Management of nonalcoholic fatty liver disease in the Middle East
Faisal M Sanai, Faisal Abaalkhail, Fuad Hasan, Muhammad Hamed Farooqi, Nawal AI Nahdi, Zobair M Younossi
Abstract The prevalence of nonalcoholic fatty liver disease (NAFLD) in the Middle East is increasing in parallel to an increase in the prevalence of associated risk factors such as obesity, metabolic syndrome, and type 2 diabetes mellitus. About 20% to 30% of the patients progress to develop nonalcoholic steatohepatitis (NASH), a histological subtype of NAFLD, with features of hepatocyte injury such as hepatocyte ballooning. NASH can progress to fibrosis, cirrhosis, and even hepatocellular carcinoma. NAFLD thus causes a substantial burden on healthcare systems and it is imperative that appropriate strategies are discussed at a regional level to facilitate effective management tailored to the needs of the region. To fulfil this unmet need, expert gastroenterologists, hepatologists, and endocrinologists from the region came together in three advisory board meetings that were conducted in Saudi Arabia, United Arab Emirates, and Kuwait, to discuss current local challenges in NAFLD screening and diagnosis, and the different available management options. The experts discussed the disease burden of NAFLD/NASH in the Middle East; screening, diagnosis, and referral patterns in NAFLD; and available treatment options for NAFLD and NASH. This paper summarizes the discussions and opinion of the expert panel on the management of NAFLD/NASH and also presents an extensive literature review on the topic.
Key words: Nonalcoholic fatty liver disease; Nonalcoholic steatohepatitis; Middle East; Expert opinion
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide and is characterized by fatty infiltration of the liver in the absence of alcohol abuse or other causes of hepatic steatosis such as medications and viral or autoimmune hepatitis[1]. It encompasses the entire spectrum of manifestations from steatosis, steatohepatitis, and fibrosis to cirrhosis[1]. NAFLD can be categorized as nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) based on histopathological criteria. NAFL is defined as the presence of more than 5% of hepatic steatosis without evidence of hepatocellular injury and is generally a nonprogressive condition. On the other hand, NASH implies the presence of hepatic steatosis (> 5%), in conjunction with features of hepatocyte injury (such as hepatocyte ballooning), and can progress to fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC)[1,2]. Although patients with only hepatic steatosis (NAFL) typically do not develop cirrhosis or liver-related complications, about 20% to 30% of NAFLD can be classified as NASH[3,4]. It is important to distinguish between the different types of NAFLD as it has an impact on prognosis and management[5].
The estimated prevalence of NAFLD varies worldwide, ranging from 6% to 35%[6,7]. In a recent meta-analysis on the global epidemiology of NAFLD, Younossiet al[8]reported its prevalence to be highest (32%) in the Middle East. Also, with an increase in the risk factors of NAFLD and NASH such as obesity, metabolic syndrome (MetS), and type 2 diabetes mellitus (T2DM), the prevalence is estimated to grow further[6]. Alswatet al[9]conducted an analysis to estimate the burden of NAFLD in Saudi Arabia and United Arab Emirates (UAE) by the year 2030[9]. The analysis projected that by 2030 there will be over 12 million individuals (an increase by 48% from 2017) with NAFLD in Saudi Arabia and 372000 (an increase by 46% from 2017) in UAE[9]. This projected NAFLD prevalence corresponds to predicted increase in the burden of obesity and T2DM. Furthermore, NASH prevalence, which was 4.2% and 4.1% of the total population in Saudi Arabia and UAE, respectively, in 2017, is expected to rise by 96% and 87%, respectively, by 2030. This model also predicts that the percentage of patients with F3/F4 fibrosis or advanced liver disease (decompensated cirrhosis or HCC) is anticipated to increase to 21.8% (13.5% in 2017) and 21.1% (13.6% in 2017) of the NASH cases in Saudi Arabia and UAE, respectively, by 2030. In Saudi Arabia, incident decompensated cirrhosis is expected to rise by 273% with almost seven thousand cases in 2030. In UAE, the incident decompensation is anticipated to rise by 241%. NAFLD related HCC cases are projected to increase by 209% (580 to 1790) in Saudi Arabia and by 181% (18 to 51) in UAE. By 2030, annual liver-related deaths are estimated to rise by 295% in Saudi Arabia and by 270% in UAE[9].
It is important to note that the clinical outcomes of NAFLD can vary across the globe. In a recent study of the Global NASH Registry?, NAFLD subjects enrolled from 14 different countries showed some clinical similarities but also a number of differences[10]. These data suggest that the epidemiology of NAFLD must be considered in the context of regional and country-specific factors that impact the incidence, prevalence, and natural history of NAFLD.
