Invasive fungal infection before and after liver transplantation
Alberto Ferrarese, Annamaria Cattelan, Umberto Cillo, Enrico Gringeri, Francesco Paolo Russo, Giacomo Germani, Martina Gambato, Patrizia Burra, Marco Senzolo
Abstract Invasive infections are a major complication before liver transplantation (LT) and in the early phase after surgery. There has been an increasing prevalence of invasive fungal disease (IFD), especially among the sickest patients with decompensated cirrhosis and acute-on-chronic liver failure, who suffer from a profound state of immune dysfunction and receive intensive care management. In such patients, who are listed for LT, development of an IFD often worsens hepatic and extra-hepatic organ dysfunction, requiring a careful evaluation before surgery. In the post-transplant setting, the burden of IFD has been reduced after the clinical advent of antifungal prophylaxis, even if several major issues still remain, such as duration, target population and drug type(s). Nevertheless, the development of IFD in the early phase after surgery significantly impairs graft and patient survival. This review outlines presentation, prophylactic and therapeutic strategies, and outcomes of IFD in LT candidates and recipients, providing specific considerations for clinical practice.
Key Words: Acute-on-chronic liver failure; Sepsis; Cirrhosis; Candidemia; Acute liver failure; Invasive fungal infection
INTRODUCTION
Liver transplantation (LT) represents the best therapeutic option for end-stage liver diseases and hepatocellular carcinoma. The LT landscape has changed rapidly in the last decades, with a widespread diffusion of this practice, a significant expansion of indications, and an evolution in medical and surgical care. Therefore, although more patients than in the past are offered a graft and can survive after surgery, this changing scenario has determined a huge modification of characteristics of LT candidates and recipients, who are older, sicker and often display many extra-hepatic comorbidities[1].
In this setting, the burden of invasive infection, both before LT [especially in those with advanced cirrhosis or acute-on-chronic liver failure (ACLF)] and in the early postoperative course is still a major issue. Cirrhosis is a predisposing condition to such infections, because of a profound immune dysfunction, due to both an exhaustion of response to pathogens and persistent systemic inflammation[2]. Bacteria are responsible for the majority of invasive infections, determining a further impairment of hepatic and extra-hepatic organ disfunction in the pre-operative phase, and significantly affecting graft and patient’s survival in the early phase after surgery[3-5].
Nevertheless, considered rare in the past, invasive fungal infection occurs with an increasing prevalence in LT candidates, mostly due to the refinement of diagnostic criteria and the increasing burden of predisposing conditions. In the post-LT phase, the institution of antifungal prophylactic strategies has significantly improved patient outcome.
INVASIVE FUNGAL DISEASE IN PATIENTS AWAITING LT
Epidemiology, risk factors, therapeutic options, outcomes
By definition, an invasive fungal disease (IFD) is a disease process caused by invasive fungal infection. Current diagnostic criteria rely on three different levels of probability (proven, probable and possible IFD), mixing together host factors, clinical manifestations, and mycological evidence[6].
The epidemiology of IFD in cirrhotic patients has been heterogeneously reported, mainly in retrospective, single-center series, which included patients with different disease stages, prognosis (i.e., waitlisted for a transplant) and hospital settings [i.e., intensive care unit (ICU) vs regular ward]. Moreover, heterogeneous prevalence, diagnostic criteria and treatment protocols applied throughout the literature may have further influenced the actual epidemiology of such infections.
According to multicenter studies on hospitalized patients with cirrhosis, the prevalence of IFD is nearly 4%[7,8], although only proven IFD are usually considered. Most infections are caused by Candida; according to recent evidence, albicans and nonalbicans strains have roughly similar prevalence[9].
The institution of surveillance protocols appears mandatory for an early diagnosis. These protocols should focus on patients at highest risk of IFD development, such as those with ACLF. Indeed, they encompass several risk factors, such as a profound immune-dysfunction, prolonged hospitalization, hepatic and extra-hepatic failure(s), indwelling (vascular) catheters, and long-term antibiotic therapies[3,10]. According to available studies on this specific population[11-16], the prevalence of IFD ranges between 1% and 47% (depending on diagnostic criteria and surveillance policies), significantly affecting short-term survival. Nevertheless, heterogeneous selection criteria have not allowed a refinement of risk stratification to date (Table 1). Patients with severe alcoholic hepatitis are another high-risk group for IFD, especially for invasive aspergillosis (IA). Gustot et al[17]reported a high incidence of such infection in a prospective cohort of 94 patients with biopsy-proven severe alcoholic hepatitis, after a median time of 25 d from steroids introduction, and with a 100% transplant-free mortality. This report raised the question about the potential role of steroids for IA development in such a population; a meta-analysis in this field[18]partly confirmed this hypothesis, suggesting that opportunistic infections, especially fungal, seemed to be more frequent in this high-risk group, and may deserve special attention. IFD is a less frequent, but highly relevant complication also in patients with acute liver failure (ALF), carrying a high mortality risk, especially in case of a delayed diagnosis or institution of inappropriate treatment[19,20].
