Current and future applications of ultrasound imaging in peripheral nerve disorders
Antonia S Carroll,Neil G Simon
Antonia S Carroll,Brain and Mind Research Centre,University of Sydney,Camperdown 2050,NSW,Australia
Antonia S Carroll,Department of Neurology,Westmead Hospital,University of Sydney,Westmead 2145,NSW,Australia
Antonia S Carroll,Department of Neurology,St Vincent's Hospital,Sydney,Darlinghurst 2010,NSW,Australia
Neil G Simon,Northern Clinical School,University of Sydney,Frenchs Forest 2086,NSW,Australia
Abstract
Key words:Neuromuscular ultrasound;Peripheral neuropathy;Polyneuropathy;Entrapment neuropathy;Immune-mediated neuropathy;Hereditary neuropathy
INTRODUCTION
Ultrasound (US) technology was initially developed for medical use in the 1940s,where A-mode linear recordings displayed alterations in echointensity as amplitude.Here,a single pulse of US is transmitted to the tissue,resulting in a one-dimensional linear representation of reflected US beams as a function of depth.Further technological advances allowed for the development of B-mode or 2D-mode,still used today,where multiple linear data channels are superimposed to create an image where amplitude is expressed as brightness.However,US was not applied to peripheral nerves prior to the 1980s[1,2]due to inadequate image resolution and difficulty discerning nerve from surrounding tissues of similar echogenicity[3].The first report of the diagnostic utility of peripheral nerve US,used to detect carpal tunnel syndrome (CTS),was published in 1991[4].Since then,US technology has continued to evolve with the development of broadband high-frequency linear-array transducers,Doppler technology and post-processing software programs,all of which have increased the ability of US to identify anatomic and structural abnormalities in nerves.
Today US imaging is increasingly and commonly used as an adjunct to clinical and neurophysiological studies in the evaluation of neuromuscular diseases,so called neuromuscular ultrasound (NMUS).Advantages of NMUS include being a noninvasive,painless,economical,portable and fast test,whilst allowing a high resolution,dynamic assessment of the complete nerve in real-time.Thus,NMUS is considered an ideal screening,diagnostic and monitoring tool.An alternative imaging modality is Magnetic resonance (MR) neurography which provides exceptional threedimensional images of neural anatomy as well as functional characteristics with the advent of tractography and diffusion tensor imaging[5,6].Furthermore quantitative techniques,such as the fat fraction of muscles and nerve size,can be used to monitor disease progression,and may be beneficial as biomarkers in future clinical trials[7].However,MR Neurography is expensive,time-consuming,poorly tolerated in children and claustrophobic individuals,limited in availability and,in clinical practice,restricted to the pre-specified region.Compared to MR Neurography,NMUS provides improved spatial resolution with extremely small nerves being amenable to investigation;however,deep neural structures,such as the lumbosacral plexus and proximal sciatic nerve,or those deep to osseous structures are unable to be imaged.Hence there is an ongoing complementary role for both NMUS and MR Neurography;however,the use of each imaging modality needs to be tailored to the correct clinical situation.
CURRENT AND FUTURE APPROACHES AND TECHNIQUES
B-mode
NMUS is predominantly performed in B- or 2D-Mode using a linear array transducer with an upper frequency of 15-18 MHz.Anatomy is assessed,along with the shape,echogenicity and cross-sectional area (CSA) of the nerve.Typically,a distal to proximal approach is followed to identify any abnormality along the course of the nerve.Structures should be assessed in two orthogonal planes,usually with crosssectional (Figure 1A) and longitudinal (Figure 1B) views,and these images should be saved as documentation and to examine temporal trends.Comparison with the contralateral nerve is clinically useful for the evaluation of unilateral diseases.The mobility of the nerve,particularly around joints,can also be dynamically assessed,if relevant,such as the ulnar nerve at the elbow and median nerve at the wrist.In addition,muscles can be evaluated for neurogenic or myopathic changes.In neurogenic conditions fasciculations,atrophy and a patchy increase in echogenicity is typically demonstrated[8,9].In contrast,in myopathic conditions homogenously increased echogenicity and increased Doppler signal is observed[8,9].The specific US features of different muscle disorders is beyond the scope of this review and readers are directed to specific reviews on this topic[10-13].
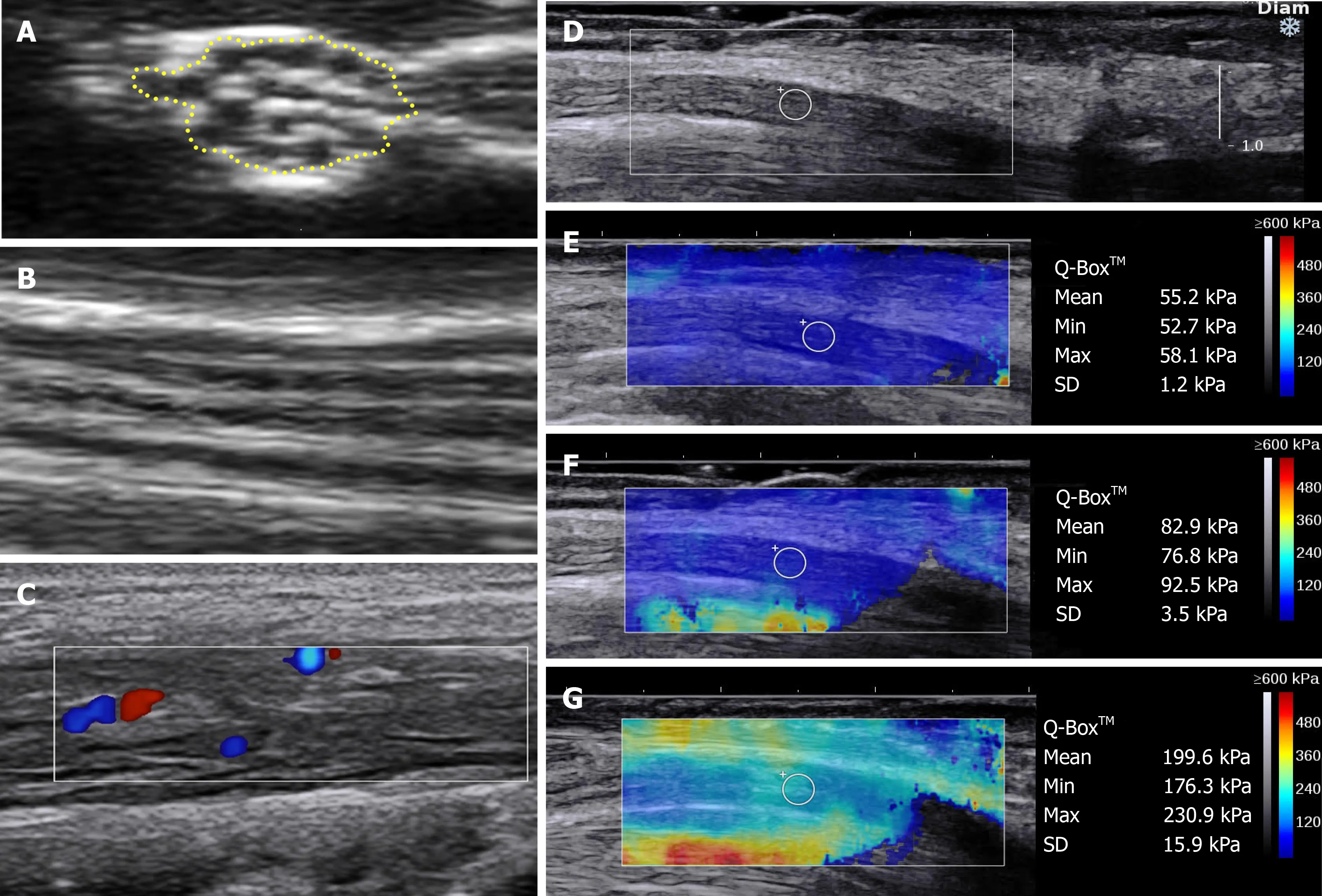
Figure 1 Approaches and techniques used in neuromuscular ultrasound.A and B:Normal nerve ultrasound appearances of the median nerve in the forearm in B-mode.A:Cross-sectional area traced within the hyperechoic rim;B:Longitudinal view demonstrating the normal linear fascicular pattern;C:Doppler signal in the tibial nerve of a patient with hypertrophic perineuritis;D-G:Sheer wave elastography in carpal tunnel syndrome;D:Longitudinal B-mode image of the median nerve at the carpal tunnel above the lunate bone,a typical location for elastography measurements;E:Elastography values in a normal wrist;F:increased median nerve stiffness in mild carpal tunnel syndrome;G:further increased median nerve stiffness in severe carpal tunnel syndrome.D-G:Citation:Wee TC,Simon NG.Ultrasound elastography for the evaluation of peripheral nerves:A systematic review.Muscle Nerve 2019;60:501-512.Copyright? The Authors 2020.Published by John Wiley and Sons.
