Role for contrast-enhanced ultrasound in assessing complications after kidney transplant
Giuseppe Como, Jacopo Da Re, Gian Luigi Adani, Chiara Zuiani, Rossano Girometti
Abstract
Key words: Contrast-enhanced ultrasound; Kidney transplant; Post-renal transplant complications; Graft function; Ultrasound contrast agents
INTRODUCTION
Kidney transplantation (KT) is an effective treatment for end-stage renal disease(ESRD), having the potential of reducing mortality and improving the quality of life as compared to chronic dialysis. Despite the improvements in surgical techniques and immunosuppression regimens occurred over the last decades, post-transplant complications still represent a major clinical issue in KT recipients. Thus, early diagnosis of complications is of paramount importance in improving patients’outcomes.
Ultrasound (US) with color Doppler analysis has become the preferred imaging tool to evaluate the graft status within the first 24 h after KT. Together with serial biopsies of the allograft parenchyma, this technique plays also a pivotal role in post-KT surveillance, aiming to diagnose post-surgical complications, acute rejection, or chronic allograft nephropathy. On the other hand, color Doppler US is characterized by a low specificity, with Doppler-derived measures, such as the resistance index (RI),not directly reflecting the status of microcirculation. Computed tomography (CT)and magnetic resonance imaging (MRI) represent second-line imaging modalities used for assessing inconclusive US findings, providing a panoramic representation of vascular complications, and characterizing perirenal collections or renal masses.However, those modalities are rarely used because of the need to administer contrast medium, which is nephrotoxic in the case of CT, and at risk of raising gadoliniumrelated safety issues when MRI is planned in patients with impaired renal function.
Contrast-enhanced ultrasound (CEUS) is gaining ever-increasing acceptance as a complement to color Doppler US in the KT setting. In subspecialized centers, this technique is used to assess the graft status after KT due to its portability, safety profile of US contrast agents (UCAs), rapidity of execution, and diagnostic accuracy. Indeed,CEUS allows the evaluation of both macro- and micro-circulation, as well as perirenal collections or parenchymal abnormalities such as those related to rejection, acute tubular necrosis (ATN), impaired perfusion, and focal lesions. CEUS candidates as the ideal first-line examination to screen for post-KT complications and to refer positive patients to prompt intervention.
In this review, we present CEUS technique in brief, KT-related advantages and disadvantages of this tool, and its potential applications in KT recipients in the postoperative period.
CEUS TECHNIQUE
The current standard for UCAs is represented by second-generation compounds,consisting of a phospholipidic or albumin shell containing microbubbles of an inert lipophilic gas, such as sulfur hexafluoride [SonoVue/Lumason(Bracco, Milan,Italy)], perfluorocarbons [, Definity/Luminity(Lantheus Medical Imaging, North Billerica, MA, United States) or Optison(GE Healthcare, Oslo, Norway)]. UCAs used in the KT setting are administered intravenously and show blood-pool pharmacokinetics,, they last in the intravascular compartment for some minutes before dissolving (the gas is excreted through the lungs, while the bio-compatible shell is metabolized by the liver).
After preliminary B-mode and color Doppler evaluation, CEUS is performed with a bolus injection of contrast through a ≥ 20 Gauge catheter inserted in the antecubital vein of the arm. On average, we used a per-injection dose of 1.8 mL of Sonovuefor KT-related applications, followed by a 5-10 mL saline flush. CEUS requires a specific contrast imaging mode to avoid microbubbles being destroyed by excessive acoustic power,, by setting a low mechanical index.
The following post-contrast phases can be observed: (1) Corticomedullary phase,characterized by an increased contrast between enhancing cortex and still hypoperfused medulla, lasting between 15 and 30 s after injection; and (2)Nephrographic phase, showing an homogeneous enhancement across the cortex and medulla, lasting between 30 and 70 s after injection. Imaging performed after > 70 s from contrast injection is referred to as delayed phase. Of note, there is no urographic phase, since UCAs are not excreted by the kidney.
ADVANTAGES AND DISADVANTAGES OF CEUS IN THE KT SCENARIO
If planning to use CEUS, one should consider the related advantages and disadvantages in order to forecast the expected results in the KT scenario.
Advantages
CEUS is portable near the patient’s bedside, thus avoiding transporting critically ill patients from the intensive care unit to the radiology department. The examination of the transplanted kidney in the iliac fossa can be performed rapidly and without the need for respiratory collaboration. This is an advantage compared to more timeconsuming imaging procedures, especially MRI, and can translate into a decreased rate of low image quality examinations.
Contrary to contrast-enhanced CT or MRI, in which the acquisition is performed at definite time-points, CEUS provides a real-time representation of contrast distribution,with the possibility of recording the whole process under the form of dynamic cineloop. Real-time acquisition allows a correct representation of the corticomedullary phase in all examinations, independently from the patient’s hemodynamic status,without the need for bolus tracking. Continuous data acquisition can be also translated into time-intensity curves (TIC) plotting contrast enhancement intensity versus time,which in turn are the base for extracting quantitative indexes of perfusion with the proper software.