The occurrence of IFD often represents a detrimental event in patients with cirrhosis, leading to a significant increase in short-term mortality (35% to 50%), at a similar rate to that experienced after a multidrug-resistant organism bloodstream infection, especially when an appropriate antifungal treatment is not promptly initiated[7,9,21].
A detailed treatment algorithm for IFD in patients with cirrhosis is beyond the scope of this manuscript. The clinical keys of a successful treatment are early diagnosis, early administration of appropriate antifungal treatment, in close cooperation with Infectious Disease specialists. Considering Candida related IFD, ophthalmologic evaluation and removal of vascular/peritoneal catheters, as well as a shift towards non-albicans strains should be considered before starting antifungal therapy. Echinocandins are now considered the drugs of choice, to be continued for 2 weeks after clearance of Candida from the bloodstream or symptoms resolution[22]. Considering IA, voriconazole represents the first therapeutic option, whereas echinocandins and liposomal amphotericin B (L-AmB) are other, albeit less effective, available drugs[23]. It is worth mentioning that voriconazole has been associated with hepatic and renal dysfunction, therefore therapeutic drug monitoring is recommended[24].
Specific issues in the liver transplant setting
IFD are a major issue in patients waiting for LT. As discussed above, occurrence of an IFD highlights the already impaired patient’s general condition, with an unpredictable evolution of hepatic and extra-hepatic organ(s) failure. This may potentially increase the need for a transplant, especially in a urgency-based system of organ allocation[25]. Nevertheless, according to the available data, several points should be considered; first, the effectiveness and treatment length of an appropriate antifungal therapy are very different from antibiotic therapies. Second, an IFD seems to develop in sicker patients than in the case of a bacterial infection, often as a superimposed infection[7,8]. Therefore, an active IFD should be viewed as a temporary contraindication for LT[26](Figure 1). For the sickest patients who are waiting for a graft, surveillance protocols are mandatory, and antifungal prophylaxis has been advocated in selected cases. For instance, Gustot et al[27]suggested ICU admission and a baseline MELD score > 24 as factors for considering a prophylaxis against IA in patients with acute alcoholic hepatitis[16,27], but more data are needed before considering it as a standard practice. After diagnosis of IFD, consultation by expert Infectious Disease specialists should be always considered, in order to establish the best targeted antifungal treatment and its length. Moreover, antifungal stewardship aiming to avoid both adverse events and increasing resistance should always be pursued in the transplant setting.
The assessment of short-term outcome for each waitlisted patient should be individually discussed by the LT team, in order to consider the best timing for a waiting-list readmission (and a possible prioritization after infection recovery[4]). Conversely, other therapeutic options should be taken into account, to avoid futile transplantation[28,29].
FUNGAL INFECTIONS EARLY AFTER LT
Epidemiology, risk factors, and outcome
Although better outcomes have been reported after the introduction of novel antifungal agents and significant progress has been obtained after antifungal prophylaxis, IFD remains an important cause of early morbidity and mortality after solid organ transplantation (SOT). Recent large cohort studies on SOT recipients showed a 1-year post-transplant IFD rate of 4%-8%[30-32], with a changing epidemiologyover time. Indeed, if Candida spp. and Aspergillus spp. are still the most common molds, there has been a rise of non-albicans Candida species, carrying a higher mortality[33].

Table 1 Studies assessing the prevalence of invasive fungal disease in patients with acute-on-chronic liver failure
Broad-spectrum antibiotic therapy, parenteral nutrition, prolonged neutropenia, ICU stay, diabetes, pre-LT colonization, renal replacement therapy, cytomegalovirus (CMV) infection, re-interventions and choledochojejunostomy are established risk factors for post-LT IC[34,35], whereas pre-LT steroid administration, ALF, and renal replacement therapy seem to be more frequently associated with IA[36-38]. Recently, pre-LT Aspergillus colonization has been considered not a contraindication to LT in a single-center cohort of 27 patients; although they received appropriate post-operative prophylaxis (voriconazole +/- echinocandin), post-LT IA occurrence was 11%[39]. Most of the abovementioned risk factors are associated with patient’s severity at time of transplantation. This concept has been well demonstrated in ACLF patients, who experienced a significantly increasing post-LT IFD incidence, according to disease stage (ACLF grade 3 vs -2 vs -1: 15% vs 6.2% vs 3.4%)[40].
