Decomposition of a gas mixture of four n-alkanes using a DBD reactor
Boqiong JIANG(江博瓊),Xiaodan FEI(費(fèi)小丹),Shuiliang YAO(姚水良),Qinmin WANG(王欽民),Xinlei YAO(姚馨蕾),Kai XU(徐鍇) and Zhizong CHEN(陳摯宗)
1 School of Environmental Science and Engineering,Zhejiang Gongshang University,Hangzhou 310018,People’s Republic of China
2 School of Environmental and Safety Engineering,Changzhou University,Changzhou 213164,People’s Republic of China
Abstract
Keywords:multiple components,n-alkane decomposition,dielectric barrier discharge,energy efficiency,activation energy
1.Introduction
Atmospheric environmental problems due to particulate matter(PM)represent a serious health risk[1].It has been found that PM1(particulate matter that can enter the lung),PM2.5(fine particulate matter),and PM10(inhalable particulate matter)have the greatest effect on children aged 0 to 3 years.Most commonly,when the PM concentration increases by 3.4 μg m?3,the odds ratio reaches 1.78,and the risk of autism increases by 78%[2].Volatile organic compounds(VOCs)not only give rise to an increase in PM2.5,but also cause environmental problems such as photochemical smog,acid rain,and ozone depletion[3-5],and diseases such as childhood leukemia,asthma,and cancer[6-8].The control of VOC emissions into the atmosphere has therefore justifiably become a key issue as a means of improving both the atmospheric environment and public health.
Alkanes are the largest category overall among VOCs.Cai et al monitored the components of VOCs in the center of Shanghai from 2007 to 2010.They found that atmospheric VOCs were dominated by alkanes(43%)and aromatic hydrocarbons(30%),followed by halogenated hydrocarbons(14%)and olefins(6%)[9].An et al also found that the total concentration of VOCs in the Nanjing industrial area was 43.5 ppbv,with alkanes,olefins,alkyne,and aromatics content accounting for 45.1%,25.3%,7.3%,and 22.3%,respectively[10].Recently,Li et al analyzed the atmospheric VOC species and meteorological conditions in Beijing from March 2016 to January 2017; it was found that the proportions of four VOC classes during the four seasons were similar,with alkanes being the most abundant species at 54.1%-64.7%,followed by aromatics at 18.5%-27.5%,alkenes at 11.0%-15.0%,and acetylene at 3.2%-5.2%[11].
Technologies for VOC control include absorption,condensation,membrane separation,catalytic combustion,nonthermal plasma(NTP)decomposition,and photocatalysis[12,13].Of these,NTP decomposition technology is primarily used for the end control of low-concentration VOC exhausts[14].Previous research on the subject of controlling VOCs using NTP technology have mainly focused on the decomposition of VOC gas containing a single VOC pollutant[15],such as benzene,toluene,xylene,and other aromatic compounds[16-24],formaldehyde[25],and hydrocarbon derivatives[26].A few studies have been conducted in relation to alkane decomposition using NTP decomposition technology.Jin et al developed a dielectric barrier discharge(DBD)reactor with filled with porous alumina balls to decompose n-hexane,finding that n-hexane could thereby be oxidized to CO and CO2with low nanoparticle emission[27].Jin also studied the decomposition of n-hexane,n-heptane,n-octane,n-nonane,and n-decane using a tubular DBD reactor,establishing a kinetic model for each alkane decomposition[28].Rousso et al studied the chemical kinetics of low temperature oxidation and pyrolysis of pentane in a nanosecond repetitively-pulsed DBD reactor,finding that direct electron impact dissociation pathways play a critical role in plasma assisted fuel pyrolysis and oxidation,and that plasma-generated radicals and excited species such as O and O(1D)enhance low temperature fuel oxidation[29].A short review has also been provided by Yao et al,in which the mechanism of alkane decomposition is summarized[30].As some VOC sources emit the mixture of various kinds of VOCs[31],it is necessary to explore the decomposition of a VOC mixture comprising several VOCs,rather than a single form of VOC.
In this study,the decomposition of a gas mixture of n-alkanes(n-heptane(C7H16),n-octane(C8H18),n-nonane(C9H20),and n-decane(C10H22))was carried out using a DBD reactor.The influences of reaction temperature and O2concentration were also investigated.