NAFLD and NASH pose major challenges in both diagnosis and treatment. There is an ongoing debate as to whether at-risk patients should be screened for NAFLD/NASH in the absence of effective, approved pharmacological treatment options[2,11]. In such a scenario, screening of high-risk groups could be a more practical option. Also, there are uncertainties regarding appropriate referral, management, and follow-up of these patients. With the rising prevalence of NAFLD and NASH in the Middle East, it is imperative that these concerns are discussed at a regional level to facilitate effective strategies tailored to the needs of the region.
With this objective, advisory board meetings were conducted between May 2018 and February 2019 in Saudi Arabia, UAE, and Kuwait, to discuss current local challenges in NASH screening and diagnosis and the different available management options. These meetings were attended by expert gastroenterologists, hepatologists, and endocrinologists from the region. This paper summarizes the discussions in the meetings, along with a comprehensive literature review on the topic. For the review, PubMed and Google Scholar were searched employing the Boolean operators, “AND” and “OR”, and using the following search terms: “nonalcoholic fatty liver disease”, “NAFLD”, “nonalcoholic steatohepatitis”, “NASH”, “type 2 diabetes mellitus”, “T2DM”, “metabolic syndrome”, “obesity”, “guidelines”. The bibliographies of the key references were also searched manually for additional relevant references.
During the meetings, the experts discussed the following topics: (1) Disease burden of NAFLD/NASH in the Middle East; (2) Screening, diagnosis, and referral patterns in NAFLD; and (3) Available treatment options for NAFL and NASH.
DISEASE BURDEN OF NONALCOHOLIC FATTY LIVER DISEASE/ NONALCOHOLIC STEATOHEPATITIS IN THE MIDDLE EAST
NAFLD/NASH burden in the Middle East countries is growing with the increase in the prevalence of risk factors such as obesity, MetS, and T2DM
A population-wide screening program in UAE found that 35% of the population had obesity, 32% were overweight, 55% had central obesity, 18% had diabetes, 27% were prediabetic, and 44% had dyslipidemia[12](Table 1). Further, Radwanet al[13]reported a two to three-fold increase in the prevalence of obesity in the UAE between 1989 and 2017[13]. In a survey conducted in 2013 in Saudi Arabia, 28.7% of the 10735 participants were obese (body mass index, ≥ 30 kg/m2)[14]. Moreover, Al-Quwaidhiet al[15]projected an increase in the overall prevalence of obesity in Saudi Arabia to 41% in men, and 78% in women by 2022[15]. Obesity has been strongly linked with NAFLD and is known to be associated with the entire histological spectrum from steatosis, hepatocellular injury, fibrosis, cirrhosis, and even HCC[16]. In a study (n= 296), Alqahtaniet al[17]found that 65% of severely obese young children in Saudi Arabia had NASH and 60% had clinically significant liver fibrosis[17].
Similarly, regional disease burden from diabetes has been steadily increasing. As per the World Health Organization, in the Middle East and North Africa region, a 72% increase in the prevalence of diabetes from 39 million adults in 2017 to 85 million in 2045 is estimated[18]. In Saudi Arabia, the prevalence of diabetes has risen almost 10 fold over the past three decades[19]. There are several contributors to this, including rapid urbanization, unhealthy eating habits, and sedentary lifestyle[18]. Patients with T2DM have a high prevalence of NAFLD and advanced fibrosis. In a meta-analysis of 24 studies, the pooled prevalence of NAFLD in T2DM patients was almost 60%[20]. In another more recent (2019) meta-analysis, the global prevalence of NAFLD among T2DM patients was 55.5%[21]. Similarly, a meta-analysis that included 17 studies with T2DM patients found the prevalence of NAFLD to be 54%[22]. All these studies mayhave slightly different methodology but, as is evident, prevalence of NAFLD in individuals with T2DM was quite high. Moreover, presence of NAFLD has been reported to be associated with a twofold increased risk of incident diabetes[23]. Further, the prevalence of NASH in diabetic patients with NAFLD has also been evaluated. In a recent meta-analysis by Younossiet al[21], while the global prevalence of NASH in patients with T2DM was found to be 37.3%, the prevalence of advanced fibrosis was 17%[21]. In a study derived from the NASH Clinical Research Network, of 346 patients with diabetes and NAFLD, the prevalence of NASH and advanced fibrosis was 69.2% and 41%, respectively[24].