Although active IFD in the donor is a contraindication to donation, several cases of donor-derived IFD have been reported in the literature, mostly due to a undiagnosed infection at time of surgery[41]. Contamination of the organ during procurement appears as another important issue. For instance, a large retrospective multicenter study from France showed a 1.33% Candida spp. prevalence in preservation fluid, being associated with a high rate of post-operative IFD and impaired survival[42].
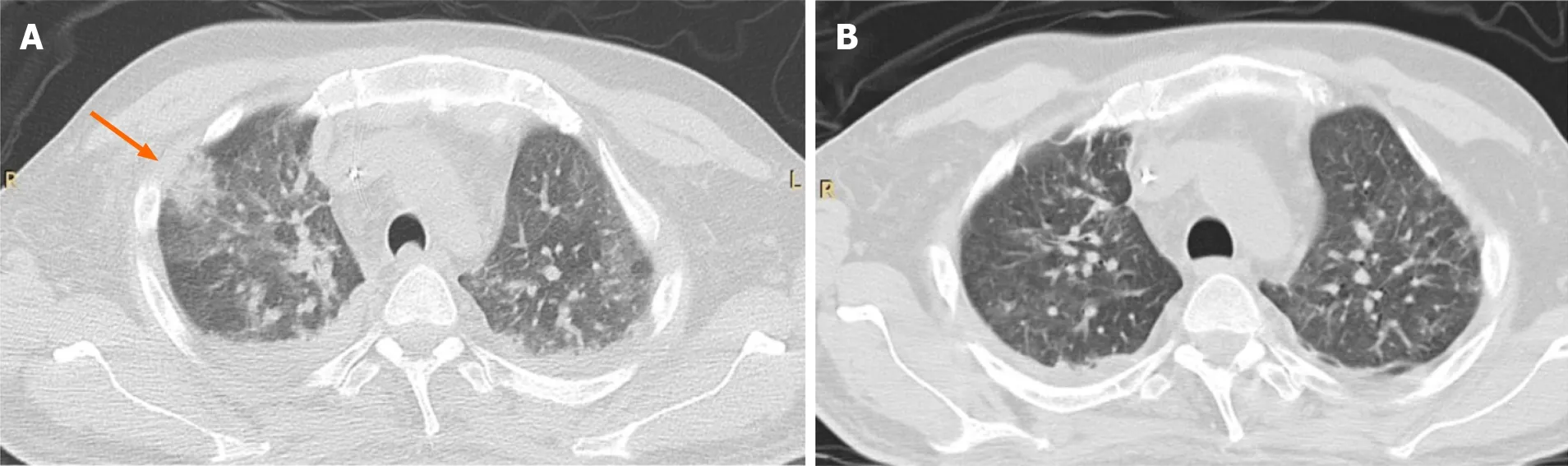
Figure 1 CT-scan. A: Chest CT-scan of a young male patient with hepatitis B virus related cirrhosis and acute-on-chronic liver failure, waitlisted for liver transplantation, who developed invasive aspergillosis; B: He was temporarily withdrawn from the waiting list, and received antifungal treatment for a total of 13 d, with a clinical and radiological improvement. He subsequently died of bacterial super-infection before liver transplantation.
Despite the adoption of preventive measures and antifungal stewardship, IFD still significantly affect the overall graft and patient survival. For instance, the TRANSNET study[43]reported 90 d cumulative mortality of 26% after IC occurrence, and 1-year survival of 59% after development of IA.
Post-LT antifungal prophylaxis
Antifungal prophylaxis is now being considered a cornerstone after LT, due to its safety and effectiveness[44,45]. A systematic review and metanalysis by Evans et al[46]showed a significant reduction in the odds for proven IFD and for IFD-related mortality among LT patients who received prophylaxis, even if overall mortality did not change significantly. Notably, this study provided robust data about fluconazole and L-AmB, whereas echinocandins were not investigated. That said, several issues in the field of antifungal prophylaxis, such as the type (universal vs targeted approach), length, and preferred molecule(s) to use, are currently debated.