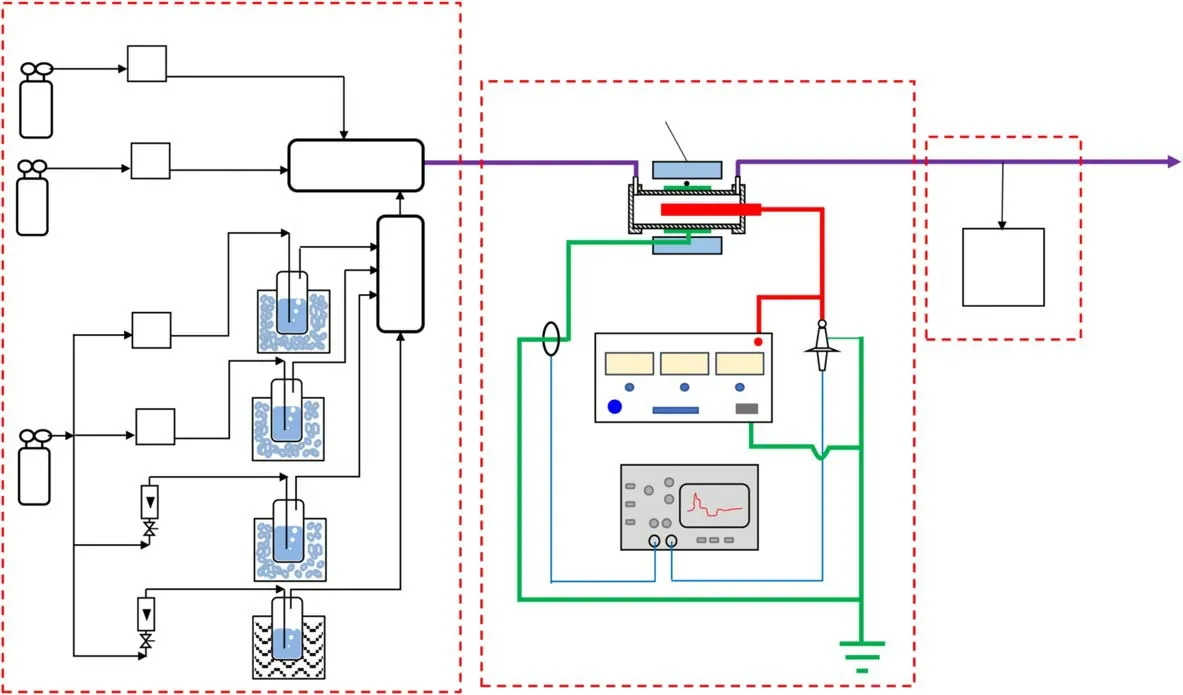
Figure 1.Schematic diagram of the experimental setup.
2.Experimental devices and methods
The experimental system is shown in figure 1.This includes a simulated gas supply system,a plasma reaction system,and an analysis system.Six gas flow meters(MFC1-4,D07-19B,Sevenstar,China; RFC1-2,LZB-3WB,Changzhou Xinwang,China)were used to generate a simulated air mixture(total flow rate 500 ml min?1)of n-heptane(MCF3,3.3 ml min?1),n-octane(MCF4,13.3 ml min?1),n-nonane(RFC1,53.8 ml min?1),and n-decane(RFC2,29.25 ml min?1)using four bubblers held either in an ice bath or in three water baths.The concentration of each alkane was 100 ppmv,and 410,467,525,and 582 mg m?3for n-heptane,n-octane,n-nonane,and n-decane,respectively;the concentration of each alkane C′in ppmv was calculated using equation(1):
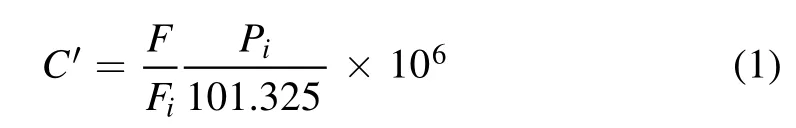
where F,Fi,and Pidenote the total gas flow rate in ml min?1,the flow rates(ml min?1)of each alkane,and the saturated pressures(kPa)of each alkane,respectively; 101.325 is the total gas pressure in kPa.Piwas calculated using the Antoine equation,with the Antoine coefficients being obtained from the handbook[32].The flow rates of O2(MCF1)and N2(MCF2)were 100 ml min?1,and 300.4 ml min?1,respectively.When performing O2concentration effect experiments,O2(MCF1)and N2(MCF2)flow rates were changed proportionally,but the total flow rate of O2(MCF1)and N2(MCF2)was controlled to be 400.4 ml min?1.