CSA:Nerve size is measured using CSA,measured in mm2,which is traced within the hyperechoic epineurial rim of the nerve with the transducer perpendicular to the nerve.Care must be taken to measure a true CSA as CSA is frequently overestimated when using an oblique view of the nerve.CSA is the most commonly used measure to quantify abnormalities in nerve diseases,due to its reproducibility and high inter- and intra-assessor reliability[14].Normative reference values are available for the main limb nerves,the brachial plexus and cervical roots[15-18].Physical characteristics,such as age,gender,height and body-mass index,and temperature have been identified,in some publications,to alter nerve size[15,16,19],therefore,ideally standardised conditions should be used for all tests,and local normative values should be collected.
Echogenicity:Echogenicity of a peripheral nerve is commonly described and typically varies in normal individuals along the length of a nerve,with the proximal segments appearing more hypoechoic than the distal segments[20].The echotexture of the nerve can be semi-quantitatively evaluated by assessing the mean grey scale value of a selected image,or can be quantified by post-processing software using thresholding techniques to determine the hypoechoic fraction and density of the nerve[21,22].Of note quantitative measures of echotexture are not comparable and vary with US systems,settings and processing software,which limits its utility as a potential disease biomarker.Figure 2 demonstrates qualitative alterations in nerve echotexture in different pathological conditions.
Ultra-high frequency B-mode:Since the development of US,continual modification of US probes and signal processing has resulted in improved image resolution and quantification.In 2016 the first ultra-high frequency transducer (Vevo MD ultrasound device,FujiFilm Visual Sonics,Toronto,Ontario,Canada),with a maximum frequency of 70 MHz,was approved for clinical use by the Food and Drug Administration (FDA) allowing extremely high-resolution imaging of neural and other structures.However,this dramatic enhancement of resolution to 30 μm,comesat the expense of penetration depth.Specifically,the 70 MHz probes are only able to image superficial structures,up to a maximum of 3 cm in depth.However,despite this limitation,the use of ultra-high frequency US allows significant insights into the internal neural structures providing detailed data on the size,number and density of fascicles,echogenicity and intraneural vascularisation.To date this technology has been used to accumulate normative data in median and ulnar nerves and to evaluate chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and spinal muscular atrophy (SMA)[23-26].
Fascicular US:Improvements in US resolution has facilitated further in-depth analysis of smaller neural structures,such as individual nerve fascicles.Variation of individual nerve fascicle size will likely provide pathological insights in peripheral neuropathies,nerve tumours and nerve trauma.In the context of CSA enlargement,nerve fascicles are often enlarged;however,this is not always homogenous[27].For example,fascicular size has been evaluated in Charcot Marie Tooth disease type 1A(CMT1A),revealing significant diffuse fascicular enlargement across nerves,but interestingly,non-homogenous enlargement across the fascicles of an individual nerve[28].Similar,but more pronounced,variability in fascicular enlargement has been seen in CIDP,multifocal motor neuropathy (MMN) and vasculitis,with regional and differential enlargements observed[13,25,28,29].However,the interpretation of fascicular size is complex,as the calibre of the fascicles can vary between different nerves,the same nerve in different locations,and between individuals.For example,the brachial plexus trunks are typically comprised of two large fascicles,whereas the median nerve in the forearm is comprised of a multitude of smaller fascicles,up to 30 in one study[23].As a result,a standardised approach and normative values are required for future utility of this approach.
Recently normal values have been published for the upper limit of normal fascicular sizes in the median,ulnar and peroneal nerves[28].In addition a fascicle pattern grading system was proposed,using the summed proportion of enlarged fascicles in the median,ulnar and peroneal nerves and the proportion of enlarged fascicles within the individual nerve,to distinguish between inherited and acquired neuropathies[28].Further large prospective studies using various US probes and assessing several anatomical locations will be required to validate the proposed fascicle pattern grading system,provide further normative data and solidify the use of fascicle size as a monitoring or diagnostic biomarker.
Doppler US
B-mode can be used with Colour or Power Doppler to quantify blood flow (Figure 1C).Blood flow,both intraneural or epineural,is not able to be detected in normal nerves using current non-contrast imaging[30].Increased blood flow,described qualitatively or quantitatively,can be seen in acquired inflammatory neuropathies,neurolymphomatosis or in nerve entrapment[31-38],and is best evaluated with Power Doppler which is more sensitive for low flow states,such as is found intraneurally[39].Autonomic activation,by provocative manoeuvres,exercise,or electrical stimuli,can alter neural blood flow,as can tremor and abnormal arterial anatomy,both congenital and iatrogenic,hence,patient selection and positioning is necessary to ensure the generalisability of results[40].
Currently used quantitative measures are surrogates for intraneural blood flow including manual counts of Doppler signals or the analysis of Doppler waveforms to assess the velocity of blood flow.These measures more correctly indicate hypervascularity,rather than a definitive increase in blood volume within the nerve,and,due to the slow flow within nerves,likely underestimate intraneural blood flow due to detection thresholds of flow velocity[41].Recently additional techniques have been used to more accurately assess intraneural blood flow including contrast enhanced ultrasound (CEUS) and measurement of maximum perfusion intensity(MPI)[41,42].
CEUS:Novel approaches to measuring intraneural blood flow have been recently published in attempts to improve the accuracy and precision of vascularity measures and better understand pathophysiology and treatment response in neuropathies.Two pilot studies of CEUS in normal median nerves,were able to safely and reproducibly detect microvascularity in asymptomatic cohorts[42,43].CEUS has been subsequently used to evaluate animal models with CTS and sciatic nerve crush injuries,and in humans with CTS and for intra-operative differentiation of peripheral nerve tumours[44-47].High-resolution CEUS has allowed the quantification of previously undetectable low volume intraneural blood flow;however,further studies are required to evaluate its use in the assessment of disease severity and prognosis in peripheral nerve diseases.To date studies have been limited due to FDA restrictions on the use of contrast agents,despite its approval and safety record for use in echocardiography[48].
MPI:Similar methods have been proposed to quantify intraneural blood flow without using contrast,including the measurement of MPI.This uses a semiautomated,validated perfusion measurement software,such as PixelFlux (Chameleon Software,Munster,Germany),to measure the MPI by quantifying blood flow using the intensity of colour hues of the pixels within a specified region of interest in an uploaded Doppler US video file[41].An initial study identified improved reliability of MPI compared to previous semi-quantitative vessel scores in CTS,and correlations with neurophysiologically determined CTS severity and nerve CSA[41].MPI has subsequently been applied in diabetic neuropathy and end-stage kidney disease,finding correlations between intraneural blood flow measurements,nerve size,symptomatology and neuropathy severity scores[49,50].Interestingly,in the haemodialysis cohort,blood flow measurements were significantly improved afterdialysis,suggesting that this measure may have future utility as a biomarker of both disease severity and treatment response[50].
Elastography
US Elastography is an additional functional technique which has garnered increasing interest as a potential prognostic and monitoring biomarker in neuromuscular diseases.Elastography non-invasively measures the stiffness of tissues,which reflects underlying pathological changes in tissue composition and architecture.Stiffness tends to increase in peripheral neuropathy of all causes,consistent with the replacement of compliant myelin with various less-compliant connective and other tissues[51].Strain and shear-wave elastography (SWE) are the two major techniques,differing in measurement methodology.
Strain elastography:Strain elastography measures stiffness by recording the displacement of a tissue by either a manual compression or by physiologic tissue movement,such as cardiorespiratory pulsations.The latter method,specifically known as ambient strain elastography (ASE) is preferred for neuromuscular evaluation as it is more reproducible and reliable for clinical practice,than manual compression techniques where the quantification and standardisation of force is challenging[52].
SWE:Alternatively,SWE uses a standardised high-intensity acoustic or vibration pulse to cause tissue displacement generating a quantitative measure of stiffness(Figure 1D-G),with similarly reproducible and reliable findings to ASE[51].
The output of these different elastography modalities is not comparable and at present,despite the benefit of quantitative results with SWE,the choice of technique is most frequently determined by the availability of elastography modules and local expertise.US elastography techniques have predominantly investigated the median nerve at the wrist,to date,finding elevated stiffness in CTS compared with control wrists,and in some studies,severity of CTS correlating with increasing nerve stiffness(Figure 1E-G)[53-59].However,further studies evaluating elastography as a diagnostic and monitoring biomarker are required to cement its use in clinical practice.
Applications
NMUS also allows for the improved evaluation of children and phobic individuals who tolerate electrodiagnostics (EDX) poorly,as well as access to technically challenging or inaccessible sites.The diaphragm,for example,is technically difficult to assess,with nerve conduction studies being unreliable and affected by body habitus,and electromyography (EMG) risky in the context of critical respiratory insufficiency and the potential to precipitate a pneumothorax[60].The diaphragm is easy to US and demonstrates both acute or chronic changes characterised by the degree of movement and alterations in thickness during the respiratory cycle.The ratio of inspiratory-expiratory thickness has a sensitivity of 93% and specificity of 100% for neuromuscular diaphragmatic dysfunction[61].US can also be used in this setting to reduce risk by guiding EMG,and as an alternative to radiological respiratory function tests,such as the “sniff test”[62].