UCAs have a favorable safety profile, as demonstrated by a 0.009% rate of major adverse reactions, a rate lower than that reported for CT and comparable to that of MRI contrast agents. Compared to CT, CEUS determines no radiation exposure,especially in case of repeated examinations, and avoids potential nephrotoxic effects of iodinate contrast agents. This is of special importance during the postoperative recovery of renal function, as well as in the follow-up of patients with chronic graft dysfunction. Compared to gadolinium-based contrast agents, UCAs can be administered without the risk of retention or accumulation in the central nervous system or other tissues, as potentially relevant for patients receiving multiple examinations over time. UCAs can be also administered in the case of a glomerular filtration rate < 30 mL/min, which currently represents the threshold for caution in administering MRI contrast agents, given the risk of systemic nephrogenic fibrosis.A robust safety profile translates into the possibility of performing multiple contrast injections within the same examination session. This allows for a better characterization of abnormal findings and/or the evaluation of more than one finding at a time. Differently from the liver or spleen, in which there is late contrast accumulation of UCAs in the sinusoids, in the kidney contrast enhancement decreases with decreasing microbubble blood concentrations. This is of help when performing multiple injections while avoiding interpretative pitfalls related to late UCAs accumulation.
In other clinical scenarios (, assessment of focal liver lesions), CEUS has been advocated as a tool to improve the speed and cost-effectiveness of the diagnostic process. One might expect a similar role for CEUS in other abdominopelvic indications, including the KT-related ones.
Disadvantages
Similar to grayscale US, the image quality of CEUS can be impaired by patient-related factors, such as presence of bowel gas, large body habitus, or limited cooperation and physical obstacles in the early postoperative period (wounds or surgical bandage). It should be pointed out that those factors are of less impact on image quality than in other body districts, since the renal graft is usually placed extraperitoneally into the right or left iliac fossa,, in a superficial and fixed position making the graft easily accessible by US waves.
CEUS requires skilled operators, with adequate knowledge of the examination technique, UCAs characteristics, post-surgical anatomy, and patterns of presentation of KT-related complications. Previous experience in US and color Doppler examinations are a pre-requisite to achieve those goals and perform proper training with CEUS.
CEUS is less panoramic than CT and MRI. While this is not a limitationwhen examining one single renal graft, it can make the evaluation of patients with multiple organ transplantations more difficult (, dual kidney transplant or combined liver and kidney transplant). In those cases, multiple contrast injections are required, thus increasing the examination time because of the delay needed to wait for microbubbles to disappear between the injections.
Lastly, there are some CEUS-specific artifacts that can affect image quality and interpretation. Operators should be aware of potential interpretative pitfalls and the technical adjustments needed to minimize their occurrence.
POST-TRANSPLANT ANATOMY
Knowledge of post-transplant anatomy is of paramount importance to interpret CEUS findings (Figure 1). Cadaveric renal graft is usually placed in the extraperitoneal space in the right iliac fossa. The left iliac fossa can be chosen in case of previous surgery on the right side or an atheromatous burden of left arterial axis carrying the risk of suboptimal graft vascularization. The left-sided kidney is the preferred one for living-donor KT because of the longer renal vein, which reduces the risk of postoperative thrombosis. When a dual transplant is planned, both kidneys are usually placed in the same renal fossa.
Arterial anatomy varies depending on the transplant type. In cadaveric KT, the transplanted renal artery (TRA) is linked to a an oval portion of the donor’s aorta surrounding the renal ostium (aortic patch), which is in turn anastomosed with the recipient external iliac artery in an end-to-side fashion(Figure 1). This aortic cuff increases the surface of vascular anastomosis, thus reducing the risk for strictures or thrombosis. In living-donor KT, there is a direct end-to-side anastomosis between the TRA and external iliac artery. The iliac internal artery can be the site of anastomosis if the external iliac artery is athermanous or has been used for a previous transplant. In case of accessory renal arteries, a variety of surgical techniques can be used, such as performing multiple individual anastomoses or harvesting a larger aortic patch including the origin of any accessory artery.
The transplanted renal vein (TRV) is normally anastomosed with the external iliac vein in an end-to-side approach. Urinary drainage is obtained by performing ureteroneocystostomy, usually with an anti-reflux technique (, by tunneling the ureter into the bladder dome). For a variable period of time (, one month in our center), a temporary stent is left in place to prevent ureteral strictures or urinary leakage.
CLINICAL APPLICATIONS
The European Federation of Societies for Ultrasound in Medicine and Biology(EFSUMB) recommends using CEUS to rule out vascular complications, inflammatory complications, and different post-KT parenchymal abnormalities, including renal lesions. CEUS is recommended as an extension of preliminary grayscale US and color Doppler to be interpreted in light of the overall clinical picture and laboratory tests.
Vascular complications
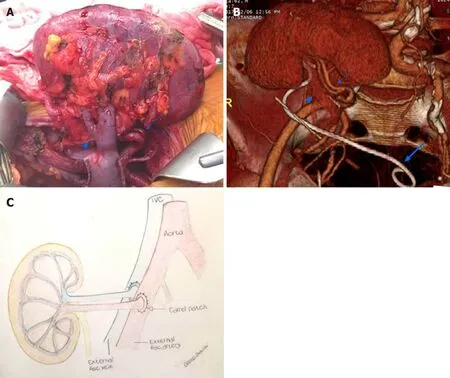
Figure 1 Renal allograft anatomy. A: In the graft photo there is clear visualization of the transplanted renal vein (arrowhead) and transplanted renal artery(arrow); B: The corresponding volume rendering reconstruction from a computed tomography post-transplant scan shows the graft position in the right iliac fossa,transplanted renal vein (arrowhead), transplanted renal artery (arrow), and an ureteral stent temporarily left in place to favor urine output (large arrow); C: The drawing illustrates the end-to-end arterial anastomosis performed with a carrel patch (donor’s aortic patch attached to the transplanted renal artery) (arrow).
