Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
Julien Pestel, Maud Robert, Hubert Vidal, Assia Eljaafari
Julien Pestel, Maud Robert, Hubert Vidal, Assia Eljaafari, INSERM U1060 CarMen, Batiment CENS-ELI, Centre Hospitalier Lyon Sud, Pierre Bénite 69310, France
Julien Pestel, Maud Robert, Hubert Vidal, Assia Eljaafari, Faculty of Medicine, Université Claude Bernard Lyon 1, Batiment CENS-ELI, Centre Hospitalier Lyon Sud, Pierre Bénite 69310, France
Maud Robert, Department of Surgery in Gastro-enterology, Edouard Herriot Hospital, Lyon 69003, France
Sara Corbin, Public Health Department, Hospices Civils de Lyon, 1 quai des célestins Lyon 69002, France
Assia Eljaafari, DO-IT Research Team, Hospices Civils de Lyon, 1 quai des célestins, Lyon 69002, France
Abstract
Key words: Interleukin 17 secreting T helper cells; Lean adipose tissue; Type 1 diabetes; Advanced glycation end products; Adipose-derived stem cells
INTRODUCTION
Glycated proteins result from non-enzymatic Maillard reactions between sugars and amine residues, mostly lysine and arginine[1].While in the healthy body all proteins can be modified by non-enzymatic glycation reactions, advanced glycation end products (AGE) are known to exert deleterious effects on human health when they are too abundant, as observed in diabetes, arteriosclerosis, renal failure and also in Alzheimer, and Parkinson diseases[2-4].Although glycated haemoglobin is a major biomarker for diabetes mellitus diagnosis[5], the role of glycated albumin as a potential diagnostic marker[6]is currently under investigation, due to the higher levels of albumin in blood, its shorter life, and its independence from haemolytic processes[7-9].In addition to modifications of protein structure and function, AGE pathogenic effects mostly result from binding and activation of specific receptors, named receptor of advanced glycated end products (RAGE)[10,11].Those receptors belong to the immunoglobulin superfamily of transmembrane proteins[12].Besides AGE, RAGE can bind a variety of molecules, such as the high mobility group box-1, the β-amyloid peptide and the S100/calgranulin[13].Interaction of RAGE ligands with RAGE, initiate a cascade of signalization leading to activation of p21ras, p44/p42 mitogen-activated protein kinases and nuclear factor-kappa B (NFKB), which generally results in the synthesis of proinflammatory cytokines[14-16].The implication of AGE/RAGE in diabetes pathophysiology has been demonstrated using RAGE blockade experiments able to inhibit diabetes dysfunctions in vessels or in organs, while AGE injection in mice provoked such dysfunctions[17-19].
T-lymphocytes play an important role in diabetes, either through activation of autoimmune cells directed against beta-pancreatic cells in the case of type 1 diabetes (T1D), or through infiltration of tissues or organs such as adipose tissue (AT) in type 2 diabetes (T2D).In T1D, contribution of AGE/RAGE to diabetes evolution has been clearly demonstrated.For example, RAGE blockade experiments prevented diabetes transfer with diabetogenic T cells in non-obese diabetic/severe combined immunodeficiency mice[20].Moreover, T cells from T1D patients or from at risk diabetes relatives, have been shown to express elevated levels of intra-cellular RAGE associated with increased T cell survival and inflammatory cytokine release[21].AGE/RAGE interaction is also known to play a role in interleukin (IL)-17 immune responses as shown by AGE-mediated up-regulation of RAGE expression in T cells of T1D patients, which resulted in increased IL-17A secretion[22].
The interleukin 17 secreting T helper (Th17) cell subset has been recently discovered as a T-cell inflammatory lineage that mainly secretes IL-17A and IL-17F cytokine whose receptors are ubiquitously expressed[23].Those receptors are able to spread inflammation due to their ability to activate secretion of pro-inflammatory cytokines and metalloproteinases following IL-17A binding[24].
We have recently implicated adipose-derived-stem cells (ASC) and adipocytes (AD) in the promotion of Th17 cells through cell-to-cell contact-dependent interactions with blood mononuclear cells (MNC)[25,26].This function was likely to be mostly displayed by ASC obtained from obese rather than from lean individuals and resulted in inhibiting adipogenesis and insulin response of obese ASC and AD, respectively.In the present study, we aimed to determine the potential role of the AGE/RAGE axis on ASC-mediated Th17 promotion in lean individuals.Therefore, we investigated herein whether glycated albumin would induce IL-17A secretion by T cells, and whether anti-RAGE monoclonal antibody (mAb) would prevent this activation.To this purpose, we co-cultured lean ASC with MNC and treated them with glycated human serum albumin (G-HSA).We observed that G-HSA increased IL-17A secretion but also, Interferon gamma (IFNγ), and Tumor necrosis factor alpha (TNFα) secretion and that anti-RAGE mAb specifically inhibited IL-17A secretion.