NORMAL SONOGRAPHIC APPEARANCE OF PERIPHERAL NERVE
Ultrasonographic appearances of normal peripheral nerve parallel the anatomy of the nerve at both a micro- and macroscopic level[1].In cross-section nerves display a“honeycomb” appearance reflecting the hypoechoic fascicles surrounded by hyperechoic epineurium and perineurium (Figure 1A).In longitudinal view a nerve appearance is described as a “train-track”,with a linear hypoechoic fascicle with hyperechoic edges (Figure 1B).Proximal segments of nerve,such as the roots and plexus,appear more hypoechoic due to reduced connective tissue composition and increased fascicle density[20,63].As a normal nerve progresses distally the CSA tapers[15].This finding is more pronounced in the lower limb nerves than in the upper limbs.In healthy individuals,nerves can have focal enlargements closer to entrapment sites[15].
The hyperechoic appearance of the epineurium is caused by the high impedance of the densely packed connective tissue.This characteristic finding is useful to differentiate nerves from other structures.Anisotropy,an artefact generated by tilting the probe,can also be used to distinguish nerves.Nerves typically have low anisotropy,meaning that there is minimal alteration in echogenicity with change of the transducer angle.This low anisotropy aids to distinguish nerve from tendons and muscle,which have high anisotropy,changing from hyperechoic to hypoechoicappearance with movement of the transducer angle from perpendicular to parallel.Vessels can be differentiated from nerve by the identification of pulsatile movement,collapsibility under pressure or flow on Doppler.
US APPEARANCES IN PERIPHERAL NERVE DISORDERS
Typical morphological changes in the nerve are seen after nerve injury of many causes,including enlargement in nerve size and reduced echogenicity.The latter is proposed to be due to intraneural oedema caused by thickening of connective tissues,increased vascular leak and/or vascular proliferation[3,64].Injury to the nerve also typically cause alterations in fascicular architecture and intraneural blood flow.In the following sections the specific US findings observed in entrapment,hereditary,immune-mediated and axonal neuropathies,and motor neuron diseases will be discussed.Figure 2A-F outlines the typical sonographic patterns identified on NMUS in various polyneuropathies.Figure 3 provides an overview of the degree and distribution of nerve enlargement in inherited and acquired polyneuropathies.
Peripheral nerve entrapment
In compressive neuropathies localised enlargement,just proximal to the site of compression,decreased echogenicity,loss of the internal fascicular structure and increased blood flow can all be commonly observed[3,65].However,a maximum point of enlargement should be identified along the course proximal and distal to the site of compression as enlargement can be present distal to the entrapment site,and nerve mobility may alter the positioning of such enlargements[66].The entrapment site may display an hour-glass or spindle-shaped appearance in longitudinal views[66].Strictures,ganglion cysts,tumours,thrombosed arteries and other extrinsically or intrinsically compressive lesions can be identified by US as the cause of symptomatology,and hence can drastically alter the management paradigm.In one study of mononeuropathies the diagnostic or management trajectory was altered by peripheral nerve US in over 42% of cases[67].The use of peripheral nerve US assists to identify early or neurophysiologically normal disease,localises the lesion,and can identify the cause of compression,and hence complements traditional EDX techniques.
The earliest identifiable ultrasonographic sign of compression is reduced echogenicity within the nerve followed by nerve enlargement.Pressure of the US probe over the compressed section of nerve can reproduce clinical symptoms,a so called “Sonographic Tinel” sign.With ongoing progression of entrapment denervation features of the distal muscles can develop including increased muscle echogenicity and fasciculation,and later atrophy.The characteristic ultrasonographic findings seen in specific compressive neuropathies is summarised in Table 1.
Hereditary neuropathies
Peripheral nerve US has been used as a diagnostic tool in Charcot-Marie-Tooth disease (CMT),Hereditary neuropathy with liability to pressure palsies (HNPP) and more recently in hereditary transthyretin (ATTRv) amyloidosis and sensory neuropathies and neuronopathies associated with cerebellar ataxia,neuropathy,and vestibular areflexia syndrome (CANVAS),spinocerebellar ataxias (SCA) and Freidrich's ataxia (FRDA).
CMT:An early study using a 7.5 MHz linear array probe to investigate the role of peripheral nerve US in CMT suggested that the technique had limited utility in distinguishing CMT from healthy subjects[148].The diameter and echogenicity of nerves,but not the CSA,was evaluated and found not to differ from healthy controls,and the increased echogenicity of adjacent muscles made neural structures more difficult to assess in the CMT cohort.However,with the advent of higher frequency probes,which have improved US resolution,characteristic findings have been identified in subtypes of CMT.
CMT1A:In CMT1A,the most common demyelinating form of CMT caused by a duplication the peripheral myelin protein 22 gene,the CSA of proximal and distal nerves,plexuses and roots are typically found to be diffusely,uniformly and symmetrically enlarged,analogous to the typical uniformity seen in nerve conduction velocities (Figure 2A)[3,149-151].In general the CSA enlargement in CMT1A is greater than twice that of healthy controls (Figure 3D)[152].Furthermore,the CSA enlargement has been found to parallel clinical and electrodiagnostic parameters in CMT.Specifically,the CSA of the median nerve correlates with a CMT severity rating score,the CMT neuropathy score,and greater CSA correlates with reduced motorconduction velocities and compound muscle action potential amplitudes[153].Thus,suggesting that US findings reflect both the physiological function and disease severity in CMT1A.
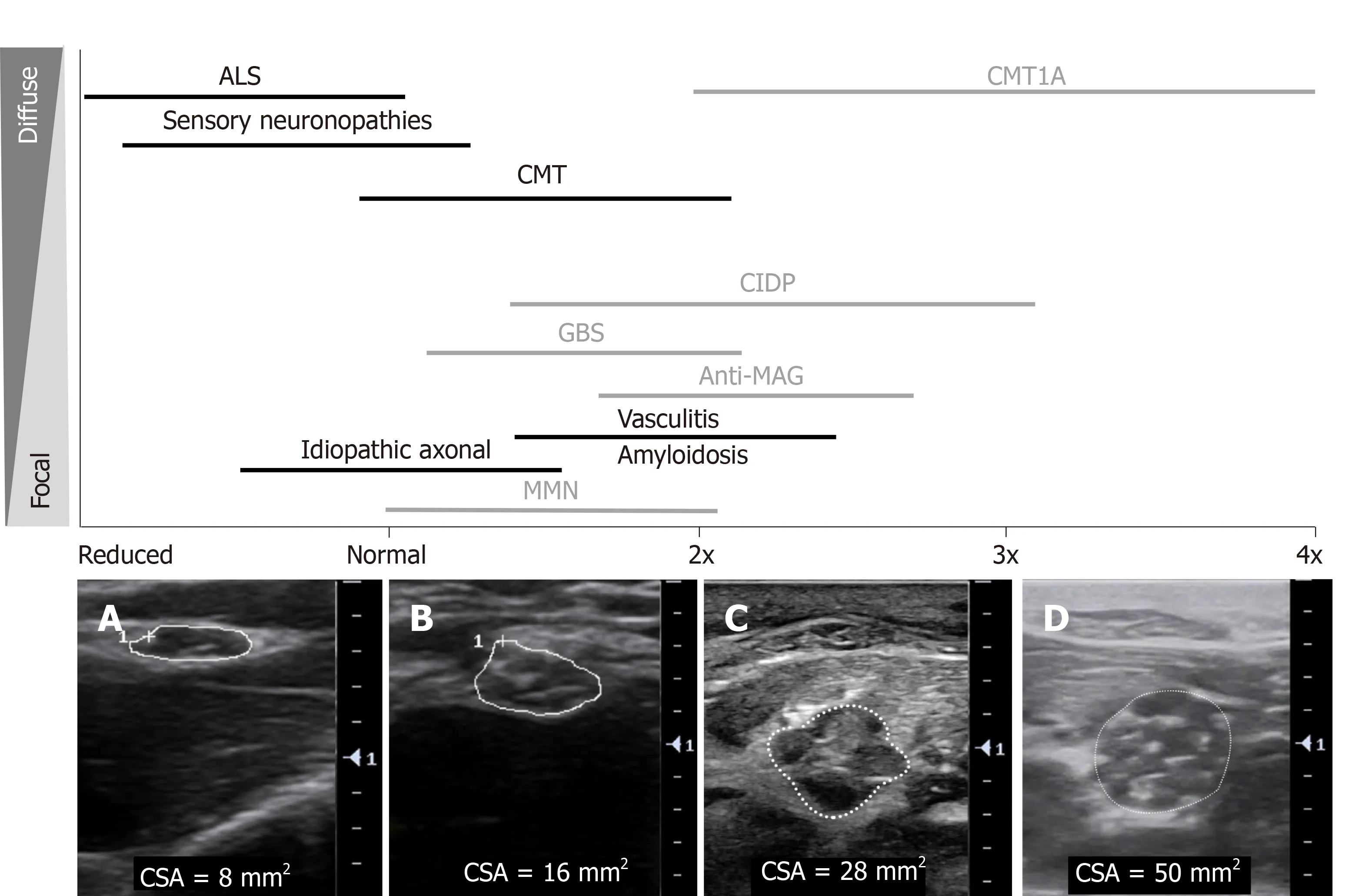
Figure 3 An overview of sonographic findings in inherited and acquired polyneuropathies:degree and distribution of nerve enlargement.Black:Axonal neuropathies,Grey:Demyelinating neuropathies.Cross-sectional area (CSA) shown in:A:Median nerve at the elbow in a patient with amyotrophic lateral sclerosis,CSA 8 mm2;B:Median nerve at the elbow in a healthy control,CSA 16 mm2;C:Median nerve in the forearm in a patient with chronic inflammatory demyelinating polyradiculoneuropathy,CSA 28 mm2,with heterogeneously enlarged,hypoechoic fascicles;D:Median nerve in the forearm in a patient with Charcot Marie Tooth disease type 1A,CSA 50 mm2,with more homogenously enlarged,hypoechoic fascicles.ALS:Amyotrophic lateral sclerosis;CMT1A:Charcot Marie Tooth disease type 1A;CMT:Charcot-Marie-Tooth disease;CIDP:Chronic inflammatory demyelinating polyradiculoneuropathy;GBS:Guillain-Barre syndrome;MAG:Myelinassociated glycoprotein;MMN:Multifocal motor neuropathy;CSA:Cross-sectional area.