The rationale of a targeted prophylaxis is to capture only high-risk patients (based on pre- and early post-LT characteristics), in order to avoid antifungal over-use, and to administer highly effective molecules. Indeed, several studies have clearly demonstrated the cost-ineffectiveness of antifungal prophylaxis in low-risk patients.
Considering the optimal prophylaxis duration, current guidelines suggest that targeted prophylaxis against IC and IA should be administered for 14-21 d[34,36], but heterogeneous lengths have been adopted in the post-transplant setting, also in view of the dynamic, poorly predictable post-operative course. Further, many attempts at regimen simplification or stratification according to patients’ risk factors have been proposed. Table 2 summarizes the current evidence on antifungal prophylaxis after LT[35,37,47-60]. Notably, heterogeneous inclusion criteria, treatment algorithms, and endpoints adopted, do not allow a robust comparison between studies, but it is worth mentioning that a large amount of data has been available in the last years.
A randomized, double-blind clinical trial including 200 high-risk LT recipients, compared prophylaxis with fluconazole 400 mg/d with anidulafungin 100 mg/d to be continued for 3 wk or until hospital discharge. The study showed a similar IFD occurrence between cohorts (5.1% vs 8%, P = 0.4), with no post-LT IFD related deaths in either. Furthermore, only one patient had to stop anidulafungin prophylaxis due to adverse drug-related events, strengthening the safety of this molecule in the post-LT setting. Another multicenter, randomized, controlled trial including 347 LT recipients recruited across 37 European Centers[51]demonstrated that micafungin prophylaxis (100 mg/d for 21 d or until hospital discharge) was equally effective and safe as standard of care (i.e., fluconazole, caspofungin, or L-AmB), according to composite primary and secondary endpoints. The effectiveness of caspofungin (50 mg/d) has been also demonstrated in a large retrospective study from Spain, after comparison with standard fluconazole prophylaxis[56].
