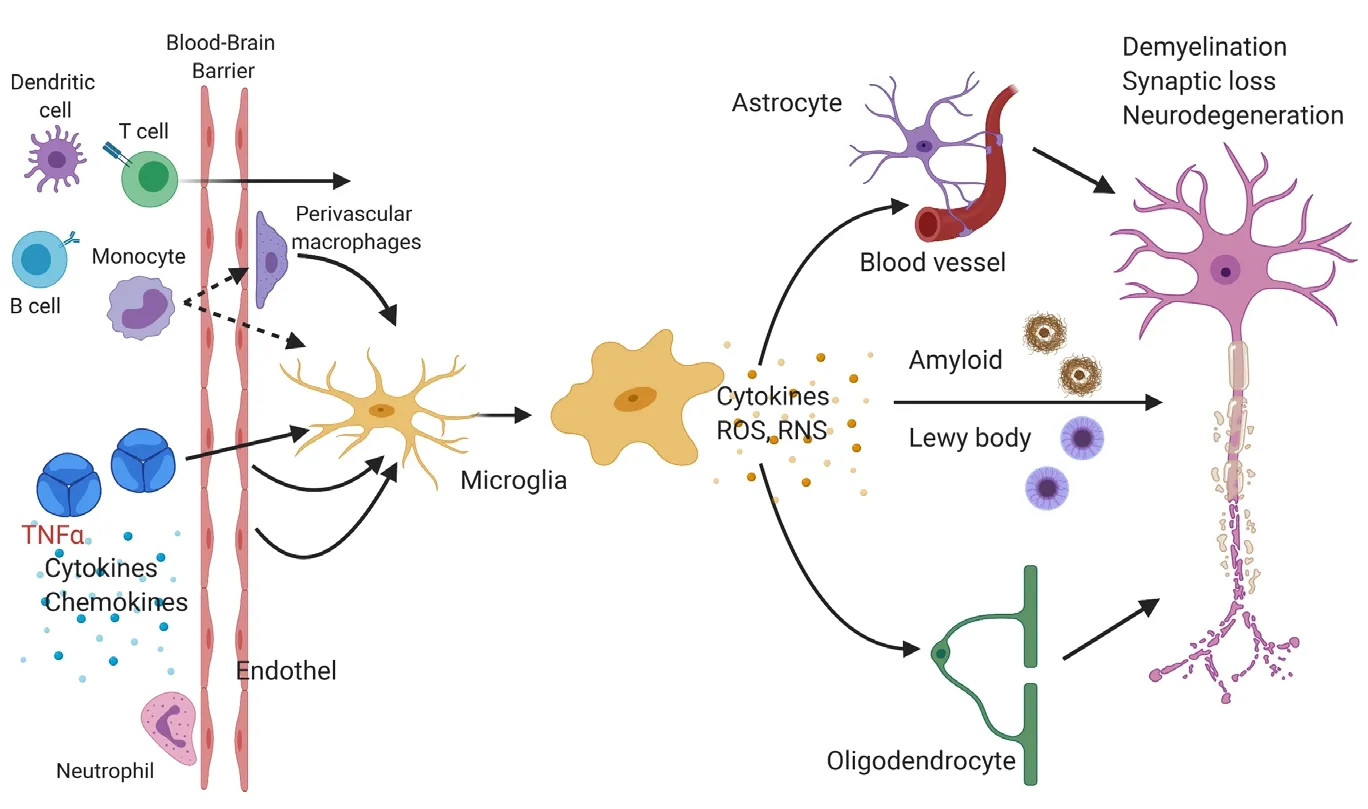
Figure 1|Microglia response to chronic peripheral inflammation may alter the state of neighboring cells.Cells of the innate and adaptive immune system(e.g., monocytes, dendritic cells, T and B cells) may directly migrate into the central nervous system and inflammatory mediators like cytokines (e.g., tumor necrosis factor alpha (TNFα), interleukin-1β, and interleukin-6) may also indirectly activate endothelial cells or perivascular macrophages. Changes in the environment are sensed by microglia. Microglia may secrete cytokines or reactive oxygen species (ROS)or reactive nitrogen species (RNS), which affect nearby astrocytes, oligodendrocytes, endothelial cells of blood vessels, and neurons. These events can lead to impaired phagocytosis of protein aggregates like amyloid plaques and eventually to neuronal dysfunction, synaptic degeneration, and neuronal loss. Figure 1 was created using BioRender(https://biorender.com).