However,variability has been identified in the CSA of the sural nerve in patients with CMT1A,with studies identifying either no,or significant,differences when compared with healthy controls,which may reflect differing techniques for CSA measurement[153,154].In addition,in CMT1A there is diffuse fascicular enlargement across nerves;however,within an individual nerve there is variability in the extent of fascicular enlargement[27,28].Nerves are reported to be more hypoechogenic and display no increase in vascularity[151].
CMT1A can be distinguished from other axonal and intermediate subtypes of CMT,including CMT2 and CMTX1,by a greater CSA or fascicular diameter of the median nerve and minimal variation between entrapment and non-entrapment sites[27,155].In addition,the CSA of the median,ulnar and peroneal nerves has been found to be significantly greater in CMT1A than in other demyelinating forms of CMT,including CMT1B,demyelinating CMTX and CMT4C;however,more overlap in CSA is observed[149].
CMT1B:In demyelinating CMT1B,due to mutations in Myelin Protein Zero (MPZ),the median and vagus nerve CSAs were enlarged compared to normal individuals[156].However,across the spectrum of CMT caused by mutations in MPZ,which can cause a demyelinating (CMT1B),axonal (CMT2I/2J) or intermediate (CMTDID)phenotype[157],the CSA of only the demyelinating subgroup was found to be enlarged in the median and ulnar nerves,in particular at proximal nerve sites[158].Similarly the C5 nerve root CSA was enlarged in both the demyelinating and intermediate groups[158].NMUS of lower limb nerves in CMT1B have consistently revealed reduced calibre nerves,with considerable overlap between the demyelinating,axonal and intermediate subgroups,thought indicative of a floor-effect due to length dependent axonal losses[156,158].Taken together,this suggests that peripheral nerve US comparing distal to proximal segments of upper limb nerves may be most useful to differentiate the subtypes of CMT associated with MPZ mutations,and that intranerve CSA variability could assist to distinguish CMT1B from CMT1A.
CMT1X:CMT1X,the second most common form of CMT,is an X-linked intermediate CMT associated with mutations in gap junction-associated protein B1[157].Initialreports,which evaluated median nerves to the mid forearm,did not find any significant differences between median nerve CSA and fascicle diameters in CMTX cohorts and healthy controls[27,150].However,more recent studies,which evaluated both proximal and distal sites in the upper and lower limbs,identified consistent symmetrical enlargement in proximal nerve segments and lower limb nerves[155,159].This finding was more pronounced in men,who are typically more significantly affected[159].
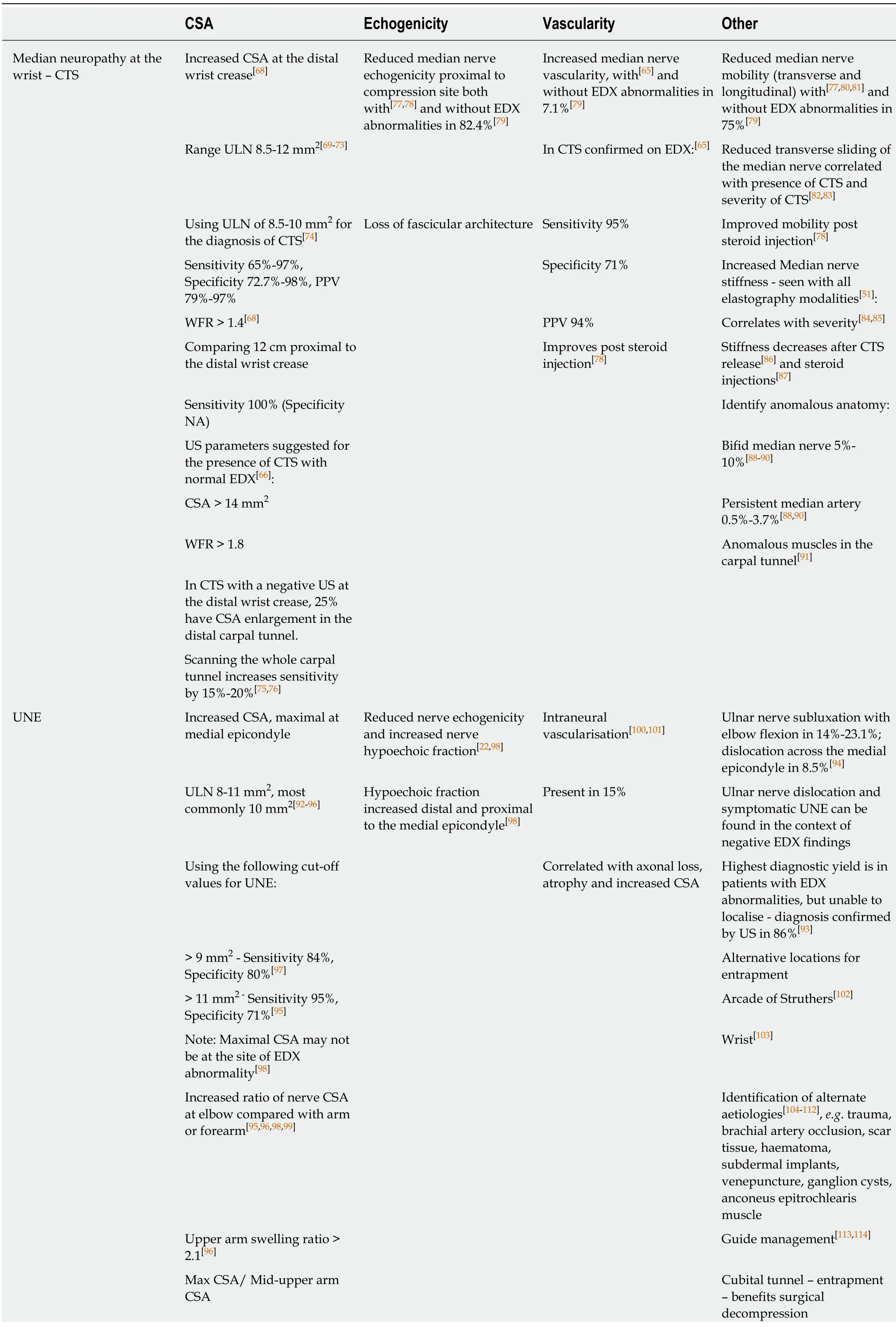
Table 1 Characteristic ultrasound findings in entrapment neuropathies

Sensitivity 60%,Specificity 74%Epicondylar groove -external compression or stretching - no benefit from surgery Forearm swelling ratio >2.3[96]Max CSA/ Mid-forearm CSA Sensitivity 65%,Specificity 79%,PPV 89.2%Radial neuropathy at the spiral groove or PIN Increased CSA[16,66,115,116]:Loss of echogenicity with PIN at level of maximal CSA[117]Intraneural hypervascularity at site of compression[118]Loss of fascicular structure with PIN at site of maximal CSA[117]Radial nerve in spiral groove and antecubital fossa - ULN 9-10 mm2 Provides additional information complementing EDX in 84%[116]Posterior interosseous nerve -ULN 1-2 mm2 Superficial radial nerve -ULN 1-3 mm2 Common peroneal neuropathy at fibular head Increased CSA at fibular head Loss of echogenicity at the site of maximal compression,with and without a normal CSA[121,124]NA Sensitivity for diagnosis[125]:90% (95%CI,79.7%- 97.3%)ULN 8-14 mm2,commonly 11 mm2[16,119-122]US localisation[120]:Using the following cut-off values for the common peroneal nerve at the fibular head:Fibular head 55%> 8 mm2 - Sensitivity 90%,Specificity 69%[120]Above fibular head 71%> 11.5 mm2 - Sensitivity 89%,Specificity 99%[121]Addition of US to EDX allowed for localisation in 88%,compared with 65.5%EDX alone[120]Suggested cut-offs:Alternate aetiology[16,126,127]:8 mm2 if abnormal EDX[120] Ganglion cysts (18% of cases)lipomas,scar tissue after knee surgery,abnormal biceps femoris anatomy 11 mm2 for normal EDX[120] Guides requirement for surgical intervention[128]CSA may be normal if conduction block,but no axonal loss,on EDX[123]LCFN - MP Increased CSA at anterior superior iliac spine[129,130]Intraneural hypoechogenicity[129]NA Anatomical variants in 20%[131]4-5 mm2[129,130] LFC nerve closer to/lateral to the Anterior superior iliac spine in patients with idiopathic MP[132,133]ULN 5 mm2 - Sensitivity 95.7%,Specificity 95.5%,PPV 95.7%[130]Neuroma[131]Increased nerve diameter 4 mm[129]Tibial neuropathy - TTS or Proximal tibial neuropathy Increased CSA at or distal to the tarsal tunnel (cf.median nerve) can be seen in the[134]:Intraneural and fascicular hypoechogenicity[135]NA Identification of underlying aetiology of TTS Tibial nerve,Medial plantar and Lateral plantar nerves Ultrasound identifies 60-80%[136,137]Using the following cut-off values for the tibial nerve within the tarsal tunnel[134]:Preoperative US assessment identifies[136,138-140]:Ganglia(7-58%),Talocalcaneal coalition +/- ganglia,Varicose veins (18- 6%),Accessory soleus and flexor digitorum muscles (6%)

CTS:Carpal tunnel syndrome;CSA:Cross-sectional area;ULN:Upper limit of normal;WFR:Wrist to forearm ratio;US:Ultrasound;EDX:Electrodiagnostics;PPV:Positive predictive value;UNE:Ulnar neuropathy at the elbow;PIN:Posterior interosseous syndrome;NA:Not available;LFCN:Lateral femoral cutaneous neuropathy;MP:Meralgia paraesthetica;TTS:Tarsal tunnel syndrome;LFC:Lateral femoral cutaneous;CI:Confidence interval.