TRA stenosis (TRAS) is the most common vascular complication post-KT, with an incidence up to 23%. Depending on the cause, TRAS can occur at any time, although it is more frequent between 3 mo and 2 years after transplantation. Early TRAS is usually located at the site of end-to-side anastomosis and can be related to intraoperative complications such as trauma to the donor or recipient vessels during clamping or suturing. Late TRAS occurs in patients with atherosclerotic disease in the recipient or immune-mediated endothelial damage.The clinical manifestation is most frequently severe hypertension with or without graft dysfunction, which can progress to graft loss if radiologic or surgical intervention is not performed.
Color Doppler US can show increased TRA velocity, distal spectral broadening, and increased arterial acceleration time of intra-parenchymal arterial vessels. The cutoff for pathologic TRA velocity varies between 200 and 300 cm/sec according to different Authors. The lower limit suffers from low specificity, and in turn can cause an excessive number of unnecessary procedures. Controversy exists also with regard to the best RI cut-off. CT angiography can be used to define the site and severity of the stenosis,as a road-map for subsequent interventional digital subtraction angiography (DSA). MRI angiography is a valid alternative for diagnosing TRAS,although this technique is less readily available and accessible, and tends to overestimate the degree of strictures. Given the high negative predictive value,CEUS can be used as a complement to color Doppler to rapidly rule out TRAS within a single examination session, thus also sparing unnecessary CT angiography or DSA.
After contrast injection, normal TRA can be identified as a tubular enhancing structure running from the external iliac artery to the hilum of the renal allograft.Artery length and tortuosity are quite variable, depending on the surgical technique and length of the vascular pedicle in the transplanted kidney. According to Pan, quantitative measurement of strictures using CEUS achieves a 87.5% sensitivity for TRAS, with a specificity significantly higher (95.7%) than that of Doppler-derived indexes, such as peak systolic velocity (PSV)-TRA (67.4%) and PSV-ratio. Diagnosis of TRAS can be made when luminal narrowing exceeds 50% as compared to normal poststenotic TRA (for strictures affecting the anastomotic site), or pre-stenotic TRA (for strictures affecting the main artery course) (Figure 2). Of note, about half of TRAS occurs at the anastomotic site.
TRA thrombosis (TRAT) is less common than TRAS, as it was reported to occur in 0.4% of patients. Factors related to TRAT development are hyperacute rejection, anastomotic occlusion, arterial kinking, and intimal flap. This condition may translate into diffuse or segmental parenchymal infarcts presenting with anuria, as well as swelling and tenderness over the graft.
The main color Doppler criterion for diagnosing TRAT is the absence of flow in TRA and intrarenal arteries. However, this finding lacks specificity and can be found also in acute rejection. CEUS can confirm arterial occlusion by showing the absence of contrast enhancement, and by reliably defining the site and extent of endoluminal defects. Additionally, CEUS can exploit hypoperfused or infarcted parenchymal areas (see below), which might be the only imaging findings when TRAT involves a polar artery(Figure 3). In the setting of TRAT, CEUS is expected to provide a better representation of the presence and extent of renal infarction than grayscale US and color Doppler US.
TRV thrombosis (TRVT) occurs in 0.2% of patients. Etiology is multifactorial and includes donor-age > 60 years, recipient’s related factors (age > 50 years, diabetic nephropathy, thrombophilia), intra- and postoperative hemodynamic instability, deceased donor, and cold ischemia time > 24 h. Usually, TRVT manifests in the first post-operative week with reduced urinary output as well as swelling and tenderness over the graft. This complication is a major cause of graft loss and should be treated promptly with thrombectomy.
On grayscale US, the early phase of disease is characterized by enlargement of the graft and hypoechoic appearance of the cortex. On Doppler examination, the thrombus can be difficult to visualize if venous anastomosis is located deeply in the pelvis. Indirect signs rising the suspicion of TRVT are reversed arterial diastolic flow,spike-like systolic component, and elevated resistive indexes. However, while those findings have good sensitivity, they lack specificity as they occur also in other complications, including allograft torsion, severe rejection, and ATN.
CEUS can depict non-enhancing vein defects and hypoperfused parenchymal areas,increasing their conspicuity compared to color Doppler analysis. During the earliest moments of the corticomedullary phase, one can appreciate pulsatile rather than continuous renal enhancement, possibly because of graft congestion and increased resistance to arterial flow. This finding is associated with patchy cortical enhancement.
Acute cortical necrosis is a rare complication resulting from technical surgical problems, transplantation across ABO-incompatible blood groups,positive cytotoxic antibody crossmatch, pre-existent perfusion lesions in the kidney donor, and prolonged cold ischemia time of the cadaveric allograft. This condition causes irreversible renal failure, thus requiring prompt diagnosis and transplantectomy.