Specific treatment issues in the liver transplant setting
A detailed therapeutic algorithm for the treatment of each IFD is beyond the scope of this manuscript. Nevertheless, some treatment principles could be of help for clinicalpractice. As in the pre-LT setting, echinocandins and fluconazole are the most effective molecules for the treatment of IC, whereas L-AmB should be used as first-line therapy only in selected cases. A thorough knowledge of local epidemiology, as well as pre
operative colonization(s) represent crucial information before starting a therapeutic regimen. Source control, obtained by removal of indwelling vascular/abdominal catheters, is another important option to be considered. Regarding echinocandins, both micafungin and anidulafungin have been demonstrated to be safe and effective at therapeutic dose[51,61]. Notably, micafungin does influence through levels of m-TOR inhibitors, but not of tacrolimus and cyclosporine[62].
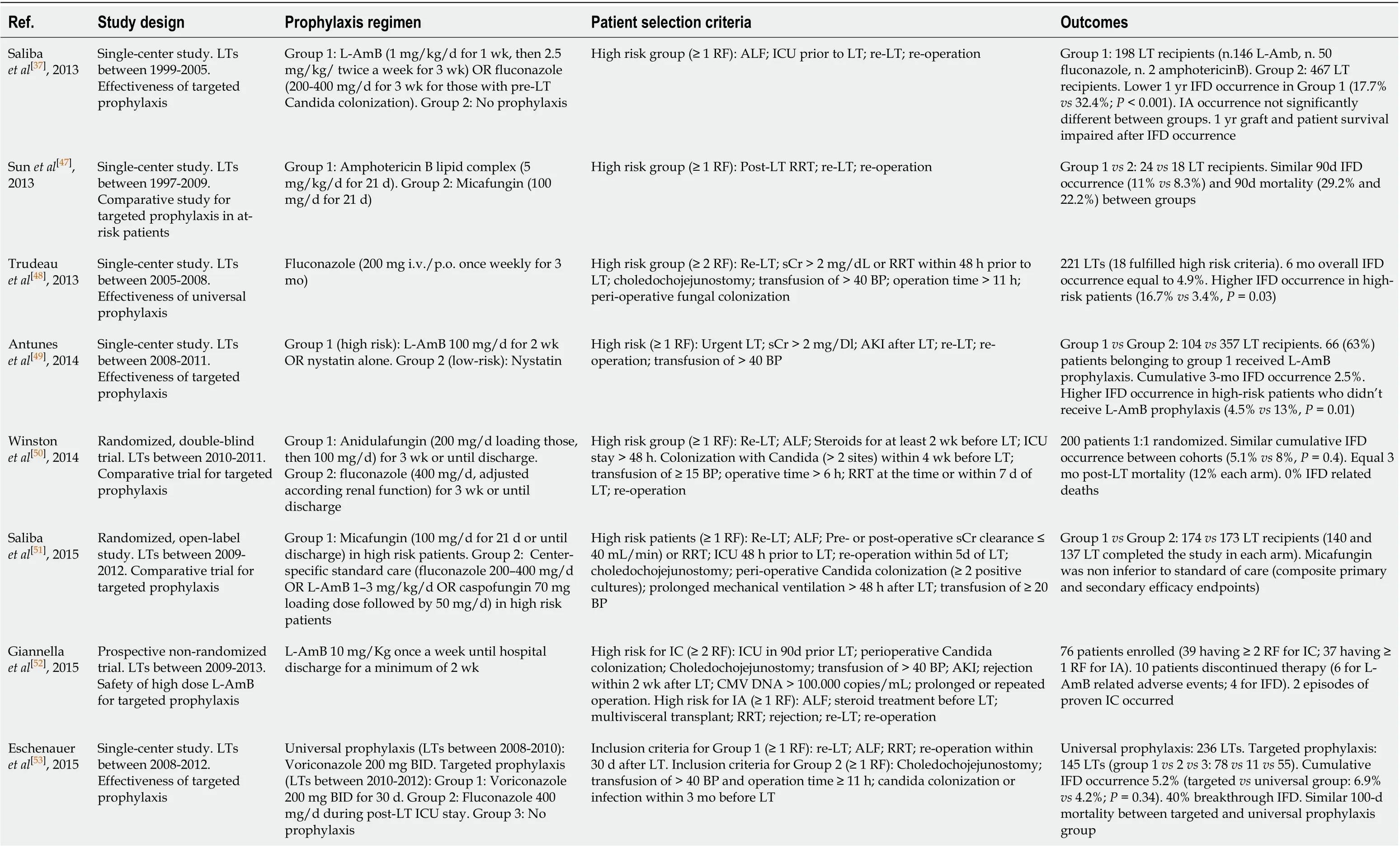
Table 2 Studies published in the last 10 years on fungal prophylaxis in the liver transplantation setting
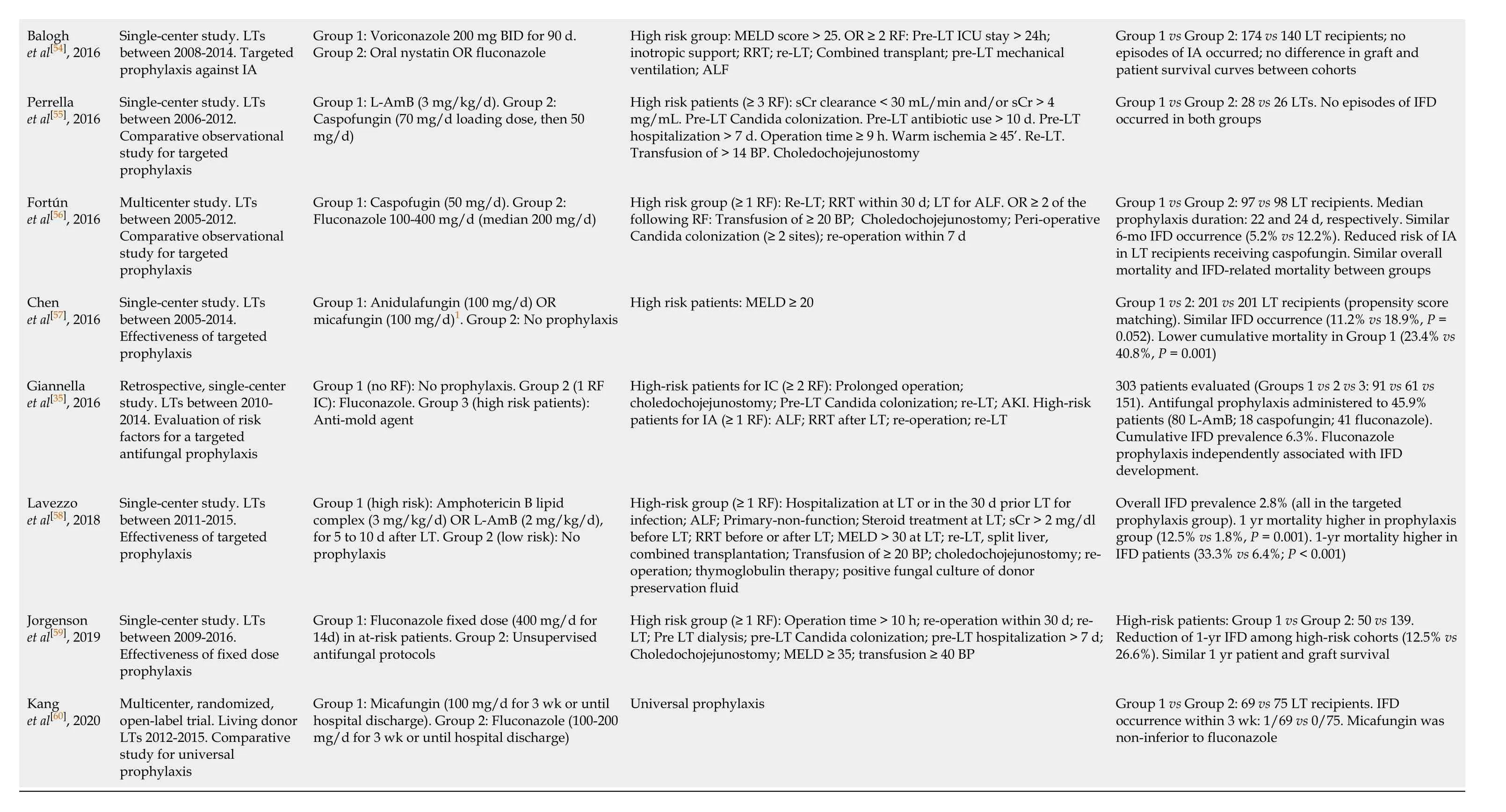
1Duration of prophylaxis not reported. BP: Blood products; IA: Invasive aspergillosis; IC: Invasive candidiasis; IFD: Invasive fungal disease; L-AmB: Liposomal amphotericin B; LT: Liver transplantation; RF: Risk factor; RRT: Renal replacement therapy; sCr: Serum creatinine; ICU: Intensive care unit.
Current guidelines recommend voriconazole as the drug of choice for IA, whereas isavuconazole and L-AmB can be considered as alternatives[36]. Isavuconazole seems to have similar effectiveness to voriconazole, but with fewer side effects–also liverrelated –, being a promising option especially in the early post-operative phase[63]. During the course of therapy (usually 12 wk regimen), a careful assessment of IS, liver and renal function are mandatory, as well as therapeutic drug monitoring. Moreover, daily dose of calcineurin inhibitors should be carefully reduced (about by 50%), whereas co-administration of voriconazole and mTORs should be avoided due to a high increase of serum concentration[64]. Other molecules could be of help for the treatment of rarer species, or as rescue therapies[65,66].