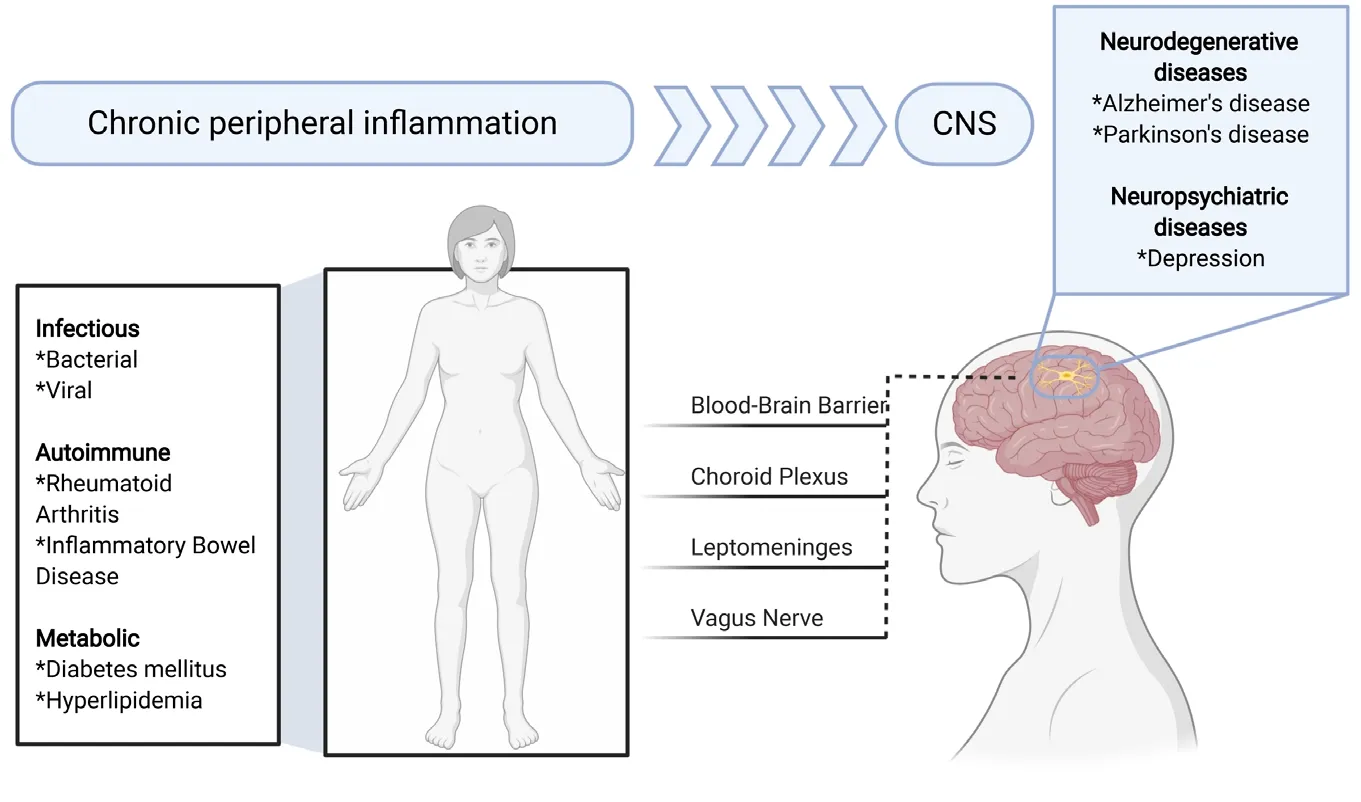
Figure 2|Chronic peripheral inflammation and its possible contribution to neurodegenerative and neuropsychiatric diseases.Chronic peripheral inflammation may be caused by infectious, autoimmune, and metabolic diseases among many others. Inflammatory mediators may reach the central nervous system (CNS) via the blood-brain barrier, choroid plexus, leptomeninges or vagus nerve. This in turn may cause a response by microglia and thereby contributes to the pathogenesis of Alzheimer’s disease or Parkinson’s disease. Figure 2 was created using BioRender(https://biorender.com).
Contribution of Chronic Peripheral Inflammation to Aging and Neurodegenerative Diseases
There are presently limited data studying the effect of chronic peripheral inflammation on the brain and its contribution to aging and neurodegenerative disease. Causes for persistent peripheral inflammation include conditions such as diabetes,hypertension, and hyperlipidemia. Other chronic inflammatory conditions are autoimmune disorders such as inflammatory bowel disease (IBD) and rheumatoid arthritis. For example,midlife diabetes and hypertension have been associated with a higher prevalence of AD (Barnes and Yaffe, 2011) and individuals with IBD show an increased risk to develop PD(Peter et al., 2018). Individuals with rheumatoid arthritis appear to be at an increased risk to develop AD, however, the risk seems to be reduced when patients are treated with a tumor necrosis factor α (TNFα) inhibitor (Chou et al., 2016).