Figure 2 shows the geometric structure of the DBD reactor,comprising a stainless steel foil(50 mm),an aluminum rod electrode(260 mm in length,6 mm in diameter),a quartz tube(inner diameter of 10 mm,outer diameter of 12 mm,and length of 370 mm),and two stainless steel unions.There was a 2 mm discharge gap between the inner wall of the quartz tube and the aluminum rod.The aluminum rod and stainless steel foil were connected to the high-voltage and ground terminals of a pulse power supply.The pulse power supply(HV-10-08,Suzhou Allftek,China)was used to supply pulse voltage to the DBD reactor to generate barrier discharges within the discharge gap.The waveforms of the discharge voltage and current were measured using a voltage probe(P6015A,Tektronix,USA)and a current transformer(TCP0030,Tektronix,USA),and recorded with an oscilloscope(DPO 3032,Tektronix,USA).The gas from the outlet of the DBD reactor was analyzed online,using a gas chromatograph(GC1690,Hangzhou Kexiao,China)with an analysis error of about 10%.
The discharge power P in W from the pulse power supply to the DBD reactor was calculated using equation(2):
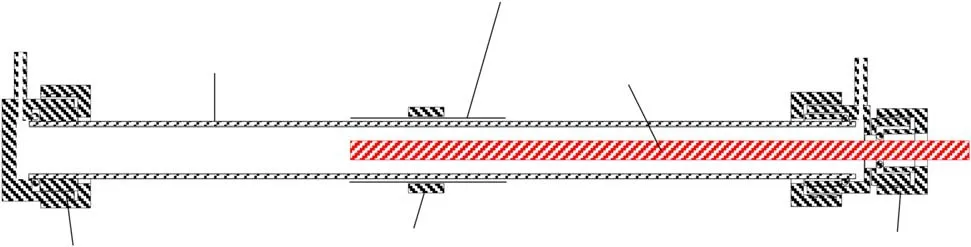
Figure 2.Geometric structure of the DBD reactor.
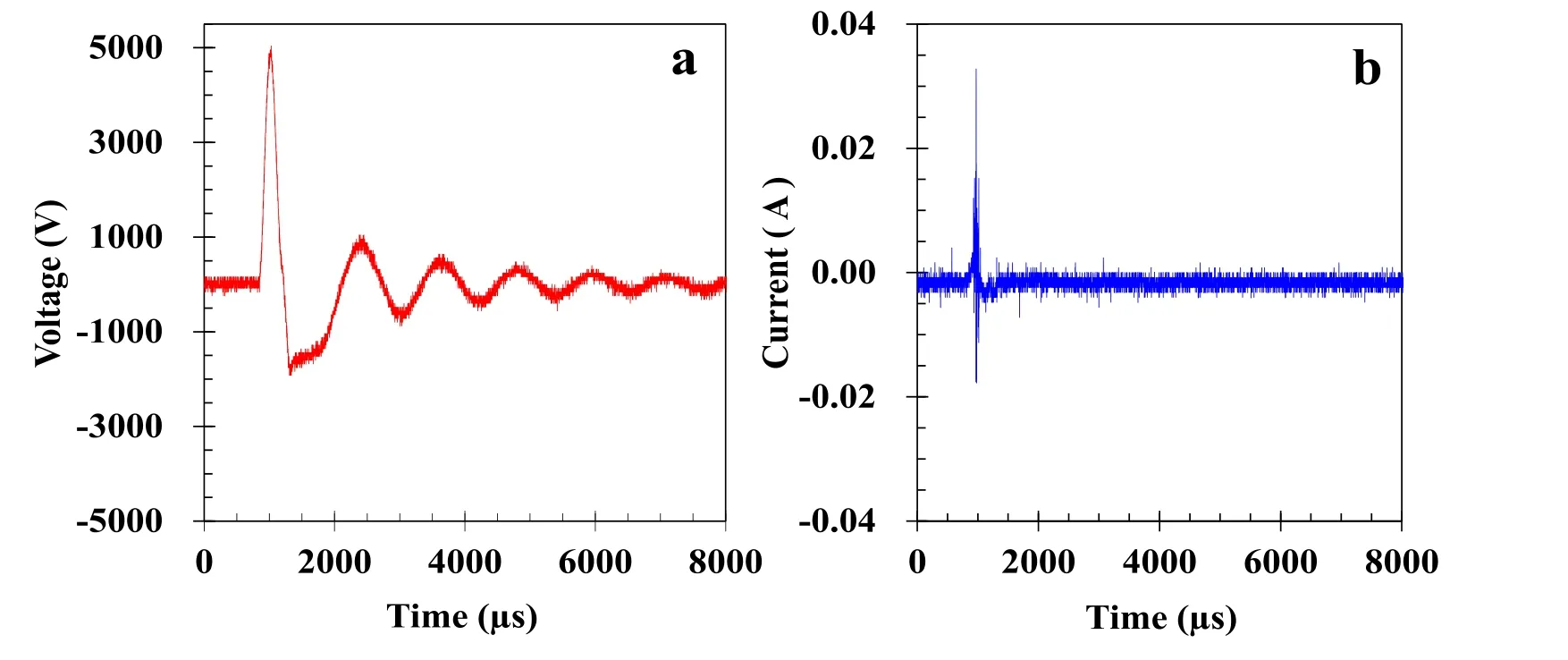
Figure 3.Typical waveforms of discharge(a)voltage and(b)current.Experimental condition:100 Hz,150 °C,and 20% O2.