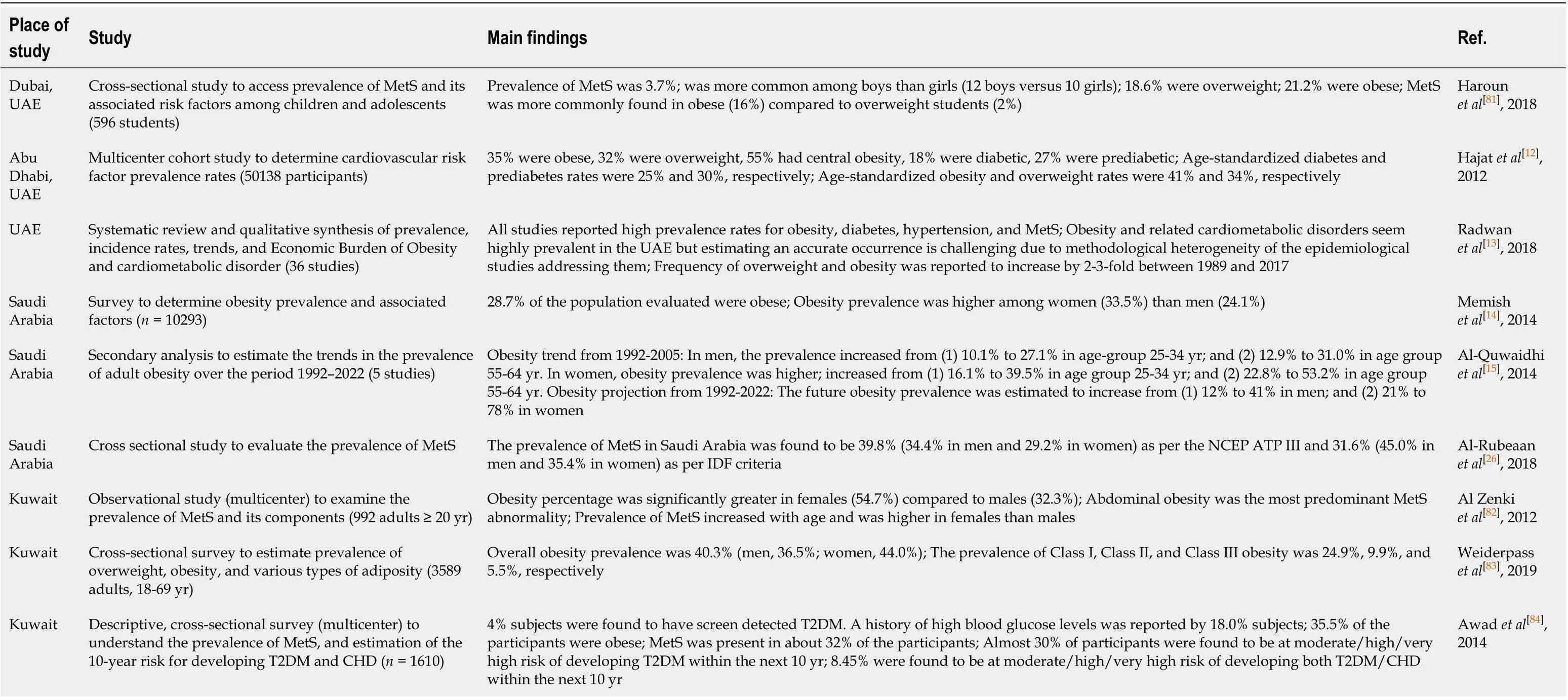
Table 1 Prevalence and Estimation Studies of Risk Factors of nonalcoholic fatty liver disease in United Arab Emirates, Kingdom of Saudi Arabia, and Kuwait
Similar to T2DM, the prevalence of MetS (i.e., abdominal obesity, hypertension, atherogenic dyslipidemia, and hyperglycemia) in the Middle East is also on the rise. A meta-analysis of cross-sectional studies from the Middle East countries reported the prevalence of MetS to be 16%-41% in Saudi-Arabia, 9%-36% in Kuwait, and 22%-50% in UAE[25]. A recent study found the prevalence of MetS in Saudi Arabia to be about 40%[26]. A strong association between MetS and NAFLD has been reported previously. In a study conducted on 304 patients with NAFLD without overt diabetes, 88% of patients who had NASH on liver biopsy had MetS. Further, the study showed that MetS was associated with a high risk of NASH among NAFLD patients [odds ratio (OR), 3.2; 95%CI: 1.2-8.9;P= 0.026][27]. Furthermore, in the NASH Clinical Research Network data, presence of NASH was significantly associated with MetS (OR: 1.43; 95%CI: 1.09-1.98;P= 0.009)[28]. In a regional study from Kuwait, T2DM (P= 0.02) and obesity (P< 0.004) were again significantly associated with NAFLD[29].