Contrast-enhanced CT and MRI can demonstrate the pathognomonic peripheral rim sign, characterized by unenhanced cortical rim surrounding a normally enhancing medullary region. While US is insensitive to this finding, CEUS can easily depict it,thus achieving prompt diagnosis without the need of further imaging or even biopsy. After administering UCAs, the peripheral rim sign manifests with prompt filling of the main arteries followed by enhancement of medullary pyramids and absent cortical enhancement. The renal cortex appears as a hypovascular hypoechoic band persisting from the corticomedullary phase up to about 5 min after contrast injection.
Arteriovenous fistula (AVF) is an iatrogenic complication related to biopsy, typically caused by biopsies performed before transplantation,intraoperatively, or during postoperative KT surveillance. AVF usually resolves spontaneously within 1-2 years, while a minority of cases persist and present with hematuria, renal failure, and hypertension. According to a survey by Furness,spontaneous major bleeding is rare, although it can represent a life-threating condition requiring prompt intervention.
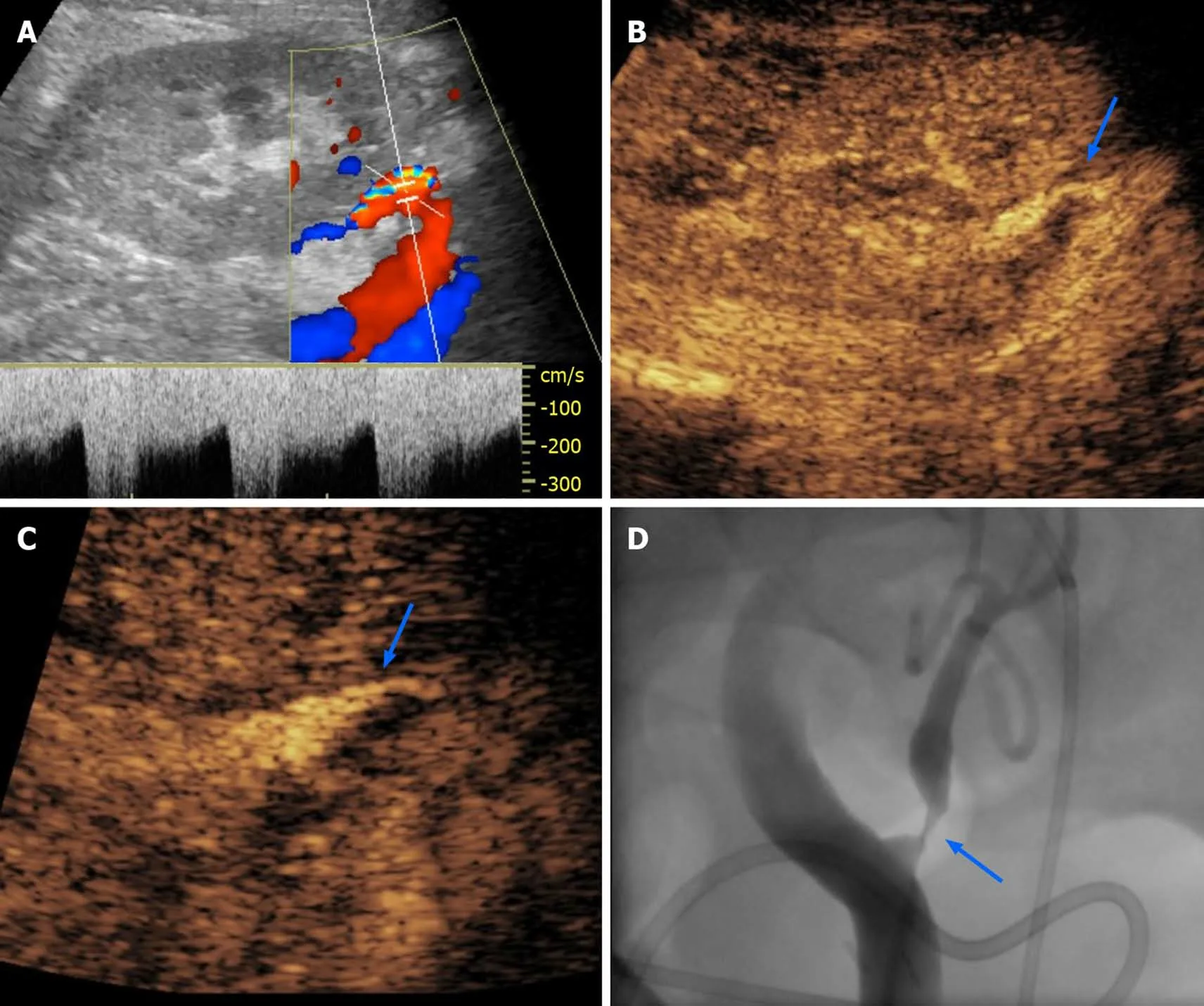
Figure 2 Transplant renal artery stenosis in a 30-year-old man with graft dysfunction, 1 year after cadaveric kidney transplantation. A:Color Doppler analysis of transplant renal artery (TRA) showed aliasing artifact and increased systolic pick velocity (300 cm/s); B-D: After the administration of an ultrasound contrast agent, there was better evidence of focal thinning of the TRA lumen (arrow in B), as evident on a magnified image (arrow in C) and subsequent digital subtraction angiography (arrow in D) performed for interventional purposes.