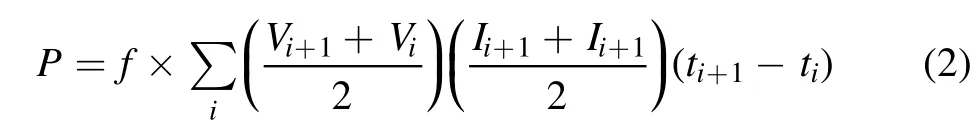
where Viand Ii,are,respectively,voltage in V,and current in A at discharge time tiin s,data having been collected from the waveforms of discharge voltage and current during a plus discharge duration.f denotes the pulse frequency in Hz.
The energy density Edin J l?1was calculated using equation(3),where F is the total gas flow rate in ml min?1:
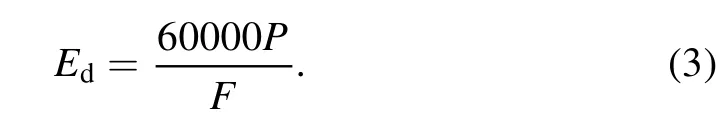
The alkane conversion X was defined as:

where C0and C in mg m?3denote the alkane concentrations in the gas mixtures from the outlet of the DBD reactor,without and with discharges,respectively.
The energy efficiency E(in mol kWh?1)was calculated as the moles of n-alkanes decomposed per unit of electrical energy as follows:

where M is molecular weight of each alkane.
Unless otherwise stated,the reaction temperature of the DBD reactor was controlled at 150 °C using an electric furnace(MXG1200-40s,Longkou Xianke,China).
3.Results and discussion
3.1.Typical discharge waveform
Positive and negative pulses are repeatedly provided by the pulse power supply to the DBD reactor.Figure 3 illustrates the typical waveforms of discharge voltage(a)and current(b).The peak voltage and rise time of the positive pulse are 5.0 kV and 1.7 μs,respectively.The maximum discharge current is 0.0328 A.The discharge power was calculated via equation(2)to be 1.4 W(at a pulse frequency of 100 Hz),and the energy density Edwas 168 J l?1.
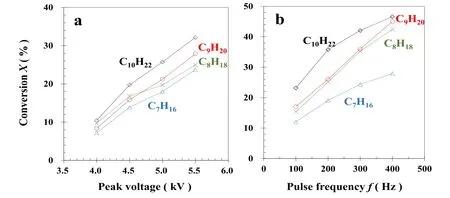
Figure 4.Alkane conversion as a function of(a)peak voltage and(b)pulse frequency.Experimental condition:(a)100 Hz,150°C,and 20%O2; and(b)5.0 kV,150 °C,and 20% O2.
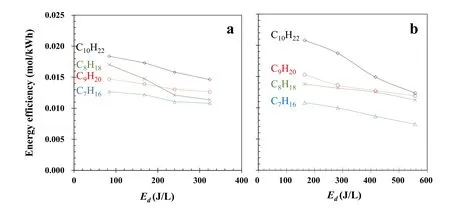
Figure 5.Energy efficiency as a function of energy density.Experimental condition:(a)peak voltage(4.0-5.5 kV),100 Hz,150 °C,and 20% O2;(b)pulse frequency(100-400 Hz),5.0 kV,150 °C,and 20% O2.
