Effect of cholesterol on in vitro cultured interstitial Cajal-like cells isolated from guinea pig gallbladders
Bei-Bei Fu,Department of Health Management,Shengjing Hospital of China Medical University,Shenyang 110004,Liaoning Province,China
Jin-Huang Xu,Shuo-Dong Wu,Ying Fan,Department of General Surgery,Shengjing Hospital of China Medical University,Shenyang 110004,Liaoning Province,China
Abstract BACKGROUND Loss and/or dysfunction of interstitial Cajal-like cells (ICLCs) in the gallbladder may promote cholesterol gallstone formation by decreasing gallbladder motility.AIM To study the effect of cholesterol on the proliferation and apoptosis of ICLCs from guinea pig gallbladders.METHODS Guinea pig gallbladder ICLCs were isolated and cultured in vitro.The cells were exposed to cholesterol solutions at different concentrations (0,25,50,and 100 mg/L) for 24 h.Then,cell proliferation was detected by the CCK-8 method and the apoptosis rate was detected by flow cytometry.Further,the expression of the c-Kit protein was detected by Western blot and the expression level of c-Kit mRNA in the cells was detected by real-time quantitative PCR.RESULTS After ICLCs were cultured with cholesterol at concentrations of 25,50,and 100 mg/L,the proliferation rates decreased significantly (P < 0.05),whereas the apoptosis rates increased significantly (P < 0.05).Moreover,the expression of c-Kit protein and mRNA decreased significantly (P < 0.05).CONCLUSION High cholesterol concentrations can inhibit the proliferation of ICLCs and promote apoptosis.This decrease in the ICLC proliferation rate might be caused by the inhibition of the stem cell factor/c-Kit signaling pathway.
Key words:Interstitial Cajal-like cells;Cholesterol;Apoptosis;c-Kit
INTRODUCTION
Cholesterol gallstone (CG) is a disease commonly seen in clinical surgery.Its etiology is complex,and a decrease in gallbladder motility is an important cause of the formation of CGs[1-3].In recent years,with the confirmation of the role of interstitial Cajal-like cells (ICLCs) in the regulation of gallbladder motility[4-7],their relationship with CG formation has been a hot research topic.Studies have found that the number of ICLCs decreases in both human and guinea pig CG model gallbladder specimens[8-10],and this decrease might be caused by the supersaturation of bile with cholesterol[11].The purpose of this study was to investigate the effect of cholesterol on the proliferation and apoptosis of ICLCs by isolating and culturing guinea pig gallbladder ICLCsin vitroand adding water soluble cholesterol to the cell culture solution to simulate a high-free cholesterol environment to which gallbladder ICLCs are exposed in CG patients.The influence of cholesterol on gallbladder ICLCs was further explored by studying its effect on the expression of c-Kit protein and mRNA.
MATERIALS AND METHODS
Main materials
Five healthy male guinea pigs weighing 300-400 g were provided by Beijing Jinmuyang Experimental Animal Breeding Co.,Ltd.,and were adaptively raised according to the standards for conventional animals in the Animal Department of the Research and Development Center of Benxi Medical and Pharmaceutical Research Base,Shengjing Hospital of China Medical University.Stem cell factor (SCF) (Santa Cruz Biotechnology,Santa Cruz,CA,United States),M199 culture solution (Hyclone),fetal bovine serum (Gemini South American),tertiary antibodies (Gibco),c-Kit monoclonal antibodies (NOVUS,United States),triton X-100 (Beyotime Biotechnology Inc.,China),DAPI (Beyotime Biotechnology Inc.,China),goat serum (Beijing Solarbio Science and Technology Co.,Ltd.,China),water-soluble cholesterol (Sigma-Aldrich Corporation,United States),a CCK-8 kit (Fuyuan Biotechnology Co.,Ltd.,China),an apoptosis kit (Fuyuan Biotechnology Co.,Ltd.,China),an RNA extraction kit(Takara),and a reverse transcription kit (Takara) were used for the following experiments.