In summary, obesity, MetS, T2DM, and NAFLD are all associated with each other. Emerging data also appears to show that the prevalence of NAFLD is growing in parallel with that of obesity, MetS, and T2DM, worldwide, with Middle East being no exception[11,30,31].
SCREENING, DIAGNOSIS, AND REFERRAL PATTERNS IN NONALCOHOLIC FATTY LIVER DISEASE
Patients with risk factors, namely obesity, MetS, and T2DM should be screened for NAFLD
Given the high prevalence of NAFLD in high-risk groups, most international guidelines including European, Asia-Pacific, and National Institute for Health and Care Excellence recommend routine screening in these patients with liver enzymes, ultrasonography, and transient elastography, where available[2,32,33]. European guidelines also recommend case finding of advanced disease (i.e. NASH with fibrosis) in high risk individuals (age > 50 years, T2DM, MetS)[2]. However, the American Association for the Study of Liver Diseases (AASLD) guidelines currently do not recommend routine screening for NAFLD given the uncertainties regarding performance characteristics of diagnostic tests and available treatment options[1]. Nevertheless, they urge clinicians to have a high index of suspicion for NAFLD and NASH in patients with diabetes even if liver enzymes are normal[1]. European guideline also recommends that individuals with persistently abnormal liver enzymes be screened for NAFLD and all those with steatosis be screened for MetS independent of liver enzymes[2]. Obtaining a random aspartate transaminase and alanine transaminase (ALT) level in individuals with obesity, MetS, and T2DM may be a reasonable approach[34]. However, as it is known that liver enzymes may not always be elevated in NAFLD, an ultrasound can be done on patients suspected to have NAFLD. Other tests can be used for screening based on availability and feasibility.
Definite diagnosis of NASH can only be made by liver biopsy. Candidates for liver biopsy should be chosen carefully based on the results of noninvasive tests.
The diagnosis of NAFLD involves clinical history to rule out secondary causes of fatty liver and to obtain information on risk factors such as age (≥ 50 years) and the presence of T2DM and MetS[35](Figure 1). Further, biochemical investigations and radiological findings aid in making the diagnosis. Patients often do not experience overt symptoms but their quality of life is impaired[36]. In most cases, the diagnosis of NAFLD is made when elevated liver enzymes are found incidentally. Also, it is important to note that liver enzymes can be within the normal range in NASH and a definitive diagnosis can only be made by histology[30].
Although liver biopsy is considered as the gold standard for establishing NASH and for staging of fibrosis, it is associated with the risk of complications such as pain, intraperitoneal bleeding, and pneumothorax[35]. Noninvasive methods of diagnosis include serum biomarkers, routine radiologic tests such as ultrasound, computed tomography, magnetic resonance imaging, and assessment of liver stiffness by transient elastography (FibroScan?) and magnetic resonance elastography[37]. Validated laboratory-based scoring systems for estimating the stage of hepatic fibrosis are also available, such as NAFLD fibrosis score, FibroMeter, and Fibrosis-4[35]. The advantages and limitations of some of the available diagnostic modalities are listed in Table 2.
In addition to these tests, a number of serum fibrosis tests are being evaluated forrisk-stratifying patients with NASH. Of these, Enhanced Liver Fibrosis (ELF?) is available in Europe and seems to be a promising non-invasive test for risk assessment in patients with NASH[38].