On grayscale US and color Doppler examination, AVF appears as a hypoechoic area showing vascular signal, typically under the form of a vortex-like image representing turbulent flow. This area extends outside the distribution of normal vascular structures and is entered by a feeding artery with high velocity and low resistance flow. The efferent vein showing arterialized waveform can also be identified. Once sonographic diagnosis is made, contrast-enhanced CT or MRI can better delineate the AVF anatomy.
On CEUS, AVF appears as an intraparenchymal pseudo-nodule with intense contrast-enhancement of vascular type, associated to early enhancement of the efferent vein. UCAs administration can also demonstrate the hemodynamic steel effect of AVF on the surrounding parenchyma, appearing as a hypoperfused area close to the AVF site. In this setting, CEUS is of help in referring patients to AVF embolization or in monitoring the effect of the procedure over time.
Urological complications
Perirenal collections are common after KT, occurring in up to 50% of patients. Urinomas and hematomas tend to present early after surgery, while lymphocele typically develops 4-8 wk after. Collections are usually asymptomatic,although large ones can displace the graft or cause vascular and/or ureteral compression. Consequently, symptomatic collections should be drained.
US shows limited sensitivity in detecting clinically significant collections, tending to underestimate their size. Moreover, while US can detect perirenal collections, it has a limited capability to distinguish among different origins and types. CT or MRI usually provide a more panoramic representation of symptomatic collections, and can better characterize the content, especially in the case of MRI.
Administering UCAs increases the conspicuity of vessels, graft parenchyma,perirenal tissues and non-enhancing collections. According to Grzelak, the use of CEUS allowed to detect 17.6% more cases of perirenal hematomas than with US alone, and allowed to visualize collection with a thickness lower than 10 mm, values that were undetectable by grayscale US. Increased conspicuity of collections allows better evaluation of their size and relationship with the major vascular structure and the ureter (Figure 4), thus potentially helping in planning an intervention without the need for further imaging.
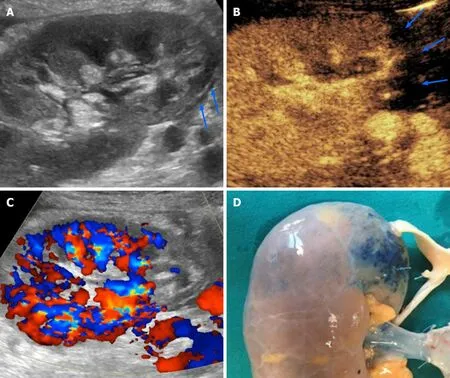
Figure 3 Renal infarction occurring 4 d after cadaveric kidney transplantation in a 53-year-old patient with early renal graft dysfunction. A and B: Grayscale ultrasound found an area of patchy echotexture (arrows in A) showing absent perfusion on contrast-enhanced ultrasound pulse inversion mode(arrows in B); C: Of note, contrast administration enhanced color signal, thus making this area clearly visible even with this technique; D: Infarction reasonably occurred in the blue-marked area in the pre-transplant image of the graft. This area was served by a small polar artery for which anastomosis was impossible.
Ureteral obstruction occurs in about 4.1% of renal transplanted patients, mainly within the first 6 mo (mean time of occurrence is 5.4 mo). The obstruction is generally due to ureteral kinking or scarring-related stenosis secondary to ischemia or rejection. Less commonly, obstructions arise from stones or extrinsic compression from perirenal collections. Clinical manifestation is unspecific,including increase in serum creatinine and reduction in urine output. Grayscale US allows to confirm hydronephrosis and search for intrinsic or extrinsic causes of obstruction along the urinary tract and bladder.
CEUS nephrostogram consists in the injection of UCAs through a percutaneous nephrostomy catheter followed by CEUS examination. This technique has been advocated as an alternative to fluoroscopic nephrostogram for a radiation-free evaluation of catheter and/or ureteral patency, showing comparable or even better performance in assessing malignant or benign ureteral obstruction. For CEUS nephrostogram, we used 0.5-1.0 mL of SonoVue diluted in 10 mL of saline solution,with slow manual injection under real-time imaging. In our experience, this technique leads to a panoramic representation of the excretory system and documentation of contrast passage into the bladder (Figure 5). Alternatively, other Authors proposed the direct injection of UCAs in the renal collecting system as a mean to enable nephrostomy placement at patient’s bedside.
Parenchymal complications
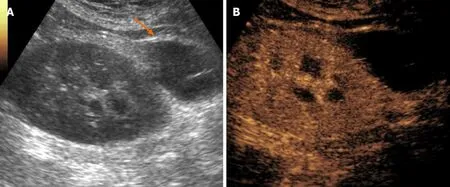
Figure 4 Postoperative lymphocele in a 62-year-old patient who underwent cadaveric kidney transplantation for end-stage renal disease.A: On B-mode ultrasound, lymphocele was visible as an anechoic collection in the perirenal space (arrow), close to the external iliac vessels of the donor; B: Contrast injection reinforced diagnosis by showing absent vascularization and improved conspicuity with better delineation of the collection size and anatomical relationships.