Isolation and culture of guinea pig gallbladder ICLCs
The guinea pigs were fasted for 12 h,intraperitoneally injected with 10% chloral hydrate for anesthesia,and then sacrificed by decapitation.The abdomens of guinea pigs were shaved,after which they were soaked in 75% alcohol for 5 min.The upper abdomens of guinea pigs were cut open to fully expose the gallbladders.Cholecystectomy was performed from the neck of the gallbladder under aseptic conditions.The gallbladders were placed in PBS containing 1% tertiary antibodies.Under a dissecting microscope,the mesocyst and blood vessels were carefully stripped off,the gallbladder tissue was cut open and rinsed three times with PBS containing 1% tertiary antibodies,and the gallbladder mucosa and submucosa were stripped off.The gallbladder muscle strips were rinsed three times with PBS containing 1% tertiary antibodies,cut into small pieces of approximately 1 mm × 1 mm in size,and added to 5 mL of type II collagenase (at a concentration of 1.3 mg/mL).The tissue was transferred to a 15-mL centrifuge tube and digested in a water bath at 37 °C for 30 min.The digested gallbladder tissue was centrifuged at 1500 r/min for 5 min and the supernatant was discarded.The precipitate was resuspended in M199 culture solution,pipetted for 5 min,and centrifuged at 1500 r/min for 5 min.The supernatant was discarded.Finally,the cells were resuspended in 5 mL mixed culture solution (containing M199 culture solution,10% FBS,1%tertiary antibodies,and 5 ng/mL SCF).Large pieces of tissue were removed by filtering through a 200-mesh screen.The filtered cell suspension was transferred to a culture flask and cultured in an incubator (the incubator temperature was 37 °C and the CO2volume fraction was 5%).After the cells were stably cultured in the incubator for 12 h,the culture solution was discarded,the cells were washed with PBS three times,5 mL of new mixed culture solution was added,and the morphology and quantity of the cultured cells were observed and recorded.The morphology and quantity of cells were observed and recorded again at 24 h and 48 h,respectively.Thereafter,the culture solution was replaced every 2 d and the culture solution in the culture flask was observed for turbidity,color fading,floating objects,and flocs,etc.
Identification of ICLCs
The third-generation cultured ICLCs were prepared as single-cell suspensions and then inoculated into 6-well plates.After the cells stabilized,the culture solution was discarded.The cells were rinsed with PBS for 2 min three times,fixed with 4%paraformaldehyde at room temperature for 20 min,and then rinsed with PBS for 5 min three times.Then,1% triton X-100 was applied to the cells for 15 min,after which the cells were rinsed with PBS for 5 min three times.Goat serum (10%) was added to block the cells at room temperature for 30 min.Then,c-Kit monoclonal antibodies were added (1:100),and the cells were placed in a refrigerator at 4 °C overnight.On the next day,the cells were taken out and rinsed with PBS for 5 min three times.FITClabeled secondary antibodies were added (1:50) for 1.5 h at room temperature,after which the cells were rinsed with PBS for 5 min three times.DAPI was used to stain the nuclei for 5 min,the cells were rinsed with PBS for 5 min three times,and 50%glycerol solution was applied for mounting.The 6-well plates were observed under a fluorescence microscope.Photos were taken from three different fields of view and saved.
CCK-8 detection of cell proliferation
After the cultured gallbladder ICLCs were prepared into single-cell suspensions,they were inoculated into 96-well plates at a density of 5000 cells per well.After 24 h of synchronization,they were cultured for 24 h in culture solutions with cholesterol at concentrations of 0,12.5,25,50,and 100 mg/L.After adding CCK-8 solution and incubating for 4 h,the absorbance at 450 nm (A450) was measured using a microplate reader.Cell proliferation activity was calculated according to the following formula:Cell proliferation activity (%) = [A (treated) - A (blank)]/[A (non-treated) - A (blank)]× 100 (A (blank) was the absorbance of wells with culture medium and CCK-8 solution but without cells).
Flow cytometric detection of apoptosis
ICLCs were cultured in culture solutions with cholesterol at concentrations of 0,25,50,and 100 mg/L.After 24 h,cells were collected from each group,and PBS was used to wash the cells twice.Specifically,5 × 105cells were collected,added to 500 μL of binding buffer,5 μL of Annexin V-FITC,and 5 μL of PI,mixed evenly,and placed at room temperature in the dark for 15 min for apoptosis detection.The detection process was repeated three times for each cholesterol concentration group.
Western blot detection of c-Kit protein expression in ICLCs
The ICLCs were cultured in culture solutions with cholesterol at concentrations of 0,25,50,and 100 mg/L.After 24 h,cells were collected from each group to extract proteins.According to the BCA kit instructions,the protein concentration was detected,the volumes of different samples were adjusted,and water and loading buffer were added for dilution,such that the protein contents of the samples were the same.
Next,a 10% separating gel and 5% stacking gel were prepared,and the samples were loaded.Electrophoresis was performed at a constant voltage of 80 V until the bromophenol blue reached the bottom of the gel.Excess gel was removed and the proteins were transferred to 0.45-μm PVDF membranes at a constant voltage of 100 V(β-actin,45 min;c-Kit,150 min).After membrane transfer was completed,the PVDF membranes were blocked with 5% skimmed milk powder solution at room temperature for 2 h.Then,the membranes were rinsed with TBST buffer,put into incubation boxes containing c-Kit and β-actin (1:100) antibodies,and incubated on a shaker with gentle shaking at 4 °C overnight.Then,the PVDF membranes were taken out,rinsed with TBST three times for 10 min each,put into the secondary antibody solution,allowed to stand at room temperature for 2 h,and then rinsed with TBST three times for 10 min each.Enhanced chemiluminescence was then used for detection.According to the kit instructions,after mixing equal amounts of reagent A and reagent B evenly,the chemiluminescence solution was aspirated with a pipette and dripped onto the washed membranes.Specific protein bands were visualized with X-ray film using the chemiluminescence detection kit (Amersham).The Western blot bands were analyzed and β-actin was probed as the internal control,with the relative expression of the target protein calculated as gray value of target protein/gray value of β-actin.