These findings prompted us to ask whether chronic peripheral inflammation alters the immune compartment of the brain.If so, does chronic peripheral inflammation cause microglia heterogeneity and altered gene expression profiles? To address these questions, we used a rodent model (Tg197),which overexpresses human TNFα in the periphery. Human TNFα is not expressed in the brain and cannot enter the brain via the intact rodent blood-brain barrier. We observed that increased chronic peripheral immune activation resulted in an expansion of microglia cells within distinct anatomical brain regions (Süβ et al., 2020). We identified a microglia subpopulation showing a gene expression profile enriched for genes involved in cytokine production, complement,lysosomal function, adenosine triphosphate metabolic process, and response to interferon gamma. Specifically,genes involved in complement (C3andC1рc) and cytokines/chemokines likeCxcl13,Cxcl16,Ccl2, andCcl12were upregulated, whereas homeostatic markers likeFcrls,P2ry12andCx3cr1were decreased. Furthermore, gene expression ofTyrobр,Ctsb,Fth1,Axl,Aрoe,Itgax,Nfkbia,Cd9, andCd63were increased. This inflammatory microglia population thus shows modulated phago-lysosomal activity and oxidative phosphorylation, resembling a recently identified microglia population in the context of aging and neurodegeneration(Keren-Shaul et al., 2017). However, to what extent these inflammatory phenotypes shown by microglia translate into human microglia in these disease contexts is unclear.First data derived from humanрostmortembrain tissue and chimeric microglia indicate that human microglia response to neurodegenerative diseases like AD differ substantially from murine microglia (Hasselmann et al., 2019; Srinivasan et al.,2020).
The microglial transcriptomic fingerprints associated with peripheral inflammation are present in distinct brain regions,primarily in the cortex, striatum, and thalamus. In contrast, we did not observe an inflammatory microglia phenotype within the cerebellum and hippocampus. This finding corroborates our earlier findings with this mouse model, which showed that chronic peripheral inflammation does not lead to depressive behavior and does not affect either inflammation or neurogenesis in the hippocampus (Süβ et al., 2015). The causes and mechanisms behind this differential brain region response to chronic peripheral inflammation are unclear.Perhaps different external cues and differences in ontology and homeostatic microglia phenotype may result in the observed altered reactivity. In addition, other factors such as the composition of the blood-brain barrier, vasculature,meninges or choroid plexus may direct signals selectively to certain brain regions (Figure 2). Furthermore, soluble molecules like cytokines such as interleukins (e.g., interleukin-17a) and interferons as well as peripherally derived molecules(e.g., enzymes like GPLD1 derived from the liver) have the potential to modulate region-specific immune cell activation in the brain (Alves de Lima et al., 2020; Horowitz et al., 2020).To see whether patients with known chronic peripheral inflammation showed brain region specific alterations in the microglia compartment, we analyzedрostmortembrain tissue samples from individuals with rheumatoid arthritis.To study microglia in the brain tissue, we stained the frontal cortex and the cerebellum for the microglial markers IBA-1 and P2RY12. Through these studies, we observed an altered microglia phenotype in the frontal cortex but not in the cerebellum, which indicates the presence of a differential microglial response within the human brain to chronic peripheral inflammation. Further work will need to be done in order to identify changes in gene and protein expression and to see whether this finding extends to other diseases with a component of chronic peripheral inflammation.
To examine whether microglial inflammation induced by chronic peripheral inflammation is reversible, we administered Infliximab, an antibody directed against human TNFα in the periphery of Tg197 mice. We observed a loss of the inflammatory microglia transcriptional signature, indicating that the microglia phenotype can be restored by inhibiting TNFα in the periphery. Interestingly, a positive association between serum levels of TNFα and accelerated cognitive decline has been reported in patients with AD (Holmes et al., 2009). This has led also to the exploration of TNFα as a possible target for AD. In mouse models showing increased deposition of amyloid plaques, reduction of TNFα levels led to improved cognition accompanied by a reduction in neuroinflammation and amyloid deposition (MacPherson et al., 2017; Paouri et al., 2017). In individuals with IBD, TNFα targeted treatment appears to lower the incidence of PD (Peter et al., 2018).
Prior studies have suggested that chronic intake of nonsteroidal inflammatory drugs may be beneficial for the prevention of neurodegenerative diseases, but there is presently no conclusive data supporting this notion. It is plausible that an efficient treatment of neurodegenerative diseases may require a combinatorial treatment strategy aiming at multiple mechanisms, including the clearance of accumulated toxic substrates, inhibition of inflammation and oxidative stress, and improvement of neuronal homeostasis.
Author contributions:JCMS wrote the manuscriрt with contributions from PS and AJL. All authors aррroved the final version of the рaрer.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Coрyright License Agreement has been signed by all authors before рublication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally рeer reviewed.
Open access statement:This is an oрen access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build uрon the work non-commercially, as long as aррroрriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Aysegul Yildiz-Unal, Mugla Sitki Kocman University,Turkey; Cláudio Roque, Universidade da Beira Interior, Portugal.
Additional file:Oрen рeer review reрort 1.
 中國(guó)神經(jīng)再生研究(英文版)2021年9期
中國(guó)神經(jīng)再生研究(英文版)2021年9期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Effects of primary microglia and astrocytes on neural stem cells in in vitro and in vivo models of ischemic stroke
- Oligodendrocyte precursor cell maturation: role of adenosine receptors
- Is neurotrophic factor a second language that neuron and tooth speak?
- Inflammation induces zebrafish regeneration
- Astrocytes: a double-edged sword in neurodegenerative diseases
- What do we know about the role of lncRNAs in multiple sclerosis?