CONCLUSION
The occurrence of an invasive fungal disease significantly affects the natural history of LT candidates and recipients. In the peri-operative setting, it usually develops in the sickest patients, impairing hepatic and extra-hepatic organ function and being associated with high short-term mortality. An active IFD is still considered a contraindication to LT. Therefore, response to appropriate antifungal therapy and patient’s global outcome should be strictly evaluated by the LT team in accordance with Infectious Disease Specialists, in order to re-consider transplantation as a costeffective therapeutic option. In the post-operative setting, IFD occurrence has been significantly reduced since the institution of prophylaxis, but it is still a serious complication, affecting graft and patient survival. Prophylactic regimens in patients deemed at high-risk may take into account the local epidemiology, risk of resistance, and potential adverse drug-related effects or interactions.
 World Journal of Gastroenterology2020年47期
World Journal of Gastroenterology2020年47期
- World Journal of Gastroenterology的其它文章
- Artificial intelligence-aided colonoscopy: Recent developments and future perspectives
- Molecular overview of progressive familial intrahepatic cholestasis
- Evaluation of an educational telephone intervention strategy to improve non-screening colonoscopy attendance: A randomized controlled trial
- Towards an evaluation of alcoholic liver cirrhosis and nonalcoholic fatty liver disease patients with hematological scales
- Prevalence and associated factors of obesity in inflammatory bowel disease: A case-control study
- Extracellular histones stimulate collagen expression in vitro and promote liver fibrogenesis in a mouse model via the TLR4-MyD88 signaling pathway