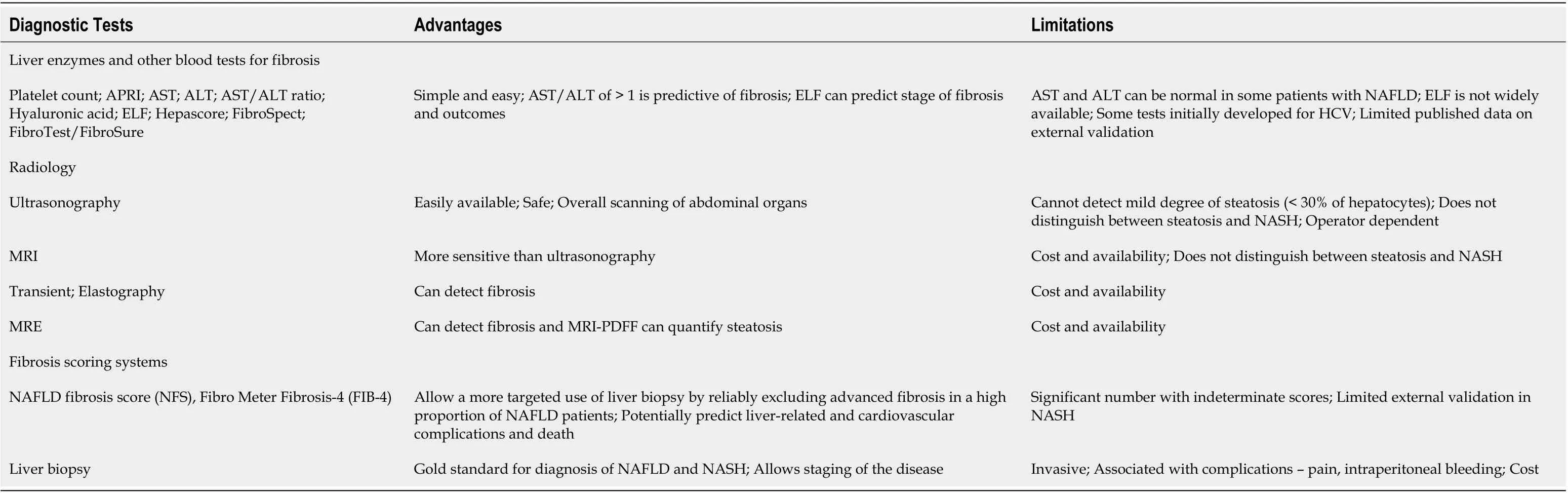
Table 2 Key diagnostic modalities for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[11,35,85,86]
Since none of the noninvasive tests can confirm a diagnosis of NASH, the only available option for definitive diagnosis is liver biopsy. However, it has been found that the combination or sequential use of different noninvasive methods or different scoring systems can increase overall diagnostic accuracy with consequent decrease in the need for liver biopsy[39-41]. In summary, diagnosis of NASH is complex and needs careful consideration of available non-invasive diagnostic tools. It is advisable to use these various modalities based on availability and carefully select the subset of patients, who will benefit from liver biopsy, based on the results of the noninvasive methods.
Liver biopsy also plays an important role in staging and risk-stratification of the disease. NASH fibrosis is divided into four stages based on severity, ranging from the less severe stages (F1 and F2) to F3 and F4 (bridging fibrosis and cirrhosis, respectively)[42]. The presence and degree of fibrosis has been shown to be the mostimportant prognostic marker in NASH[43-46]. In a study, during a follow-up of mean 20 years (range 0-40) equivalent to 139163 person-years, the risk of severe liver disease increased per stage of fibrosis (hazard ratio ranging from 1.9 in F0 to 104.9 in F4) compared to controls[44]. In another study, features of liver biopsies significantly associated with death or liver transplantation included fibrosis stage 1 (HR: 1.88; 95%CI: 1.28-2.77), stage 2 (HR: 2.89; 95%CI: 1.93-4.33), stage 3 (HR: 3.76; 95%CI: 2.40-5.89), and stage 4 (HR: 10.9; 95%CI: 6.06-19.62) compared with stage 0[43].
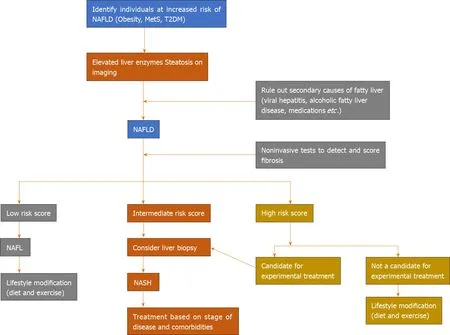
Referral of patients should be done promptly once NASH is suspected and NASH should be managed by multidisciplinary teams
Currently, NAFLD is underdiagnosed[47]. It is crucial to increase awareness regarding NAFLD and NASHviaeffective multi-disciplinary collaboration among healthcare professionals. Education of primary care physicians as well as diabetologists is important. Diabetologists and primary care physicians should be encouraged to screen at-risk patients for NAFLD, followed by time-sensitive referral, especially in patients with suspected NASH. In view of the co-morbid conditions often associated with NAFLD, a multidisciplinary management approach that includes primary care physicians, gastroenterologists, endocrinologists/diabetologists, cardiologists, and nutritionists is warranted.