Detection of c-Kit expression in ICLCs by real-time quantitative PCR
The ICLCs were cultured in culture solutions with cholesterol at concentrations of 0,25,50,and 100 mg/L.After 24 h,the culture solution was discarded and the cells were washed with PBS three times.Each culture sample was added to 1 mL of Trizol and the solution was repeatedly pipetted until the adherent cells were detached.Then,the solution was transferred to a 1.5 mL EP tube;total RNA from the cells was extracted according to the kit instructions,and cDNA was synthesized by reverse transcription according to the instructions of the reverse transcription kit.The PCR reaction was carried out with cDNA as the template.The following primers were used:Forward,5'-CCAACCAGGCTCTTCTCAACCATC-3' and reverse,5'-CCT TGT TCT CGCTCGTGTGATCC-3' forc-Kit;forward,5'-CAACACAGTGCTGTCTGGTAC-3' and reverse,5'-ATCTGCTGGAAGGTGGAGAGT GAG-3' forβ-actin.The reaction system was as follows:SYBR,10 μL;DEPC-treated water,6.4 μL;upstream primer,0.8 μL;downstream primer,0.8 μL;cDNA,2 μL.The reaction conditions were as follows:Initial denaturation at 95 °C for 5 min;denaturation at 95 °C for 5 s and annealing at 60 °C for 30 s,for a total of 35 cycles.According to the Ct value,ΔΔCt was calculated according to the formula ΔΔCt = (target gene Ct of the experimental group - internal reference Ct) - (target gene Ct of the control group - internal reference Ct).The relative expression of c-Kit mRNA was calculated through 2-(ΔΔCt)analysis.Three parallel replicate wells were set up and the mean value of detection results was adopted.
Statistical analysis
All experiments were repeated three times.The experiment results are expressed as the mean ± SD.Statistical comparisons between groups were analyzed using the analysis of variance followed by the post hoc test.Statistical significance was deemed atP< 0.05.
RESULTS
Cell culture results
After 1 wk of cell culture,based on microscopic observations (Figure1),the cells were spindle-shaped,triangular,round,or oval,with relatively large nuclei and little cytoplasm,and had 2-3 processes,which could be connected to each other.With an increase in culture time,the cell processes became thinner and elongated,and connected with each other to form a network with typical morphological characteristics of ICLCs.The cultured cells were passaged for three generations,and after immunofluorescence staining (i.e.,treatment with c-Kit monoclonal antibodies and FITC-labeled secondary antibodies),they were positive for c-Kit as shown by FITC green fluorescence,with cell nuclei stained with DAPI as shown by blue fluorescence (Figures 2 and 3).The ICLCs thus specifically expressed c-Kit.Except for ICLCs and a small number of mast cells,all other cells in the gallbladder tissue were negative for this marker.Therefore,the cells cultured were all ICLCs except for a small number of circular mast cells that were positive for c-Kit,and most ICLCs were spindle-shaped.