CMT2:CMT2 is a heterogenous group,with axonal neurophysiology,comprising of both autosomal dominant and recessive mutations in over 52 genes[157].Axonal neuropathies are typically characterised by NMUS as having reduced or normal nerve dimensions,thought secondary to progressive axonal drop out[15].However,in CMT2 variability has been seen in CSA findings with some studies identifying upper and lower limb CSAs commensurate with normal controls[155,159],and others finding slightly increased median nerve CSA compared to normal individuals[27,150].This variability may be due to the differing pathological processes occurring in the diverse genetic subtypes of CMT2 with some,but not all,displaying endoneurial swelling,hyperplasia of Schwann cells or pseudo-onion blub formation on pathological specimens[160].
In summary,NMUS can be used to differentiate axonal and demyelinating forms of CMT,and further patterns are being identified to discriminate specific genetic subtypes.Recently a scoring system has been proposed to facilitate this distinctionusing differing combinations of distal,proximal and sural nerve CSAs as well as entrapment ratios to stratify CMT1A,CMT1B,CMTX and CMT2[159].Future studies in large cohorts will be required to assess the utility of this score in differentiating between genetic subtypes of CMT,and between CMT and acquired neuropathies.In the future NMUS will be increasingly useful as a non-invasive test in children with suspected CMT and can also be used,with EDX,to guide targeted gene testing.In addition,NMUS could be used as a disease monitoring tool for clinical trials,in particular in the setting of absent motor or sensory potentials or severe muscle atrophy.A study of quantitative muscle US demonstrated that the thickness and echogenicity of the first dorsal interosseous and tibialis anterior correlated with disease severity,and this too could be used as a surrogate marker of disease progression in clinical trials[161].
HNPP:HNPP,previously known as tomaculous neuropathy due to the focal thickening of myelin (tomacula) seen on nerve biopsy,is characterised by recurrent,painless mononeuropathies at sites of entrapment[157].Multiple,focal enlargements of nerves,without increased vascularity,have consistently been identified in HNPP at typical sites of entrapment[151,155,159,162-165].However,some studies have also identified enlarged CSA at sites of non-compression,even in clinically unaffected nerves[164].Paduaet al[155]identified ulnar nerve CSA enlargement in all patients with HNPP in their cohort and suggested more frequent involvement of the ulnar nerve,than the median nerve,in HNPP.
In most cases the CSA does not correlate with electrophysiologic or clinical characteristics[162].Specifically,Gianneschiet al[165]found no sonographic changes in the distal nerves despite increased distal motor latencies.However,in one case report digital nerve enlargement has been reported[166],suggesting that further US evaluation of the distal nerves may be worthwhile in future studies.HNPP may be distinguished from forms of CMT through the use of entrapment ratios,with a CSA of the compressed nerve segment to non-compressed segment ratio greater than 1.4[150,151,159]and normal CSA at non-compressed areas.This is particularly evident as CMT1A and MPZ-associated CMTs do not typically display significant nerve enlargement at entrapment sites[149].
ATTRv amyloidosis:ATTRv amyloidosis,caused by over 130 point mutations in the transthyretin (TTR) gene,is an inherited autosomal dominant condition characterised by cardiomyopathy,frequent CTS,and an axonal,sensorimotor,peripheral neuropathy involving the large,small and autonomic nerve fibres.TTR is produced predominantly in the liver and is excreted as a tetramer,with roles in transport of Vitamin A and Thyroxine.Point mutations destabilise the molecular structure of TTR facilitating dissociation into monomers and amyloid fibril formation.Pathologically the amyloid fibrils accumulate in the extracellular space causing damage to involved tissues.Sural nerve biopsy specimens confirm intraneural amyloid accumulation with associated axonal loss.
As such,and in contrast to other typical axonal neuropathies,NMUS is expected to identify thickened peripheral nerves.Initial studies of MRI Neurography in ATTRv amyloidosis have confirmed this hypothesis (Figure 2F)[167,168].An initial study of NMUS,by Granataet al[169],of 7 patients with ATTRv identified multifocal enlargements of both distal and proximal upper limb nerves,with a normal NMUS in 1 patient with only mild sensory polyneuropathy.Interestingly the number of nerves involved on NMUS correlated with disease severity[169].Further studies in larger cohorts have confirmed significantly larger CSA in both the upper and lower limbs,with most dramatic enlargements at entrapment sites and in proximal nerves[170].Asymptomatic ATTRv carriers were compared with symptomatic ATTRv patients and healthy controls and found to have intermediate CSA,particularly in proximal nerve segments[170].Median CSA enlargement was found to correlate with reducing compound muscle action potential amplitudes[170].Ongoing research is required to confirm these initial findings;however,it is expected that NMUS could be a useful tool for monitoring progression and response to treatments in ATTRv amyloidosis.
Sensory neuronopathies
Cerebellar ataxia,neuropathy,CANVAS:CANVAS is an increasingly recognised cause of late onset ataxia with cardinal features of cerebellar ataxia,bilateral vestibulopathy and a sensory neuronopathy[171].An intronic repeat expansion(AAGGG) of the replication factor C subunit 1 gene has recently been described with a carrier frequency of 0.7% in Europeans[171].Pelosiet al[172]have evaluated the median,ulnar,tibial and sural CSAs in CANVAS patients finding significantly smaller CSAs compared with both normal controls and axonal,acquired neuropathy controls.When compared to the healthy control population,the reduction in CSA in individuals withCANVAS was sufficient to detect all but one case (93%,13/14)[172].Thus,NMUS has been proposed as a potential diagnostic tool for the sensory neuronopathy seen in CANVAS[172].A follow-up study has identified that the reduction in CSA,with a cutoff of < 5 mm2for the median and ulnar nerves at the mid-forearm and mid-humerus,has high diagnostic accuracy to discriminate CANVAS patients from healthy controls(AUC ROC 0.97-0.99)[173].
Pathological studies in CANVAS have demonstrated marked atrophy of dorsal root ganglia with corresponding near-complete neuronal loss and posterior column atrophy[174].Furthermore,sural nerve biopsy specimens in CANVAS display severe axonal loss without Schwann cell proliferation or active Wallerian degeneration[175].Suggesting that the reduction in the CSA in CANVAS is due to profound axon depletion without marked connective tissue replacement,as can be seen in some types of CMT2.