AVAILABLE TREATMENT OPTIONS FOR NONALCOHOLIC FATTY LIVER AND NONALCOHOLIC STEATOHEPATITIS
Patients with NAFL should be advised lifestyle modifications only. Patients with NASH should be considered for therapy based on the stage of the disease and the presence of comorbidities
Once NAFLD/NASH is diagnosed, the next step is to consider appropriate therapeutic intervention. This is currently limited by lack of effective treatment options to prevent or reduce progression to cirrhosis and HCC. Although all patients with NAFLD are candidates for lifestyle intervention to optimize their cardiovascular risks, only patients with NASH are candidates for pharmacotherapy. Therefore, the current treatment options for NASH include lifestyle interventions, pharmacotherapy (mostly off-label), and consideration for bariatric surgery in the context of metabolic indication for weight loss surgery. Importantly, treatment should also involve the management of comorbidities[1,2].
Patients with NAFL usually have a favorable liver-related prognosis and require management of their cardiovascular risks. In this context, NAFL patients do not require consideration of pharmacotherapy for liver disease and this treatment option should be reserved for patients diagnosed with NASH. Intervention in patients with NAFL should be limited to encouragement of appropriate lifestyle modifications[1,2]. Lifestyle interventions, including diet and exercise, have been found to be effective when adequate weight loss is achieved. In a meta-analysis that included 20 studies and 1073 NAFLD patients, exercise significantly improved aspartate transaminase and ALT (P< 0.05). Additionally, combined exercise and diet decreased ALT (P< 0.01) and improved NAFLD activity score (standardized mean difference -0.61, 95%CI: -1.09 to -0.13)[48]. Bradfordet al[49]reviewed 30 studies that used diet and/or exercise as an intervention for patients with NAFLD[49]. A total of 14 studies that included both diet and exercise showed improvement in the various outcome measures evaluated. Of the nine studies that included only exercise as an intervention, eight studies showed a positive outcome. Furthermore, all seven studies that used only diet as an intervention also showed improvement in markers of hepatic steatosis, leading the authors to conclude that lifestyle intervention is a critical component of management of patients with NAFLD[49].
European Association for the Study of the Liver, European Association for the Study of Diabetes, and European Association for the Study of Obesity and AASLD guidelines recommend a combination of daily reduction in caloric intake by 500 to 1000 kcal and moderate-intensity exercise for sustaining weight loss over time[1,2]. A systematic review and meta-analysis reported improved hepatic steatosis with ≥ 5% weight loss and improvement in NASH with a weight loss of ≥ 7%[50]. As per the AASLD guidelines, weight loss of 3%-5% of total body weight is required to improve steatosis and weight loss of 7%-10% is necessary to improve histopathological features of NASH[1]. It has also been suggested that weight loss of more than 10% may improve fibrosis[51,52].
It is important to note that currently, there are no pharmacotherapies approved for the treatment of NASH. This underscores the need for clinicians to make treatment decisions based on individual patient circumstances, taking into account the stage of NASH and safety, expected benefit, pricing, and evidence base of available off-label pharmacological treatment options. Although metformin has been widely studied in patients with NASH, it was not found to have beneficial effects on liver histology[53-55]and is therefore not recommended in patients with NASH[1,2,32]. On the other hand, the thiazolidinedione, pioglitazone has been shown to be promising in improving liver histology in NASH patients with or without T2DM[56-58]. Pioglitazone could be considered in these patients although currently AASLD guidelines recommend consideration of its use in biopsy-proven NASH only[1]. Vitamin E has also been used in patients with NAFLD and NASH. In a meta-analysis, vitamin E supplementation was associated with significant (P< 0.05) improvement in all histological parameters including steatosis, ballooning, lobular inflammation, and fibrosis in nondiabetic individuals with NASH[59]. Similar results were found in another meta-analysis, where vitamin E significantly reduced liver enzymes and improved liver histology[60]. However, concerns have been raised about the safety of vitamin E supplementation, including increased all-cause mortality with dose of > 800 IU/d and an observed association with prostate cancer[61,62]. Nevertheless, a large meta-analysis that included 57 studies including 246371 patients, who were followed for up to 10 years, did not show any association between vitamin E and all-cause mortality[63]. Consequently, it is recommended that vitamin E at a dose of 800 IU/d be considered in nondiabetic adults with biopsy-proven NASH[1]. A meta-analysis that included nine clinical trials (three with thiazolidinedione, three with metformin, two with vitamin E and one with both thiazolidinedione and vitamin E), found that thiazolidinedione and vitamin E improved liver histologic scores, but metformin did not. None of the agents improved fibrosis[64].