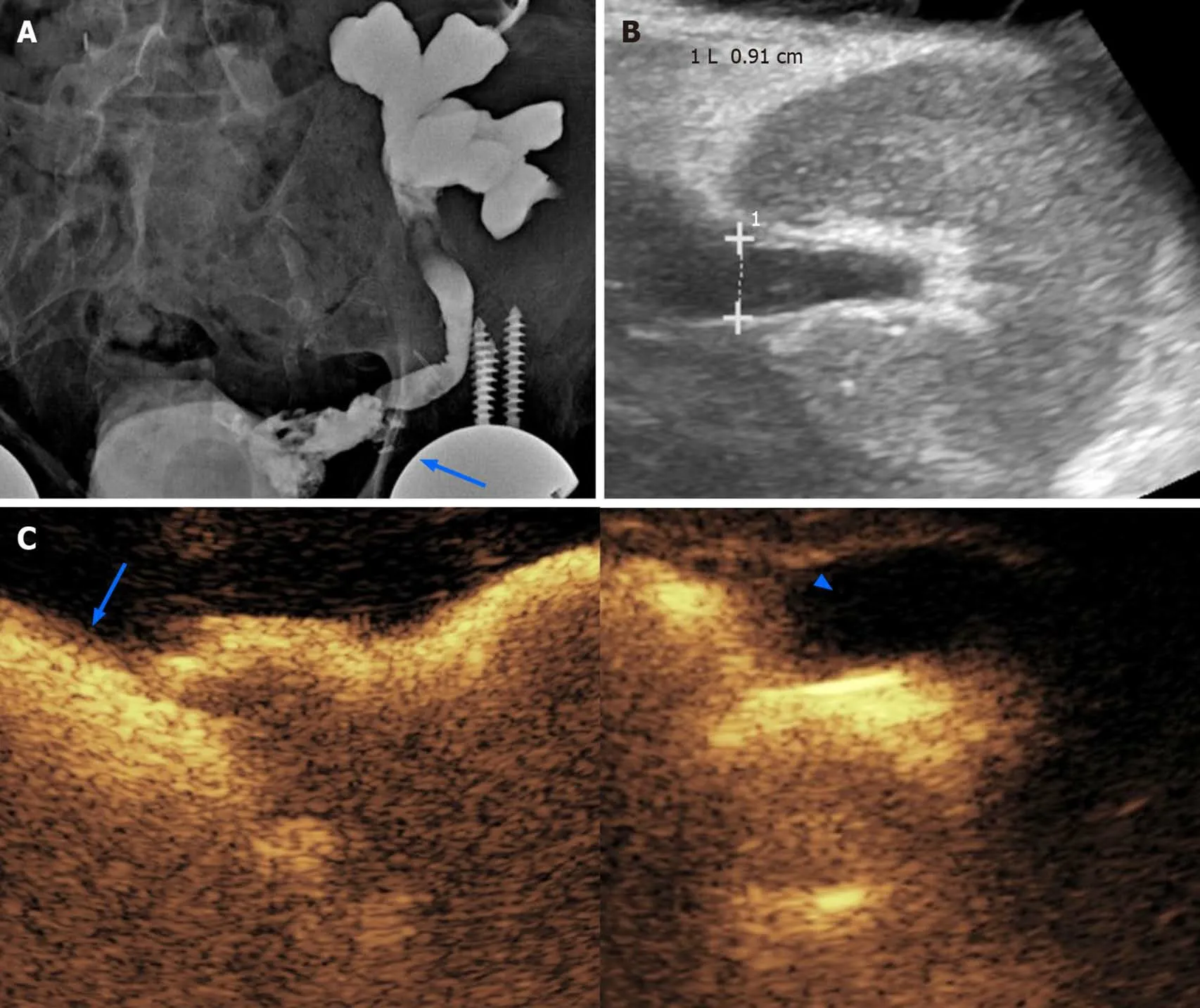
Figure 5 Obstructive renal failure occurring 3 mo after the transplantation in a 62-year-old male patient. A: A preliminary X-ray nephrostogram showed hydronephrosis with a suspicious urinary leakage at the vesico-ureteral anastomosis (arrow); B: After a conservative management of a few days, the patient was revaluated with grayscale ultrasound, showing reduced hydronephrosis; C: Subsequent contrast-enhanced ultrasound (CEUS)-nephrostogram allowed a panoramic representation of the non-dilated excretory system from the graft (arrowhead) to the bladder (arrow). Based on the resolution of dilation found on CEUS,nephrostomy was successfully removed.
Despite its decreased frequency in recent years, rejection remains one of the main complications of KT, with an incidence of 9%. This condition is currently ascribed to two different mechanisms,, T-cell-mediated rejection, and antibodymediated rejection. Based on temporal onset from KT, rejection can be classified into hyperacute (starting immediately after transplantation), acute (occurring 5-7 d after transplantation), chronic (occurring 3 mo after KT), and acute superimposed to chronic. Since clinical presentation is not specific, diagnosis is obtained with a biopsy. Thus, imaging is used to exclude alternative or coexisting complications.
On US, rejection presents with loss of corticomedullary differentiation and edematous cortical thickening. Doppler US can detect increased RI, although this finding is not specific. The main capability of CEUS in this setting is that of adding TIC-derived perfusion analysis under the form of various quantitative indexes, a feature that showed promising potential for diagnosing rejection. Benozzifound an increased time-to-peak in patients with acute rejection compared to a control group. In the same series, patients with ATN showed a different pattern of abnormalities (lower cortico-medullary ratio of mean transit time and regional blood volume), suggesting that perfusion analysis might be of help in differentiating rejection from other causes of early graft dysfunction. Although these results are promising, there are still no definite rejection-related patterns of perfusion abnormalities. On the other hand, abnormal quantitative indexes in a proper clinical context may reasonably rise the suspicion of rejection (Figure 6).