Effect of cholesterol on ICLC proliferation
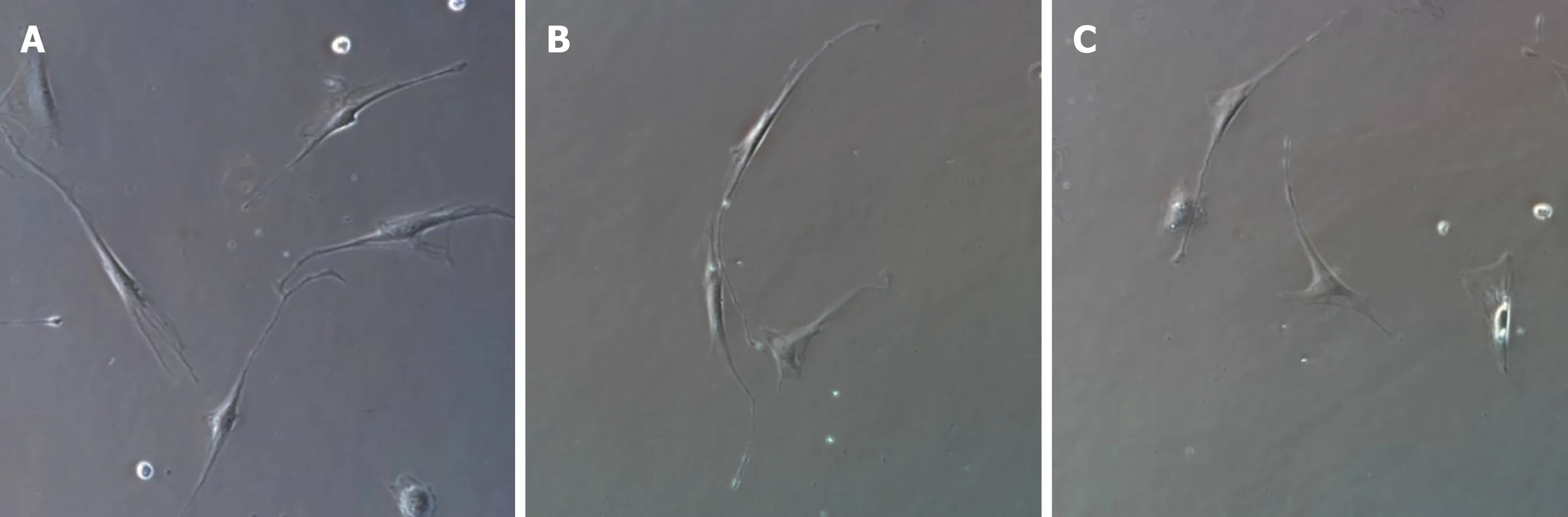
Figure1 lnverted microscopic images of interstitial Cajal-like cell culture after 1 wk (× 400).A:The cells were spindle-shaped,round,or oval,with connections between processes;B:The cells were spindle-shaped,with connections between processes;C:The cells were triangular.
The curve depicting the effect of cholesterol on ICLC proliferation was plotted according to cell proliferation activity (Figure4).The results showed that compared to that in the control group,all four concentrations of cholesterol (12.5,25,50,and 100 mg/L),set as the experimental groups,could reduce the proliferation rate of ICLCs,and these differences were statistically significant among each concentration (P<0.05).
Effect of cholesterol on ICLC apoptosis
The results of flow cytometry (Figure5) showed that cholesterol at 25,50,and 100 mg/L,comprising the experimental groups,all increased the rate of ICLC apoptosis,and these differences were statistically significant among each level (P< 0.05).
Effect of cholesterol on c-Kit protein expression in ICLCs
Quantitative Western blot results (Figure6) showed that cholesterol at 25,50,and 100 mg/L,comprising the experimental groups,all decreased the expression of c-Kit protein in ICLCs and these differences were statistically significant among each level(P< 0.05).
Effect of cholesterol on c-Kit mRNA expression in ICLCs
RT-qPCR results (Figure7) showed that cholesterol at 25,50,and 100 mg/L,comprising the experimental groups,all decreased the expression ofc-KitmRNA in ICLCs and these differences were statistically significant among each level (P< 0.05).
DISCUSSION
CGs are formed in the gallbladder as a result of a combination of multiple factors.The basic process of CG formation is as follows:The concentration ratio of cholesterol to bile acid changes under the influence of various factors,the concentration of free cholesterol in bile reaches a supersaturated state,and cholesterol crystals are precipitated from bile,which are deposited in the gallbladder due to decreased gallbladder contractile force,limited gallbladder emptying,and delayed emptying caused by decreased gallbladder motility.In the presence of mucus secreted by the gallbladder,cholesterol crystals further increase and aggregate into stones.
Interstitial cells of Cajal (ICCs) are special interstitial cells related to the activity of intestinal autonomic nerve endings,which were observed by the Spanish neuroanatomist Ramon Santiagoy Cajal in the gastrointestinal tract in 1893[12].ICCs are the pacing cells of gastrointestinal slow waves,which participate in the pacing and transmission of intestinal slow waves and are related to the conduction and regulation of intestinal signals[13].In recent years,many researchers have also found such cells in parenteral tissues and these have been referred to as ICLCs[14].It has now been confirmed that ICLCs also exist in the gallbladder.Gallbladder ICLCs function in the dynamic pacing of gallbladder smooth muscle,mediating slow wave potential transmission and regulating neurotransmitters;a decrease in the number of gallbladder ICLCs can cause a decrease in gallbladder contractility,leading to the dysregulation of gallbladder motility[8-10].Some researchers believe that some components in bile might disrupt gallbladder motility,and accordingly,the concentration of cholesterol in bile from patients with gallbladder stones was found to be significantly higher than that in bile from individuals without gallbladder stones[11].Therefore,dysregulated gallbladder contraction could be caused by the absorption of supersaturated cholesterol in bile by the gallbladder wall,which might also be the cause of reductions in gallbladder ICLCs.
Figure2 lmmunofluorescence staining of third generation interstitial Cajal-like cells (× 200).A:c-Kit fluorescent antibody staining;B:DAPI staining;C:Merged image.