NAFLD has been shown to be associated with an increased risk of cardiovascular events, regardless of the presence of coexisting metabolic syndrome, mandating consideration of interventions to treat cardiovascular risk factors[65]. Concerns persist amongst clinicians regarding statin use in patients with liver disease. However, statins can be used to treat dyslipidemia in patients with NAFLD and NASH without any concern regarding liver toxicity[1,66]. Statins have also been studied for the treatment of NAFLD and NASH but their efficacy for this indication is debated due to inconsistent results in clinical trials[66].
Sustained weight loss is indeed the most effective way to treat NAFLD and NASH. Unfortunately, this is difficult to achieve utilizing lifestyle modifications alone. Bariatric surgery, therefore, has an important role in the management of obese NASH patients. In one study, 85% of 109 morbidly obese patients with biopsy-proven NASH showed resolution of disease one year after bariatric surgery and the likelihood of improvement correlated with liver disease severity prior to surgery and degree of weight loss achieved[67]. A third of patients were also observed to have improvement in hepatic fibrosis[67]. In a systematic review and meta-analysis, bariatric surgery was associated with a significant reduction in the weighted incidence of a number of histological features of NAFLD including steatosis (50.2%), fibrosis (11.9%), hepatocyte ballooning (67.7%), and lobular inflammation (50.7%)[68]. A study conducted in the United States found bariatric surgery to be not just effective but also cost-effective in the treatment of obese patients with NASH[69].
In a study conducted in the UAE, 80 patients underwent bariatric surgery, either laparoscopic sleeve gastrectomy (n= 53) or mini-gastric bypass (n= 27). There was significant reduction in mean body weight at 3, 6, 12, and 24 mo (P< 0.001 for each time point). Of the 61.3% who had NAFLD, 22.4% showed improvement in sonographic features of hepatic steatosis with normalization of ALT[70]. In another study (n= 27) conducted in Saudi Arabia, after 3 mo of bariatric surgery, 66.6% of the patients with preoperative steatosis (median score 2) had reduced steatosis scores postoperatively (P= 0.025) and 68% of the patients had reduced fibrosis (median score 1) (P= 0.012). Also, NAFLD activity score was decreased from 4 (3-5) to 2 (1-3) (P= 0.004)[71]. However, it is also important to note that the use of bariatric surgery is limited by its cost and complications that are associated with any surgical procedure. As such, any advocacy for bariatric surgery must be tempered with a parallel education of its associated complications, which remains suboptimal in the region[72].
NASH-related cirrhosis is growing as an indication for liver transplantation
NASH is projected to become the most common indication for liver transplantation in near future[73]. With the availability of highly effective treatment options for hepatitis C virus (HCV)-related cirrhosis, NASH-related cirrhosis is emerging as the leading indication for liver transplantation worldwide.
In a study conducted in Saudi Arabia, over the course of the time period from January 2001 to December 2006, 50%, 17%, and 12% of the patients underwent liver transplant for HCV-induced cirrhosis, HBV-induced cirrhosis, and NASH-related cirrhosis, respectively. On the other hand, from January 2007 to January 2012, 35%, 16%, and 20% of the patients underwent transplantation for HCV-, HBV-, and NASHinduced cirrhosis, respectively, underscoring the evolving trends in the indications for liver transplantation[74].
Being a complex procedure, liver transplantation is associated with several limitations. Another important aspect to consider is that liver transplantation does not treat the comorbidities of NASH and therefore interventions to address the accompanying metabolic diseases is important, before and after transplantation[73].
Emerging therapies for NASH are awaited for more effective management of this disease
Currently, many new drugs are being developed with varying mechanisms of action, with the goal of improving hepatic steatosis, inflammation, liver cell injury, and fibrosis in patients with NAFLD[75]. Some of these include obeticholic acid (farnesoid X receptor agonist), elafibranor (a dual receptor peroxisome proliferator activated alpha/delta agonist), GS-9674 (farnesoid X receptor agonist), and GS-0976 (Acetyl-CoA carboxylase inhibitor). Future treatment options may also include combination therapies[75,76].