The reported cumulative incidence for acute pyelonephritis (APN) in renal transplant recipients ranges between 19% and 23%.The likelihood of developing APN is increased by episodes of asymptomatic bacteriuria, cytomegalovirus infection, immunosuppression therapy, acute rejection,urological malformations of the native kidney, and placement of a ureteric stent.APN may be asymptomatic or manifesting with bacteriuria, fever, graft pain and/or graft function impairment.
The role for grayscale US in APN is to exclude urinary obstruction and renal calculi as potential causes of infection. US can also demonstrate renal enlargement, loss of corticomedullary differentiation, and reduced visibility of renal sinus fat due to edema. CT is the preferred imaging modality for diagnosis, with higher specificity and sensitivity than US, although at the price of exposing patients to nephrotoxic contrast medium.
CEUS was shown to be as accurate as CT and MRI for assessing parenchymal changes in APN, with a 95% sensitivity and 100% specificity. Typical APN findings are round or wedge-shaped hypovascular parenchymal areas in the cortical or corticomedullary region, with increased conspicuity during delayed phase(Figure 7). Focal APN can be distinguished from abscess, since the latter presents as an anechoic area, within or outside an area of pyelonephritis, sometimes with peripheral rim enhancement or septal enhancement. CEUS is useful in monitoring the effects of therapy and regression of abscesses over time.
The overall incidence of malignancy in renal transplant recipients is about 3 to 5 times higher than in the general population. Most common malignancies are lymphoproliferative disorders and skin carcinomas, whereas tumors of the genitourinary and reproductive system prevail among visceral neoplasms. The occurrence ofrenal cell carcinoma in native kidneys and renal graft has a pooled estimated incidence of 0.7% and 0.2%, respectively. Tumors occurring in the graft are prevalently renal cell carcinomas.
US-based surveillance plays an important role in identifying tumors at an early stage. Grayscale US and color Doppler examination can rule in or out benign findings,such as simple cysts, and can refer suspicious or inconclusive findings to further imaging for characterization. In this setting, CEUS has gained a well-established role in non-transplanted patients (Figure 8), being able to differentiate solid lesions from pseudolesions, and attributing the proper Bosniak category to cystic lesions, as comprehensively described elsewhere. Findings in non-transplanted patients reasonably overlap with those of transplanted ones.
There is great interest in finding non-invasive parameters able to detect parenchymal graft dysfunction before morphological alterations become evident, or to predict renal function on a long-term basis. Some Authors advocated a role for quantitative CEUS in this scenario, based on the fact that perfusion indexes are influenced by glomerular filtration rate and urine output, which in turn are commonly used functional indexes. In a study by Schwenger, renal blood flow measured at 1 wk after transplantation was shown to correlate with renal function at 1 year.
A common cause of graft failure is related to the immunosuppressive therapy.Calcineurin-inhibitor nephrotoxicity is largely present at 10 years after transplantation,being characterized by arteriolar hyalinosis, ischemic glomerulosclerosis, and interstitial fibrosis. CEUS investigation of graft perfusion demonstrated a reduction of renal blood flow in patients receiving therapy with Cyclosporine-A early after KT,thus having the potential to represent altered micro-perfusion as a marker of graft damage.
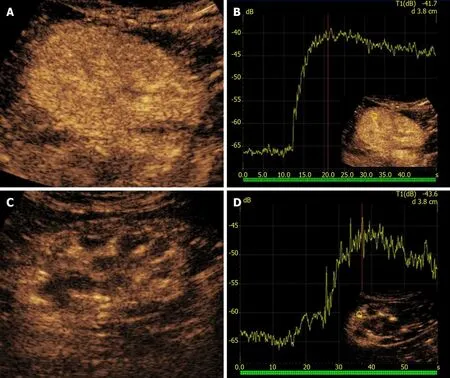
Figure 6 Rejection in a 58-year-old man who underwent two different renal transplants with a 4-year interval. A and B: After 1 mo from kidney transplantation, the left-sided retransplanted kidney showed homogeneous parenchymal enhancement on contrast-enhanced ultrasound (CEUS) examination (A),with rapid time-to-peak on the corresponding time-intensity curve (B); C: The contralateral first kidney in the right iliac fossa was affected by chronic rejection. D:CEUS showed weaker and inhomogeneous contrast enhancement as compared to the left-sided graft, with lower and delayed peak of contrast enhancement.
CONCLUSION
CEUS represents an ideal first-line tool in evaluating KT patients, improving the accuracy of conventional US for a variety of complications. Assumed that the examination is performed by experienced operators, CEUS is a fast and reliable alternative to CT and MRI, which might be contraindicated in patients with impaired renal function. While most established applications of CEUS rely on the capability of identifying major vascular and parenchymal complications, there is increasing research on quantitative perfusion indexes reflecting microcirculation abnormalities electively.