SCA:Spinocerebellar ataxia type 2 (SCA2),caused by an autosomal dominant,CAG triplet expansion in the Ataxin 2 gene,is a cerebellar plus syndrome characterised by cerebellar ataxia,sensory and/or motor peripheral deficit,pyramidal signs,extrapyramidal features and sleep disorders.Peripheral nerve involvement is thought to be present in 92% of individuals with SCA2[176],with reduced sensory action potentials seen in asymptomatic carriers up to 8 years before manifestation of cerebellar signs[177].However,it is uncertain if the sensory and motor involvement is due to a neuropathy or neuronopathy.Thus,the NMUS findings seen in CANVAS has led to further interest in US and pathological characteristics of the sensory deficits seen in SCA2.In 7 selected patients with genetically confirmed SCA2 and a sensory,axonal,non-length dependent neuropathy,Leadbeatteret al[173]identified that SCA2 patients had similarly reduced nerve CSAs to the CANVAS cohort.Specifically,all patients met the CANVAS diagnostic cut-off criteria (< 5 mm2) for the median forearm site and all but one (86%) met criteria for the median-mid humerus and ulnar sites[173].In a larger study 74% (20/27) of patients with SCA2 had reduced upper limb CSAs[178].Of the remaining 7,2 had increased and 5 normal range upper limb CSA[178].Interestingly none of the patients with small nerves had a definite length-dependent sensory neuropathy,and conversely none of the patients with a definite non-length dependent sensory neuropathy had normal or enlarged nerves[178].Taken together,this suggests that in SCA2 there may be multiple pathological contributors to the sensory deficits,including both a dorsal root ganglionopathy and peripheral neuropathy.Furthermore,these findings demonstrate that the diffuse reduction in CSA reflects a dorsal root ganglionopathy causing a sensory neuronopathy,rather than being specific for a particular disease process.
FRDA:FRDA,caused by a GAA trinucleotide repeat expansion in the frataxin gene,is the most common autosomal recessive ataxia syndrome.FRDA typically presents with cerebellar ataxia,cardiomyopathy,diabetes and skeletal malformations.Sensory deficits are common,and similar to SCA2,this may be explained by either a dorsal root ganglionopathy or peripheral neuropathy.Pathological studies have demonstrated both structurally abnormal and atrophied dorsal root ganglia and posterior columns,as well as hypomyelination on sural nerve biopsy specimens[179,180].
Interestingly Mulroyet al[181]found that in FRDA almost all tested upper limb nerves had significantly larger CSAs and common fascicular enlargement.The CSA was typically 2 standard deviations greater than the CSA of normal controls[181].However,there was no difference in the lower limb CSA in the FRDA patients compared to controls.The enlargement of CSA in the upper limbs may be due to perineural connective tissue infiltration,inflammation and dysmyelination with impaired Schwann cell interactions[182].Whereas the normal CSA seen in lower limbs may reflect the more pronounced caudal ganglionopathy as well as peripheral nerve processes[181].Further NMUS in FRDA,in particular at earlier disease stages,will be useful to delineate the pathogenesis of these findings.
Immune-mediated peripheral nerve disorders
CIDP:Clinical manifestations of typical CIDP are symmetrical,proximal and distal weakness,sensory disturbance and hypo- or areflexia.Pathological nerve specimens characteristically display focal and segmental demyelination,with subsequent remyelination,leading to the formation of onion bulbs,accompanied by endoneurial inflammation and oedema[183].As such NMUS was hypothesised to show multifocal enlargements of CSA in proximal and distal segments with hypervascularity.While the majority of patients do indeed have enlargement in CSA on NMUS,in both proximal and distal segments and at non-entrapment sites[15,184-189],there is marked variability with some patients having normal or mildly enlarged nerves (Figures 2B,2D and 3C)[190].Paduaet al[190]describe three different classes of sonographic findings,which correlate with disease duration.Specifically,Class 1 nerves have increased CSA with hypoechoic fascicles,Class 2 increased CSA with mixed hypo- and hyper-echoic fascicles,and Class 3 normal CSA with hypoechoic fascicles.The latter class was associated with more prolonged disease durations.Hence it is suggested that NMUS findings may the parallel disease stages and reflect the underlying pathophysiology in CIDP.This hypothesis is further supported by studies which have found improvement in nerve calibre in patients who reach clinical and electrophysiological remission after treatment for CIDP[185].
Hypervascularity of nerves can be seen in CIDP and correlates both with the number of nerves with enlarged CSA and cerebrospinal fluid (CSF) protein levels[38].Similarly the calibre of cervical nerve roots,which are commonly enlarged in CIDP,correlates with CSF protein levels and disease duration[186,191].Taken together,both the vascularity and number of enlarged nerves may be used in the future as objective measures of disease activity.
CSA negatively correlates,in some studies,with nerve conduction velocity[15,187,189];however,this has not been ubiquitously identified[184,192].This likely,in part,reflects the heterogeneity of US and electrodiagnostic protocols,as well as disease duration,treatment exposure and response.Further studies with more homogenous inclusion criteria will be required to delineate these findings.
Differentiation of hereditary and acquired demyelinating neuropathies and between acquired demyelinating neuropathies has recently been a focus of investigation.The major distinguishing feature between NMUS in CMT and CIDP is non-homogeneity of CSA enlargement[152].As such the use of intranerve and internerve CSA variability has been proposed,and is significantly larger in CIDP than CMT[158,193,194].A multitude of additional US rating scores have been proposed to aid the differentiation of acquired and hereditary demyelinating neuropathies including the ultrasound pattern sum score (UPSS),homogeneity score,regional nerve enlargement index,Bochum US score,and nerve abnormality index[155,188,195,196].The usefulness of these scores will require further investigation in large cohorts in order to rationalise and standardise use internationally.
Guillain-Barre syndrome:Similar to CIDP,NMUS in Guillain-Barre syndrome (GBS)typically identifies proximal more so than distal enlargement of nerve calibre.Although the degree of enlargement of CSA is less than both CMT1A and CIDP,and is patchier[15,197].Again marked variability in CSA findings are seen with the percentage of patients with enlarged CSA ranging from 0%-67%[15,152,195,197].This likely reflects heterogeneity in the clinical populations included in studies comprising of both axonal and demyelinating forms,and at differing time points of disease.Nerve enlargement can be identified as early as 1 d after onset and can be seen prior to neurophysiological changes[15,198].Persistent nerve enlargement has been reported up to 15 years,often not correlated with disability,although other reports suggest normalisation of NMUS findings with clinical remission[15,199-201].Studies evaluating NMUS across the temporal evolution of GBS are required to solidify the spectrum of normal findings in GBS.If early nerve root enlargement is consistently confirmed,NMUS could allow early diagnosis of GBS to be made,and hence limit morbidity.
A few studies have compared axonal and demyelinating forms of GBS.Razaliet al[201]found no significant differences in CSA between acute demyelinating polyneuropathy (AIDP) and acute motor (and sensory) axonal neuropathy(AMAN/AMSAN).In contrast,Moriet al[202]identified different patterns of disease with AIDP having enlarged cervical roots and proximal nerve segments,and AMAN enlarged distal segments.Enlarged vagus nerve CSA has been identified in patients with autonomic dysfunction in both demyelinating and axonal forms of GBS[188,198].
The UPSS has been proposed to differentiate CIDP,GBS and axonal neuropathies.In the UPSS enlarged cervical and vagal CSA,but minimal-moderate enlargements in the upper and lower extremity nerves is thought suggestive of GBS,compared with more marked increases in limb nerve CSA and total scores being reflective of CIDP[188].An alternate score,the Bochum ultrasound score,evaluates the CSA of the ulnar nerve at Guyon's canal and in the arm,the radial nerve at the spiral groove and the sural nerve at the calf,giving each one point if enlarged compared to normal controls[195].A cut off score of greater than 2 had a sensitivity of 90% and specificity in 90.4% in distinguishing CIDP from GBS.Further studies are required to assess the utility of scoring systems in distinguishing these inflammatory neuropathies.
Multifocal acquired demyelinating sensory and motor neuropathy:In Multifocal acquired demyelinating sensory and motor neuropathy (MADSAM),a variant of CIDP characterised by asymmetric and patchy sensorimotor impairments and neurophysiology,NMUS typically demonstrates multifocal CSA enlargement at sites of current or resolved conduction block[203-205].In one case these nerve swellings appearto be treatment responsive,with early and complete clinical and electrophysiological remission after intravenous immunoglobulin[206].Similar to CIDP,nerves in MADSAM are hypoechogenic with enlarged hypoechogenic nerve fascicles at sites of maximal CSA (Figure 2C)[203],and vagus nerve enlargement can be seen[207].However,nerve enlargements in MADSAM are more asymmetrical,heterogenous,and segmental than commonly seen in CIDP.Taken together these findings suggest that US parameters could be used in the future as a biomarker of ongoing disease activity,treatment response and prognosis in MADSAM.
MMN:MMN is a slowly progressive,immune-mediated,upper limb predominant motor neuropathy with conduction block,at sites of non-compression,typically seen on neurophysiology.MMN can be difficult to diagnose often requiring extensive proximal neurophysiology to identify demyelination and conduction block to distinguish it from mimics including amyotrophic lateral sclerosis (ALS),progressive muscular atrophy and pure-motor CIDP.NMUS has been recently evaluated as a tool to differentiate these entities.Studies have predominantly focused on CSA,identifying in the vast majority of patients with MMN moderate,segmental nerve enlargement primarily in proximal nerves,and at sites with and without neurophysiological evidence of conduction block[29,152,208-210].The presence of multifocal increases in nerve calibre distinguishes MMN from ALS and normal controls,with sensitivities of 87%-100% and specificities of 92%-100%[208,209,211].A recent comparison of MR Neurography and US,demonstrated that US was better able to distinguish inflammatory neuropathies,including CIDP and MMN,from segmental SMA (sSMA)by identifying proximal CSA enlargements[212].As such,US has been proposed as a tool to aide treatment decision making[213].The NMUS of CIDP and MMN are more similar,both showing multifocal nerve enlargements;however,studies attempting to differentiate these entities has identified greater increases in CSA in CIDP and greater intra-nerve,inter-nerve and side-to-side intra-nerve variability in MMN[193,214,215].