ADDRESSING NONALCOHOLIC FATTY LIVER DISEASE BURDEN IN THE MIDDLE EAST
Despite the high burden of NAFLD, there are no proper epidemiological studies or regional clinical guidelines that are relevant to these countries. The lack of properly carried out community-based epidemiological studies is a particular concern, and has obvious fallouts on resource planning and allocation, and poses considerable difficulties to these countries’ healthcare systems. To some extent, regional prevalence numbers remain best guess estimates, based in part on modeling analyses or otherwise on indirect markers of NAFLD such as obesity and T2DM[9]. This stresses an immediate need for adequately sampled, large-scale, nationwide surveys for NAFLD, where the prevalence across all population sectors, age groups, and geographic distributions is properly estimated. The relevant health authorities and ministries of these countries would be best positioned to lead such an initiative, executing it jointly with regional scientists and public health experts. Part of the epidemiological equation entails understanding the demographics and evolution of the disease, and towards this an access to a national registry for NASH is crucial.
There must be an urgent commitment to address the factors contributing to obesity in the Middle East, including dietary factors and inactivity, with particular focus on childhood obesity[77]. Dietary and lifestyle changes have a substantial impact on the natural course of the disease. As such, from a public health perspective, one of the most crucial aspects to focus on is to modify risk factors that are geared towards reducing obesity rates and achieving better dietary habits. Targeted measures such as taxation of beverages, marketing regulation, improving nutritional labeling, conducting awareness campaigns, and allocating subsidies to improve healthy eating could be implemented[78].
The dearth of algorithms for primary care referral is worrisome in view of the high prevalence of NAFLD in the Middle East. Primary health care should play a key role in the management of NAFLD, not only because of its pivotal role in health promotion and community care but also because specialized liver care is generally not prepared to receive such a large number of patients. Simple and affordable algorithms to identify patients at high risk of complications could be implemented in primary care to determine patients needing specialized care. A broad availability of transient elastography in primary healthcare centers must be envisioned in any NAFLD national plan, since significant hepatic disease may exist in non-obese individuals, and in those with normal liver enzymes[79]. This approach would contribute substantially to managing the intricacies of NAFLD-related disease burden within a healthcare system that provides early detection while preserving economic sustainability and equity[78].
Therefore, continuing education programs and awareness campaigns are crucial, as well as development and adaptation of clinical guidelines to protocols that identify patients who need specialist referral. Potential factors that need to be assessed include the role of non-liver specialists, and the implementation of community-based initiatives and civil society involvement aimed at NAFLD education, prevention, detection, and care.
This huge challenge facing our medical community must be effectively tackled with comprehensive, widely adopted, national (or regional) NAFLD plans, that start from awareness and education, emphasize prevention, set up early detection programs, and provide the recommended algorithms of care in a cost-effective and evidence-based manner. Such efforts should be led by robust research machinery that is able to predict, rationalize, and assess the effectiveness of hereupon-adopted strategies. Alliances will require to be forged not just across different institutes and affiliations, but also involving patients and community partners. However, most importantly, such plans would demand unprecedented attention to public health and can only succeed if they are coupled with an enforcement power that can guarantee their implementation[80].
CONCLUSION
The prevalence of NAFLD in the Middle East is increasing, along with its known associations of obesity, MetS, and T2DM, thereby placing a substantial burden on healthcare systems in the region. Suggested interventions to mitigate this challenge include education and awareness of patients and primary care providers regarding NAFLD; management of the risk factors and comorbidities; appropriate screening, evaluation, and diagnosis; and timely referral to hepatologists. This calls for a multidisciplinary approach involving primary care providers, gastroenterologists, endocrinologists, diabetologists, cardiologists, and hepatologists. There remains a need for clinical care pathways to help guide clinicians involved in the care of these patients[81-86].
Many challenges and unmet needs remain in the diagnosis and management of NAFLD. There is a lack of reliable noninvasive tests for the accurate diagnosis of NASH. Also, there is no approved therapy for the treatment of NASH and current management is largely dependent on lifestyle modifications, which are challenging to initiate and sustain, resulting in inadequate weight reduction. Many therapies are currently under development and are keenly awaited. The availability of effective pharmacotherapy holds significant promise to improve the current landscape of available treatment options in NAFLD.
 World Journal of Gastroenterology2020年25期
World Journal of Gastroenterology2020年25期
- World Journal of Gastroenterology的其它文章
- TBL1XR1 induces cell proliferation and inhibit cell apoptosis by the PI3K/AKT pathway in pancreatic ductal adenocarcinoma
- Development of innovative tools for investigation of nutrient-gut interaction
- Practical review for diagnosis and clinical management of perihilar cholangiocarcinoma
- Quality of life in patients with gastroenteropancreatic tumours: A systematic literature review
- Functionality is not an independent prognostic factor for pancreatic neuroendocrine tumors
- Risk factors associated with inflammatory bowel disease: A multicenter case-control study in Brazil