Thec-Kitgene is a proto-oncogene that encodes a class III receptor tyrosine kinase.Gallbladder ICLCs specifically express c-Kit,and currently the most common method to identify ICLCs is c-Kit immunoreactivity.SCF is a natural ligand of the c-Kit receptor and the SCF/c-Kit signaling pathway composed of c-Kit and its natural ligand SCF plays an important role in the development,differentiation,and phenotypic maintenance of ICLCs[15-17].When the expression of c-Kit or SCF decreases,alterations to the SCF/c-Kit signaling pathway might cause abnormalities in the quantity,morphology,and functions of ICLCs[18-20].
In this study,guinea pig gallbladder ICLCs were obtained throughin vitroisolation,culture,and passage.Because gallbladder ICLCs specifically express c-Kit protein,we identified ICLCs by immunofluorescence staining.The immunofluorescence results showed that the cells were positive for c-Kit as shown by FITC green fluorescence,which confirmed that the cultured cells were gallbladder ICLCs.The concentration of extracellular free cholesterol was increased by adding cholesterol of different concentrations to the culture solutions of ICLCs.This simulated the highcholesterol environment in bile to which gallbladder ICLCs are exposed in patients with cholecystolithiasis.The results of CCK-8 proliferation experiments showed that compared to that in the control group,the four cholesterol concentrations (12.5,25,50,and 100 mg/L) in the experimental groups could reduce the proliferation rates of ICLCs,which was significant for the 25,50,and 100 mg/L groups.Moreover,the results of apoptosis assays showed that compared to those in the control group,ICLC apoptosis rates in the cholesterol groups treated with concentrations of 25,50,and 100 mg/L were increased significantly.These experiments showed that culture solutions with high concentrations of cholesterol can inhibit the proliferation of ICLCs and promote apoptosis.Western blot and PCR results further showed that the relative expression of c-Kit protein and mRNA in ICLCs was decreased in cells treated with 25,50,and 100 mg/L cholesterol compared to that in the control group,suggesting that the decrease in ICLC proliferation might be due to inhibition of the SCF/c-Kit signaling pathway,which is known to affect the growth and development of ICLCs.
In summary,during the process of gallstone formation,a high-concentration cholesterol environment affects the growth and development of gallbladder ICLCs and promotes their apoptosis by inhibiting the SCF/c-Kit signaling pathway.This consequently causes a decrease in the total number of gallbladder ICLCs and the subsequent weakening of spontaneous rhythmic contraction functions in the gallbladder,thereby playing a role in gallstone formation.
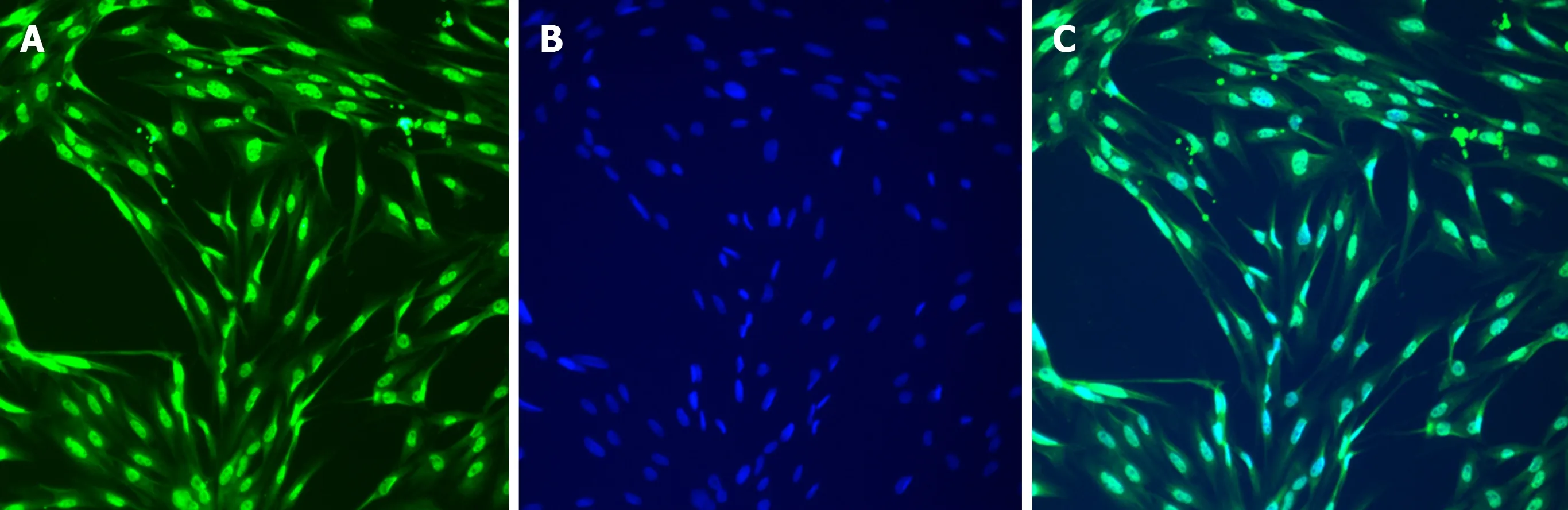
Figure3 lmmunofluorescence staining of third generation interstitial Cajal-like cells (× 400).A:c-Kit fluorescent antibody staining;B:DAPI staining;C:Merged image.