Attempts to prognosticate with NMUS has led to inconsistent results[209,210,215];however,some studies suggest that CSA reduces with more severe disease,as a correlate to axonal loss,signifying that serial NMUS in individuals with MMN could possibly play a role in prognostication and guide treatment escalation[29,214].Further evaluations in more homogenous MMN cohorts,with serial measurements prior to,and after treatment will be required to further delineate the role of NMUS in diagnostic and treatment algorithms.
Brachial neuritis:Brachial neuritis,also known as neuralgic amyotrophy or Parsonage-Turner Syndrome,is an idiopathic inflammatory condition affecting the motor and sensory nerves of the brachial plexus heralded by severe neuropathic pain.Precise localisation of a plexus lesion is often difficult with EDX alone,and NMUS has the potential to more precisely localise pathology within the plexus and identify possible compressive lesions.Initial NMUS studies suggest median,isolated anterior interosseous or posterior interosseous enlargement on the affected side compared to both healthy controls and the individuals unaffected side[216,217].A detailed study by Aranyiet al[218]has identified more varied pathologies including focal or diffuse nerve and fascicle enlargement in 57%,incomplete nerve constriction in 36%,complete nerve constriction with hour-glass torsions in 50% and fascicular entwinement in 28%.The torsions seen on NMUS were confirmed surgically,and were present in the radial nerve in 85% of cases.It is hypothesised that in brachial neuritis the nerve first enlarges,with ongoing inflammation adhesions and fixation of the fascicles cause it to constrict,and then with rotational movements of the limb nerve torsion develops.It is suggested that fascicle entwinement is most likely analogous to nerve torsion.The presence of constriction,torsion or fascicular entwinement correlates with limited functional improvement with conservative measures alone,and may even require surgical intervention[219].This suggests an important and management altering role for NMUS,or alternate imaging,in the acute evaluation of individuals with brachial neuritis.
Vasculitic neuropathy:In both systemic and isolated peripheral nerve vasculitis the typical neuromuscular presentation is of a mononeuritis multiplex due to inflammation of epineurial vessels causing an ischaemic insult to affected nerves.On NMUS focal,asymmetrical enlargement of nerve CSA can be seen in 51-71% of clinically and neurophysiologically affected nerves,with nerve hypoechogenicity and enlargement of single fascicles[39,220-223].ü?eyleret al[224]identified preferential involvement of the sural and superficial peroneal nerves.Multifocal nerve enlargement proximal to compression site was 94% sensitive and 88% specific for vasculitic neuropathy in one cohort[222].Hypervascularity has also been seen in a smaller proportion of patients with vasculitic neuropathy[222].Goedeeet al[222]proposedthat in an axonal neuropathy,multifocal enlargement of nerves proximal to entrapment sites,with sparing of the brachial plexus,may be characteristic of vasculitic neuropathies.Limited published data exists for the NMUS response to treatment;however,in one case study treatment resulted in reduced calibre of single nerve fascicles and the overall nerve,although no changes were seen in nerve echogenicity[220].
In summary,NMUS may facilitate early diagnosis,and hence earlier treatment commencement in vasculitic neuropathies,and could be used to identify optimal sites for nerve biopsy.Further studies are required to evaluate the responsiveness of NMUS to treatment and to solidify the pattern of nerve involvement in vasculitic neuropathy,in particular in comparison to other axonal neuropathies with enlarged nerve CSA,such as hereditary ATTRv amyloidosis.
Anti-myelin-associated glycoprotein neuropathy:Neuropathy associated with antimyelin-associated glycoprotein (MAG) antibodies is typically described as a distal acquired demyelinating symmetric phenotype,with characteristic prolongation of distal motor latencies on neurophysiology.One study has evaluated the NMUS properties of anti-MAG neuropathies showing segmental,but not distal,increased nerve CSA,with greater intranerve than internerve variability[225].The US phenotype was intermediate between axonal paraproteinaemic neuropathy controls and IgM paraprotein-associated CIDP.More recently Garget al[226]demonstrated no distal nerve enlargement,but significant proximal nerve enlargement compared to healthy controls,specifically of the C6 nerve root and upper trunk of the brachial plexus.To date enlarged distal nerve calibre has not been identified,which is at odds with the characteristic neurophysiological findings.This suggests that the proximal nerve segments may be more involved than previously thought and possibly underestimated by neurophysiological tests due to the prominent distal nerve slowing.
Polyneuropathy,organomegaly,endocrinopathy,M-protein,skin changes syndrome:The neuropathy of polyneuropathy,organomegaly,endocrinopathy,Mprotein,skin changes (POEMS) syndrome is characteristically of mixed axonal and demyelinating neurophysiology,with motor predominant distal weakness affecting dorsiflexion more so than plantarflexion[227].Early in the disease course,the neuropathy of POEMS can mimic CIDP,with more predominant demyelinating features.In contrast to CIDP,US of distal and proximal limb nerves identified enlargement of CSA at sites of entrapment in POEMS,but not at other sites along the nerves[228].It must be noted that in all studied patients there were clinical and neurophysiological signs of secondary axonal degeneration at the time of NMUS.Concordant with this,a large neve biopsy series recently demonstrated higher axonal degeneration,minimal endoneurial inflammation and higher numbers of epineurial vessels in POEMS than CIDP;however,the timing of biopsy after disease onset is not clear[229].While the pathophysiology of neuropathy is incompletely understood,it is thought associated with increased neovascularisation due to elevated circulating vascular endothelial growth factor,and impaired blood nerve barrier causing increased neural oedema[229].Thus,it would be postulated that in early disease increased CSA and vascularity may be appreciated.Further studies in the early diagnostic setting will be required to further evaluate the NMUS features of POEMS syndrome and may assist in clarifying the underlying pathophysiology.
Neurolymphomatosis:Nerve infiltration by lymphoma,or neurolymphomatosis,typically causes focal mononeuropathies or plexopathies,and can be the presenting symptom or site of recurrence of systemic lymphomas.Case reports and series have demonstrated enlarged nerve calibre of affected nerve segments,hypoechoic echotexture,with enlarged fascicles and hypervascularity suggestive of infiltration and neovascularisation[37,230].Further studies are required;however,in the future NMUS may play a role in diagnosis,surveillance and optimisation of nerve biopsy in neurolymphomatosis.
Motor neuron diseases
Diseases of motor neuron degeneration include ALS,and SMA,which differ in age of onset and aetiology.ALS is typified on NMUS by reduced distal and proximal nerve calibre (Figure 3A)[231-235].Analogous to what has been demonstrated in the pure sensory neuronopathies,this is hypothesised to be due to motor axon loss due to anterior horn cell degeneration.In addition a recent study identified increased wristupper arm ratios in ALS compared with controls,and a combination of reduced CSA and distal-proximal ratios had diagnostic value in distinguishing ALS from mimics[235].
In contrast,while not extensively studied to date,NMUS in sSMA hasdemonstrated reductions in CSA in the proximal nerve segments and nerve roots only,when compared to the CIDP and MMN[212].A feature which could,if confirmed in additional studies,be used to differentiate adult onset SMA from ALS.Ultra-high frequency US has been used in a small cohort of SMA types I,II and III patients,and controls to evaluate nerve area,fascicle numbers and densities[26].Interestingly differences were only observed in fascicle number between the SMA I patient and controls,suggesting that alterations in the number and density of fascicles could be used to prognosticate in SMA[26].However,further studies in larger cohorts are required to confirm this finding.
Schreiberet al[232]assessed the CSA of the median and ulnar nerves at the wrist and forearm in ALS subtypes,finding reduced ulnar,but not median CSA,in all ALS subtypes except for primary lateral sclerosis (PLS).This suggests the possible value CSA measurements as an indicator of lower motor neuron dysfunction in ALS,which has prognostic implications in particular in distinguishing upper motor neuron dominant ALS from PLS.As mentioned previously NMUS may also play a role in distinguishing potentially treatable lower motor neuron diseases,such as MMN,from non-treatable,with significant prognostic implications.
Beyond the scope of this review,but relevant in motor neuron disorders,is the use of muscle US,which has been more extensively investigated in ALS and SMA.In brief,muscle US identifies patchy increased echogenicity and atrophy in these conditions,and in some studies correlates with strength[231,233,236-238].US is a more sensitive tool than EMG for identifying fasciculations,in all muscles;however,particularly in the tongue[238-242].Hence muscle US plays an important role in ALS diagnostics as a biomarker of lower motor neurone involvement,and may allow earlier diagnosis[238,239].In addition identification of widespread fasciculations,using a score of 9 muscles,can assist to distinguish ALS and mimics with a 92% sensitivity and 100% specificity[241].