3.2.Alkane decomposition
3.2.1.Effect of discharge voltage and pulse frequency.Factors influencing alkane decomposition are pules voltage,pulse frequency,temperature,oxygen concentration,and gas flow rate.Firstly,we investigated the influence of peak voltage and pulse frequency.When the peak voltage increased from 4.0 kV to 5.5 kV,the conversion of each alkane increased from 7.2%(C7H16),9.7%(C8H18),8.4%(C9H20),and 10.5%(C10H22)to 23.8%(C7H16),25.0%(C8H18),27.9%(C9H20),and 32.1%(C10H22),in a conversion order of C10H22>C9H20>C8H18>C7H16(see figure 4(a)).Similar results were found when the pulse frequency changed(figure 4(b)).When the peak voltage increased from 4.0 kV to 5.5 kV at a fixed 100 Hz pulse frequency,Edgradually increased from 84 J l?1to 324 J l?1.When the pulse frequency increased from 100 Hz to 400 Hz,Edincreased from 164 J l?1to 557 J l?1.It is clear that these increases in conversion are due to the increase in energy injection to the DBD reactor,resulting in more reactive particles(such as energized electrons,O,O3,and ·OH)becoming available for alkane decomposition[33-35].
Figure 5 depicts energy efficiency as a function of energy density.When Edincreases,the energy efficiency of n-alkane decomposition decreases,which is similar to the results reported in the literature[36,37].This occurs primarily because when Edincreases,more discharge energy is converted into thermal energy,photons,and the production of by-products(such as O3),which cannot be used efficiently to decompose VOCs.At a voltage of 4.0 kV(Ed=84 J l?1),the energy efficiencies of n-heptane,n-octane,n-nonane,and n-decane have the maximum values of 0.013 mol kWh?1,0.017 mol kWh?1,0.015 mol kWh?1,and 0.018 mol kWh?1,respectively; the total energy efficiency for the four alkanes is 0.063 mol kWh?1.At a frequency of 100 Hz(Ed=164 J l?1),the maximum energy efficiencies of n-heptane,n-octane,n-nonane,and n-decane are 0.014 mol kWh?1,0.011 mol kWh?1,0.015 mol kWh?1,and 0.021 mol kWh?1,respectively; the total energy efficiency for the four alkanes is 0.061 mol kWh?1.This energy efficiency is close to the levels of around 0.05-0.1 mol kWh?1reported by Jin et al[27],implying that total energy efficiency is not obviously influenced by the number of alkanes.
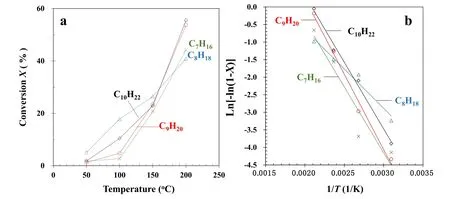
Figure 6.(a)Effect of temperature on the conversion of n-alkanes and(b)relation of ln[?ln(1?X)]and 1/T.Experimental condition:Ed 164 J l?1 and 20% O2.
3.2.2.Effect of reaction temperature.Decompositions of alkane,such as decane,initiate dehydrogenation via the interaction of decane and energized electrons,and reaction with O atoms or OH radicals[38].The relationship between alkane conversion and reaction temperature at 164 J l?1is illustrated in figure 6.Here,alkane conversion is shown to improve with an increase in temperature.This is due to the endothermic behaviour of the reaction with O atoms or OH radicals[39].The relation of alkane conversion and kinetic rate constant k under fixed experimental conditions,with the exception of reaction temperature,can be represented as[30]:
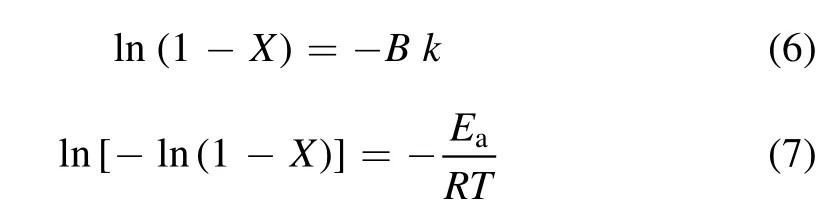
where B is a constant.