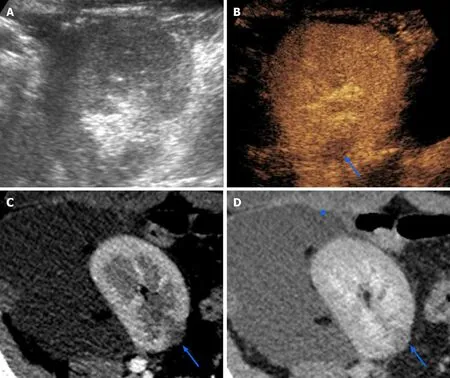
Figure 7 Focal pyelonephritis occurring 50 d after kidney transplantation in a 34-year-old female patient. While grayscale ultrasound found no abnormalities (A), contrast-enhanced ultrasound revealed a focal hypoperfused area (arrow in B) that was subsequently confirmed on both corticomedullary phase(arrow in C) and nephrographic phase (arrow in D) of a computed tomography performed to assess a large symptomatic perirenal collection (arrowhead in D).
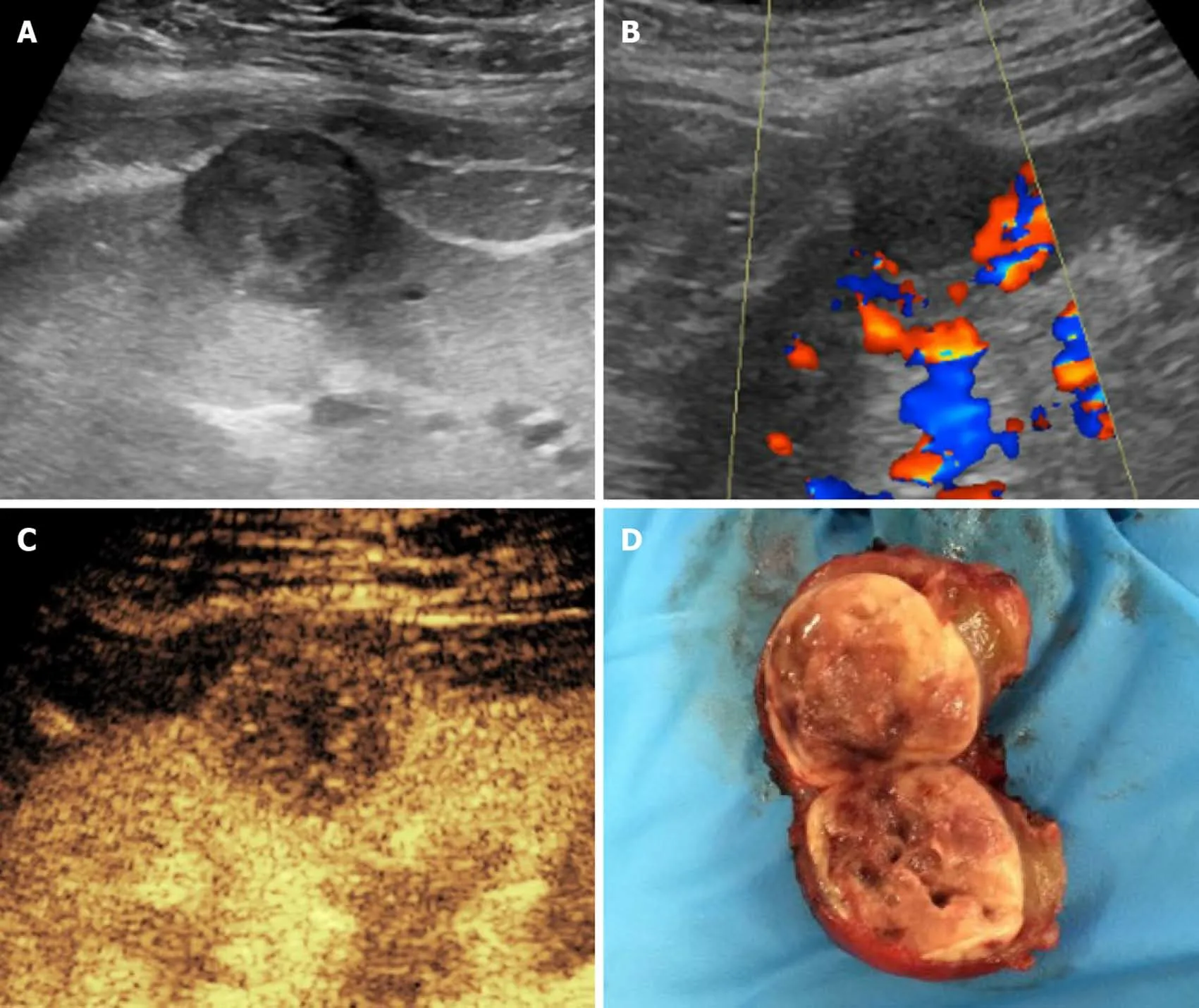
Figure 8 Histologically-proven papillary carcinoma in a 57-year-old male patient with previous kidney transplantation and impaired renal function. A and B: On grayscale ultrasound (A) the lesion appeared as a hypoechoic cortical mass with absent vascular signal at color analysis (B); C: Contrastenhanced ultrasound better assessed the solid nature of the mass by showing lower lesion vascularization as compared to normal graft parenchyma; D: The patient was referred to surgery without the need of performing computed tomography or magnetic resonance imaging.
The authors thank Viviana Moroso (MSc, PhD) of MV Medical Writing (Lule?,Sweden) for copyediting the manuscript, and Dr. Clara Zichichi (Institute of Radiology, University of Udine) for having drawn Figure 1C.