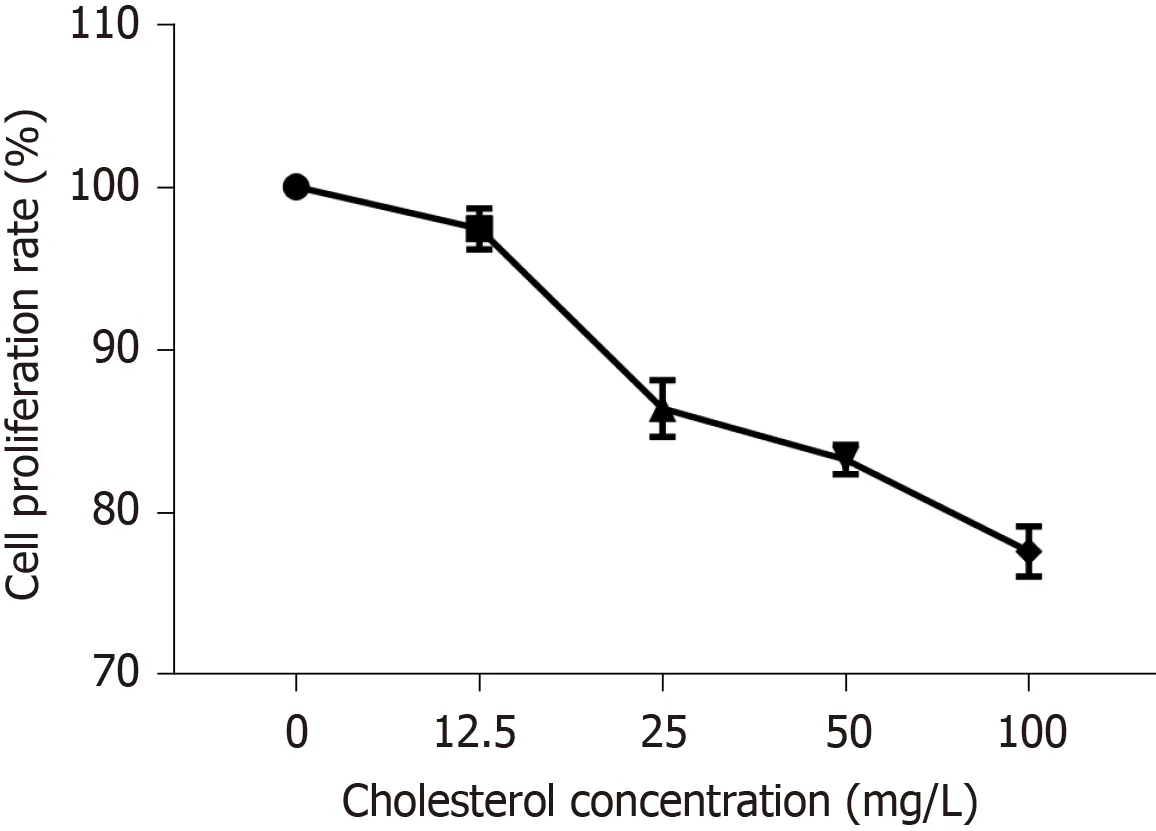
Figure4 Effect of different concentrations of cholesterol on interstitial Cajal-like cell proliferation.