Muscle US also allows the evaluation of more difficult to assess muscles,including the tongue and diaphragm,where EMG is more difficult or has potential side effects[242].This is particularly important in ALS,where dysfunction of these muscles has prognostic implications.For example the alteration in diaphragm thickness during inspiration and expiration correlates with respiratory function measures in ALS including forced vital capacity,maximum voluntary ventilation and sniff nasal inspiratory pressure,as well as diaphragm compound muscle action potential[243].A recent longitudinal study has demonstrated that serial US measurements can more accurately predict the need to initiate non-invasive ventilation when compared with respiratory function tests[244].In addition,dynamic assessment of the bulbar muscles,by evaluating the maximum thickness of the mylohyoid-geniohyoid-muscle-complex during swallowing and at rest,can be performed and correlates with the severity of upper motor neuron involvement in ALS[245].As such,NMUS will likely become an increasingly utilised non-invasive longitudinal biomarker in ALS,with use in both the diagnostic and prognostic settings.
Axonal neuropathies
To date NMUS has limited utility in axonal neuropathies.It was anticipated that,similar to ALS,NMUS findings in axonal neuropathies would identify reduced CSA of nerves.Instead normal or mildly enlarged nerve calibres are typically seen[15,39].In all subtypes of diabetic peripheral neuropathy (DPN),for example,there is mild enlargement of nerve CSA,fascicular enlargement and loss of the normal fascicular architecture,with particular enlargements seen at sites of compression[246-251].Nerve enlargement can also be seen in diabetics prior to the development of DPN[251].In some studies the CSA enlargement,thought due to increased water content,has negatively correlated with both compound muscle action potential amplitudes and motor nerve conduction velocities[247,250].Increased vascularity has also been demonstrated in diabetic nerves,and corelate with nerve calibre,sensory symptoms and the severity of neuropathy[49].Recently shear wave elastography has been assessed in the tibial nerves of healthy controls,patients with diabetes and diabetic neuropathies,finding a continuum of increasing stiffness[252].A cut-off value of 51 kPa was found to be highly specific and sensitive for the diagnosis of DPN[252].
Similarly,in chemotherapy associated neuropathies focal enlargements at sites of nerve entrapment is observed[253,254].This can be seen in asymptomatic individuals,suggesting the chemotherapeutics may increase the susceptibility of nerves to additional damage[253].While these changes are subtle,these findings suggest that NMUS may play a role in monitoring for increasing susceptibility to nerve damage,and hence may guide treatment alterations.
In axonal neuropathy associated with monoclonal gammopathy of uncertain significance and multiple myeloma typically no nerve enlargement is found[255,256].However,in two cases where nerve enlargement was identified in this axonal cohort,management was altered to include immunotherapy with a beneficial outcome[255],suggesting that nerve enlargement may also facilitate therapeutic decision making in these paraproteinaemic axonal neuropathies.However,in general the role of NMUS in the diagnosis,management or prognostication of multiple axonal neuropathies,including due to infections,inflammatory,toxic or metabolic insults,is not yet known.Future studies are required delineate the role of NMUS in these contexts.
THE CURRENT AND FUTURE ROLE OF NMUS IN THE MANAGEMENT OF PERIPHERAL NERVE DISORDERS
NMUS is now commonly used to guide procedures such as peripheral nerve blocks,steroid injections for entrapment neuropathies and botulinum toxin injections for spasticity and dystonia.There is growing interest in its use for guidance of nerve conduction and EMG.The use of NMUS to determine the optimal site for nerve and muscle biopsy and lumbar puncture is currently gaining further traction.In addition NMUS has more recently been used in the intensive care setting to identify postoperative diaphragmatic palsies,and hence is a useful tool to predict and prevent respiratory decline after extubation[257,258].
NMUS is also increasingly being used to guide surgery.NMUS has been used to identify normal neural tissue pre- and intra-operatively in the resection of nerve sheath tumours and after trauma,hence allowing the identification and informed consent for the risk of iatrogenic nerve damage,and guiding optimal micro- or macrosurgical approaches[20,259,260].In addition,pre-operative identification of nerve position and anatomical variants with NMUS can prevent iatrogenic transection,as can occur in the sural nerve,for example,during small saphenous vein stripping,and can guide further management including transposition and neurolysis[261].Furthermore,NMUS frequently identifies true entrapment of the interrogated nerve,in particular in the ulnar nerve at the elbow or peroneal neuropathy at the fibular head,and hence can stratify patients who can be managed conservatively versus those who require surgical decompression[113,114,128].
New techniques are also being developed using US technology for the management of peripheral nerve disorders.US guided minimally invasive carpal tunnel release is increasingly being used,in both surgical and primary care settings,for rapid,carpal tunnel decompression with good clinical outcomes[262-265].Specifically,these techniques allow concurrent bilateral decompressions,earlier functional recovery and less postoperative morbidity with equivalent neurological outcomes to mini-open carpal tunnel release[262-264].In addition pulsed,low-frequency US has been used for noninvasive neuromodulation in compressive neuropathy and radiculopathy reducing pain and improving quality of life in affected individuals[266].With the greater availability of NMUS,ongoing technical advances and expanding interest in the techniques,the use of NMUS for therapeutic intervention will most likely dramatically increase over the coming years.
PRACTICAL CONSIDERATIONS
NMUS is a rapidly expanding area of clinical practice and research,with significant potential to alter diagnostic and treatment paradigms in neuromuscular medicine.However,there are barriers to mainstream uptake of the technology including substantial costs of equipment,adequate teaching experience and current gaps in knowledge and applicability.Recent developments in technology have begun to reduce the financial strain associated with US unit purchase,with more economical laptop-based units and US integrated into EMG machines providing adequate resolution for the evaluation of nerves in most regions of interest.Once equipped,NMUS in mononeuropathy cohorts is reported to improve quality of life outcomes,with only modest outlays on cost-effectiveness analyses[267,268].While these initial studies suggest a positive benefit-outlay ratio,the overall cost-effectiveness of NMUS for diagnostics,monitoring and prognostication in neuromuscular diseases is not yet known.However,it seems reasonable that the initial equipment outlays are warranted in the context of improved patient care[269].
The availability of training in NMUS is another potential barrier for the widespread uptake of peripheral nerve US.At present most training is undertaken though a practical traineeship supervised by a skilled mentor and supplemented with intermittent courses.However,the availability of apprenticeships,in particular in some geographical areas,is limited,and there is no standardised curriculum or teaching structure.The American Association of Neuromuscular andElectrodiagnostic Medicine has developed guidelines to direct training in both clinical and technological aspects of NMUS[270].However,standardisation,assessment and accreditation processes should also be developed to provide certification for both supervisors and training sites.
Similarly,standardisation of processes and methodology for investigation of polyneuropathies would be worthwhile to aid research,allowing more robust comparisons to be made between sites.Furthermore,there has been a vast proliferation of scoring systems made in parallel,for different neuromuscular indications,which can lead to confusion and ultimately requires further rationalisation.In the future meta-analyses integrating geographically separate,but comparable,data and consensus scoring systems should be advocated for in order to improve our collective understanding of NMUS in different disease processes and in different ethnic groups.
Finally,Medicolegal issues may arise in NMUS if pathologies are missed,incorrectly reported or over-reported leading to morbidity or mortality.This is particularly the case for non-neural tissues.As such,it is recommended that NMUS reports stipulate that evaluation was limited to neuromuscular structures,and that any atypical features in other tissues identified should be further assessed by experienced radiologists with additional imaging modalities[269].
CONCLUSION
NMUS is becoming an integral component of the diagnostic and management algorithm in neuromuscular medicine.As a non-invasive and accessible tool,it provides excellent resolution and dynamic assessment of neural structures.Hence adding much needed information about structure and function to clinical examination and EDX.Multiple studies have shown a role for NMUS in the diagnosis,monitoring and management of peripheral nerve disorders,and the applications of NMUS continues to expand.Recognition of the patterns of sonographic changes in differing aetiologies of peripheral neuropathy may assist to streamline diagnostic processes,improving time to diagnosis,accrued disability and health-care expenditure.Further exploration of novel techniques for the evaluation of peripheral nerves,may provide additional insights into the underlying pathophysiology of peripheral nerve diseases,and targets for therapeutic modification.Increasing standardisation of existing techniques and the creation of consensus scoring systems will allow improved reproducibility of findings,and greater applicability of results to clinical reasoning.Improved understanding of current and novel NMUS techniques will provide increasing insights into the pathophysiology of nerve diseases,and comparisons with clinical,electrodiagnostic and histopathological characteristics is necessary to clarify the roles of NMUS in the future.
 World Journal of Radiology2020年6期
World Journal of Radiology2020年6期
- World Journal of Radiology的其它文章
- Multi-modality imaging of cardiac amyloidosis:Contemporary update