The activation energies Eafor the decomposition of each alkane can be calculated from the rate constants at various reaction temperatures using the Arrhenius equation(equation(7)).The activation energies are 31.3 kJ mol?1(R20.9118),18.6 kJ mol?1(R20.9689),35.7 kJ mol?1(R20.9895),and 31.6 kJ mol?1(R20.9918)for n-heptane,n-octane,n-nonane,and n-decane,respectively.Thermal decomposition of the alkanes is initialized via hydrogen abstraction by O2or OH[40].The activation energies for the hydrogen abstraction by O2are 196,196,196,and 197 kJ mol?1for n-heptane,n-octane,n-nonane,and n-decane,respectively[41].It is evident that the activation energies for alkane decomposition using the DBD reactor in this study are lower than those for alkane thermal decomposition.The decrease in activation energy is due to that the reactive species for alkane decomposition are produced by gas discharges,not the thermal heating.The activation energy for n-octane decomposition using the DBD reactor in particular is lower than for the other three alkanes; this is possibly due to the charge transfer from O2+to n-octane.A similar result was reported by Marotta and Paradisi,who found that,of all the hydrocarbons they analyzed,only in the case of n-octane was such a charge transfer not completely dissociative,and the molecular ion(M+)can be found in the atmospheric-pressure chemical ionization spectra[42].
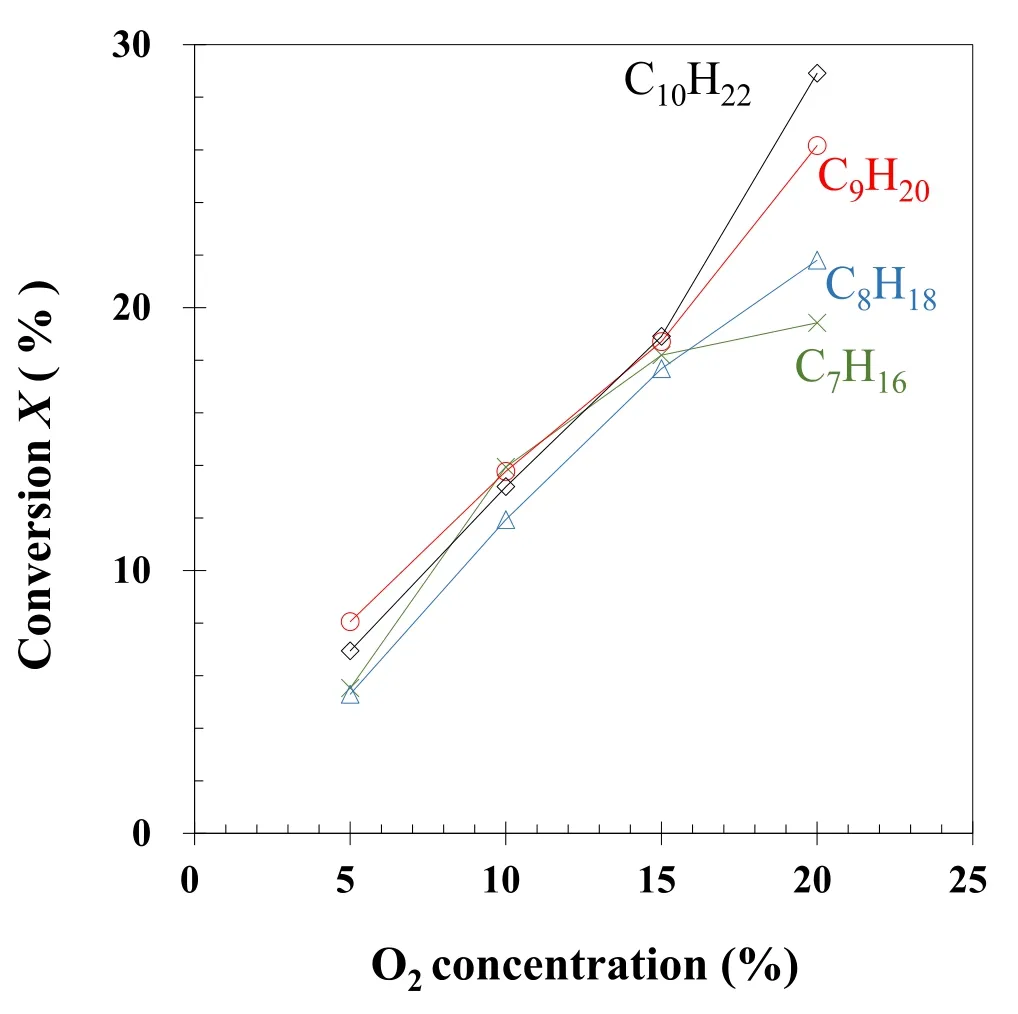
Figure 7.Effect of O2 concentration on the conversion of n-alkanes.