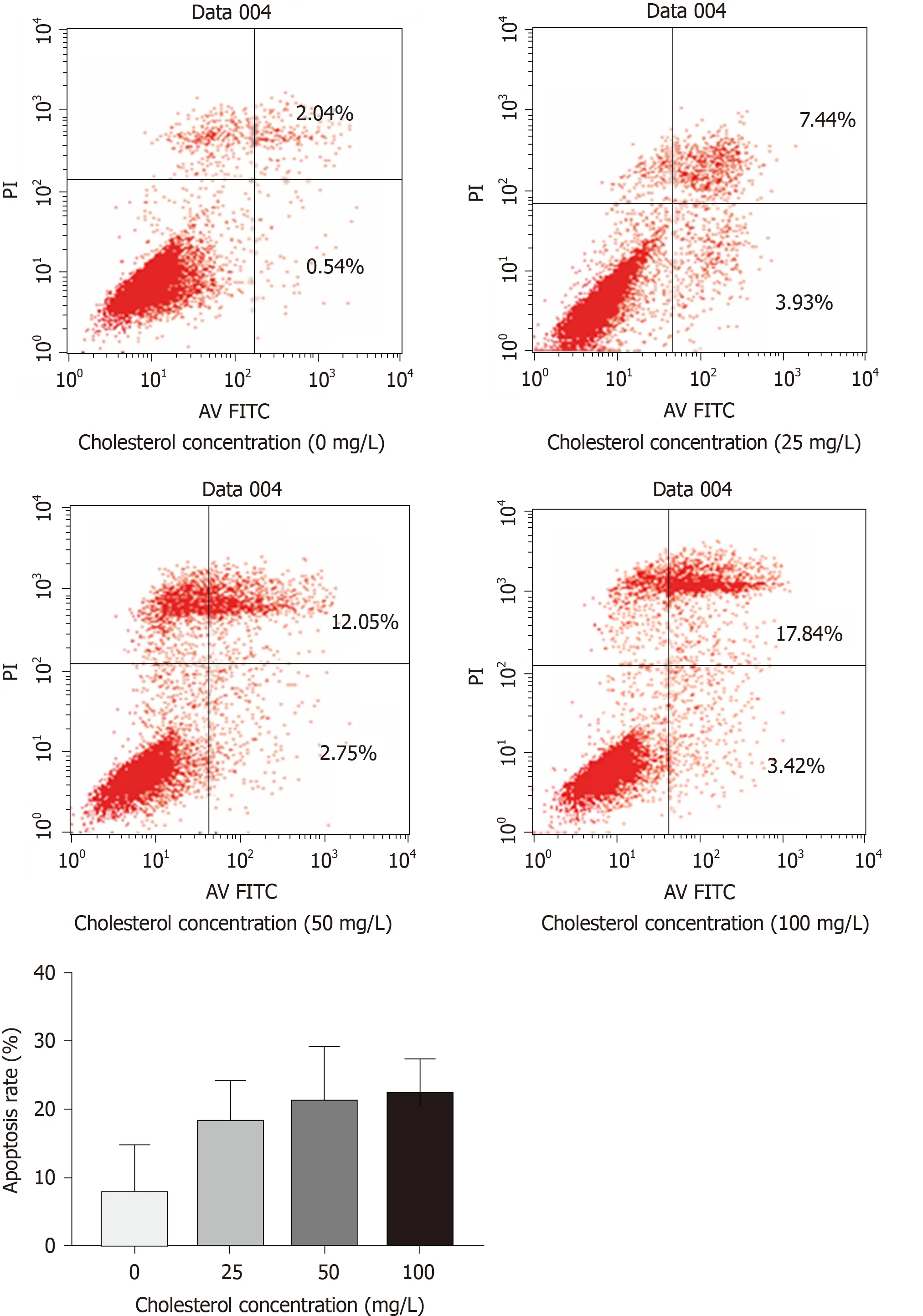
Figure5 Effect of different concentrations of cholesterol on the rate of apoptosis in interstitial Cajal-like cells.
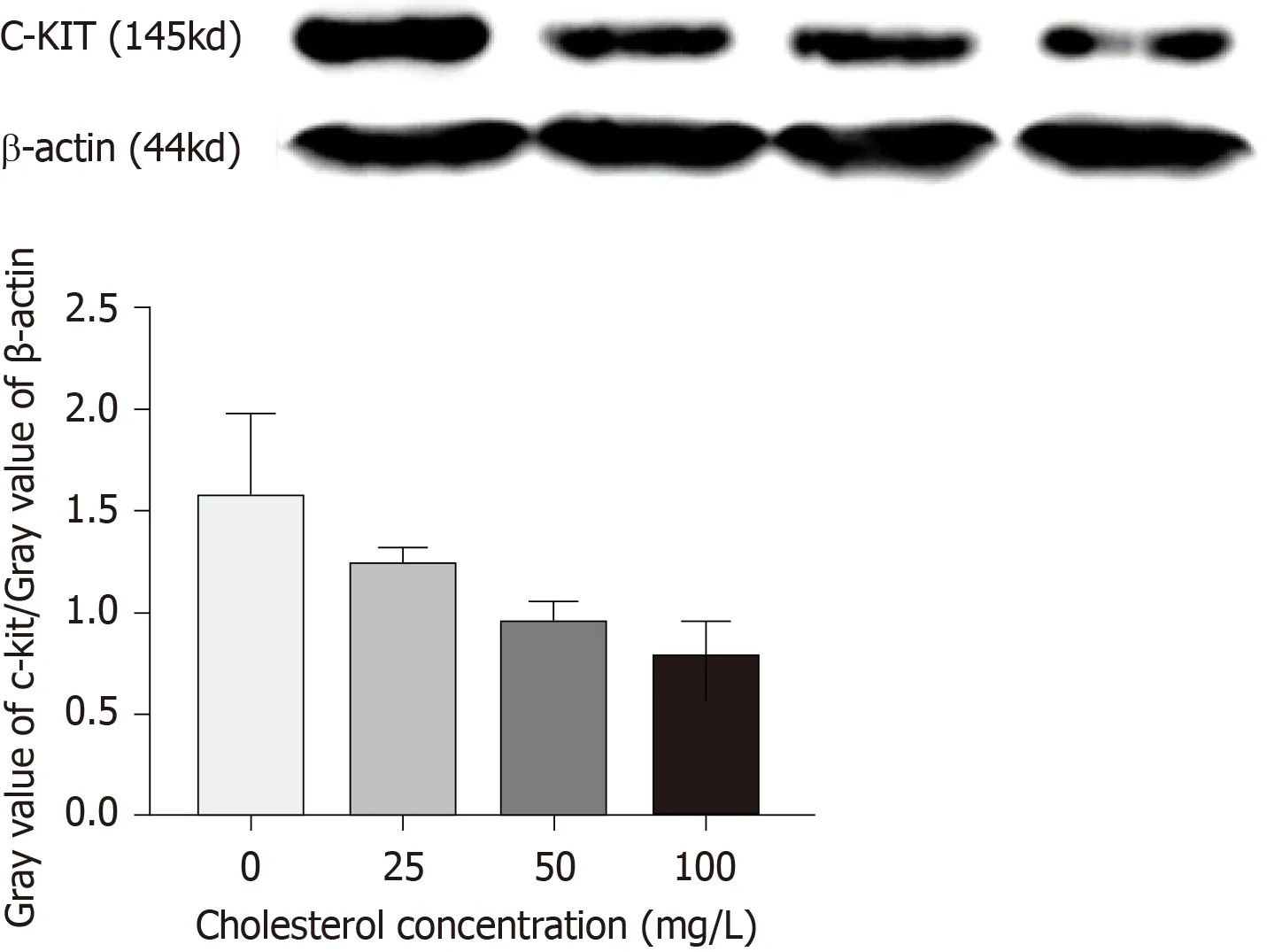
Figure6 Expression of c-Kit protein in interstitial Cajal-like cells of each group.
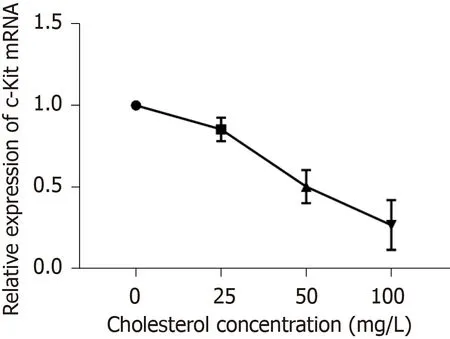
Figure7 Statistical analysis of effect of cholesterol on the expression of c-Kit mRNA in interstitial Cajal-like cells.
ARTICLE HIGHLIGHTS
Research background
Loss and/or dysfunction of interstitial Cajal-like cells (ICLCs) in the gallbladder may promote cholesterol gallstone formation by decreasing gallbladder motility.
Research motivation
Absorption of supersaturated cholesterol in bile by the gallbladder wall might be the cause of loss and/or dysfunction of gallbladder ICLCs.
Research objectives
The present study aimed to evaluate the effect of cholesterol on the proliferation and apoptosis of ICLCs from guinea pig gallbladders.
Research methods
ICLCs from guinea pig gallbladders were isolated and culturedin vitro.The cells were exposed to cholesterol solutions at different concentrations.Cell proliferation,apoptosis rate,and expression of c-Kit protein and mRNA were detected.
Research results
Compared with the control group (no treatment group),the addition of cholesterol significantly decreased the proliferation rates and the expression of c-Kit protein and mRNA in ICLCs.While,the apoptosis rates of cells were increased.
Research conclusions
High cholesterol concentrations can inhibit the proliferation of ICLCs and promote apoptosis,which might be caused by the inhibition of the stem cell factor/c-Kit signaling pathway.
Research perspectives
This is a proof-of-concept study that lacks a mechanical study.The mechanism by which cholesterol regulates the stem cell factor/c-Kit pathway remains unclear and need further investigation.
 World Journal of Gastrointestinal Surgery2020年5期
World Journal of Gastrointestinal Surgery2020年5期
- World Journal of Gastrointestinal Surgery的其它文章
- Management of synchronous lateral pelvic nodal metastasis in rectal cancer in the era of neoadjuvant chemoradiation:A systemic review
- Software improvement for evaluation of laryngopharyngeal pH testing (Restech) - a comparison between DataView 3 and 4
- When the bowel meets the bladder:Optimal management of colorectal pathology with urological involvement
- COVlD-19 outbreak and endoscopy:Considerations in patients encountered in a foregut surgery practice
- lntroduction of new techniques and technologies in surgery:Where is transanal total mesorectal excision today?