3.2.3.Effect of O2concentration.O2is the raw material of O atoms and O3,which facilitate alkane decomposition.We investigated the influence of O2concentration on alkane conversion at 164 J l?1(see figure 7).When O2concentration was gradually increased from 5%to 20%,the conversion of each alkane increased.The total conversion of n-heptane,n-octane,n-nonane,and n-decane increased from 25.8% to 96.3%,indicating that O2concentration is also an important factor in improving energy efficiency.The effect of O2concentration is due to the production of O and O3,which is proportional to the O2concentration at a fixed energy density[43].
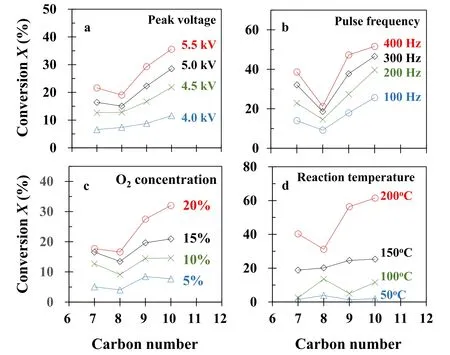
Figure 8.Relations of conversion and carbon number.Experimental conditions:(a)as per figure 4(a),(b)as per figure 4(b),(c)as per figure 6(a),(d)as per figure 7.
3.3.Relationship between conversion and carbon number
The length of an alkane molecule increases with an increase in carbon number,which further results in an increased likelihood of collisions between alkane molecules and energized electrons.The relationships between alkane conversion and carbon number under various peak voltages,pulse frequencies,reaction temperatures,and oxygen concentrations are illustrated in figure 8.
Generally speaking,all alkane conversion is proportional to carbon number,with the exception of n-octane when the reaction temperature was 50 °C.This finding suggested that the longer chain alkane of a larger carbon number decomposes more readily given its greater likelihood of collisions with energized electrons.N-octane generally exhibits a lower conversion rate than the other three alkanes;this may also be ascribed to charge transfer.Further study is required to explore the specificity of n-octane.
4.Conclusion
The decomposition of a gas mixture of n-heptane(C7H16),n-octane(C8H18),n-nonane(C9H20),and n-decane(C10H22)was carried out using a DBD reactor driven by a pulse power supply.The influence of peak voltage,pulse frequency,reaction temperature,and O2concentration was investigated.The main conclusions are as follows:
(1)Peak voltage,pulse frequency,reaction temperature,and oxygen concentration have influence on the energy efficiency of all four alkanes; in general,energy efficiency increased with an increased peak voltage,pulse frequency,reaction temperature,and oxygen concentration; the influence of pulse frequency and reaction temperature on energy efficiency is more apparent than the effects of peak voltage or oxygen concentration.
(2)The activation energies were found to be 31.3,18.6,35.7,and 31.6 kJ mol?1for the decomposition of n-heptane,n-octane,n-nonane,and n-decane,respectively.The activation energy of n-octane is markedly lower than that of the other three alkanes,possibly due to the difference in charge transfer from+O2to n-octane.Further study is required to explore the specificity of n-octane.
(3)In most cases,alkane conversion is proportional to carbon number,again with the exception of n-octane.
(4)Total energy efficiency is not obviously influenced by the number of alkanes.
Acknowledgments
This work was supported by the Zhejiang Basic Public Welfare Research Program(No.LGG19E080001)and Natural Science Foundation of Zhejiang Province(No.LY19B070002).
 Plasma Science and Technology2020年11期
Plasma Science and Technology2020年11期
- Plasma Science and Technology的其它文章
- The effect of resonant magnetic perturbation with different poloidal mode numbers on peeling-ballooning modes
- Soft landing of runaway currents by ohmic field in J-TEXT tokamak
- Numerical simulations of the wavefront distortion of inter-spacecraft laser beams caused by solar wind and magnetospheric plasmas
- Effect of insulating oil covering electrodes on the characteristics of a dielectric barrier discharge
- Numerical study on the modulation of THz wave propagation by collisional microplasma photonic crystal
- Study of partial discharge characteristics in HFO-1234ze(E)/N2 mixtures