MATERIALS AND METHODS
Isolation and expansion of ASC
Subcutaneous or visceral AT samples were isolated from residues of bariatric surgery of obese subjects (body mass index > 30 kg/m2), or visceral surgery of lean controls with the informed consent of patients.AT samples (50-100 mg) were fragmented and incubated in 2 g/L of collagenase type Ia solution (Sigma Aldrich, C2674) dissolved in Dulbecco’s modified eagles medium:Ham F12 (DMEM:F-12) medium (1:1 mL/L) (Invitrogen) for 40 min at 37 °C by mixing.Collagenase action was quenched by the addition of 1:1 mL/L of DMEM:F-12 medium supplemented with 10% heat inactivated fetal calf serum (FCS).The released stromal vascular fraction (SVF) was recovered by centrifugation (800 g for 7 min at 25 °C).Residual red blood cells were lysed by hypotonic shock and the ASC component of SVF was selectively expanded in culture medium composed of DMEM:F-12 supplemented with 10% FCS, 2 mmol/L Lglutamine and 100 U/mL penicillin-streptomycin.Half of the culture medium was changed every two to 3 d.ASC were amplified by several passages in culture (3 to 4) and directly used for experiments or stored in liquid nitrogen.The multipotent phenotype of ASC was validated by differentiating ASC into AD or osteoblasts, depending on the differentiation medium used, as previously reported[25].ASC phenotype was assessed by staining with fluorescein isothiocyanate (FITC)-conjugated, phycoerythrin (PE)-conjugated, allophycocyanin (APC)-conjugated mouse anti-human cell surface markers (from ImmunoTools GmbH, Friesoythe, Germany) as recommended by the International Society for Cellular Therapy[27], and revealed a cluster of differentiation (CD) 90+, CD105+, CD73+, and CD45- pattern (Supplement Figure 1).
Isolation of blood MNC
Blood samples were obtained through the Blood Bank Center of Lyon (France), following institutionally approved guidelines.MNC were harvested from healthy human peripheral blood by density gradient centrifugation (Ficoll-Histopaque Sigma-Aldrich, Saint-Quentin Fallavier, France).MNC were stored in liquid nitrogen prior to use.
Co-culture assays and blockade experiments
ASC were harvested and seeded in 96-well plates (20000 cells/well) for 18-24 h in 200 μL of basal culture medium (DMEM:F-12 medium, 1:1 mL/L supplemented with 10% FCS).100000 MNC were co-seeded for 48 h in the presence or absence of phytohaemagglutinin (PHA), 5 μg/mL (Sigma-Aldrich).Different ratios of ASC:MNC were used, as indicated in figure legends.Cells were incubated in Roswell Park Memorial Institute medium 1640 supplemented with either 1% human serum albumin (HSA) or 1% G-HSA, both from Sigma Aldrich (Saint Quentin-Fallavier, France).Supernatant was harvested after 48 h, and frozen.In blockade experiments anti-RAGE monoclonal antibody (RetD Systems, Lille, France) was added at 20 μg/mL during the whole period of culture.
Flow cytometry
FITC, PE, or APC conjugated mouse anti-human CD73, CD90, CD105, CD3, CD41 CD62P, human leukocyte antigen (HLA)-DR, intercellular adhesion molecule 1 (ICAM-1), CD8 (all from Immunotools) were used to label the various cells tested.Analyses were performed using the “LSR II 3 lasers” cytofluorometer and the Diva software (both were from BD Biosciences).
Cytokine secretion
IL-17A, IL-1β, IL-6 and TNFα secretions were measured by ELISA, using the corresponding antibodies (e-Biosciences, Paris, France).
Statistical analysis
One- or two-way repeated measures ANOVA, were used to compare multiple criteria.When some values were missing, mixed effects analyses were used.When the ANOVA or mixed effects analyses were significant, Bonferroni post hoc tests were used to do two-by-two analyses, taking into account the multiple comparisons.Pairedttests were used to compare two criteria, in univariate analysis.Differences were considered as statistically significant whenPvalue was < 0.05.The analyses were done using Graphpad Prism 8 software.
RESULTS
G-HSA only weakly increases the levels of IL-17A promoted by obese ASC
We have previously reported that obese ASC activate IL-17A production by T cells in the presence of PHA.To investigate whether glycated albumin would increase the levels of IL-17A, we co-cultured the cells either in the presence of 1% HSA, or 1% GHSA.Graded concentrations of ASC were co-cultured with the optimal concentration of MNC and activated with PHA.Although IL-17A secretion weakly increased, the two-way ANOVA multi-comparison tests did not show significant results whether HSA or G-HSA were added to cultures.But TNFα clearly increased (P =0.0165 in twoway ANOVA).Thus, these results demonstrated a weak, but non-significant effect of G-HSA on Th17 stimulation by obese ASC, but an increase in TNFα production.
Lean ASC mediate higher levels of IL-17A, TNFα, and IFNγ secretion by T cells, in the presence of G-HSA
Because we have previously reported that lean ASC mediate IL-17A production at much lower levels than obese ASC, we investigated whether AGE could increase this production.Therefore, we co-cultured lean ASC with MNC in the presence of HSA, or G-HSA, and activated the co-cultures with PHA.Secretion of IL-17A was measured and showed a significant increase in the presence of G-HSA (P =0.0196 in post-hoc Bonferroni tests).Interestingly, T helper 1 cytokines were also increased in the presence of G-HSA such as IFNγ (P =0.0065 in Bonferroni post-hoc tests), and TNFα (P =0.0037 in Bonferroni post-hoc tests).However, IL-6 and IL-1β, which are mostly secreted by ASC and monocytes in this model, did not show significant differences in post-hoc Bonferroni tests, even though mixed effect analyses showed significancy, suggesting a specific effect of G-HSA on T cells.
G-HSA increases RAGE and HLA-DR expression in ASC/MNC co-cultured cells
We then investigated whether RAGE expression would be increased in the co-cultures of lean ASC and T cells leading to IL-17A production.We observed that the expression of RAGE was clearly increased when G-HSA was present.Moreover, HLA-DR expression was upregulated together with RAGE expression, in the presence of GHSA.
Previous reports have demonstrated that glycated albumin induces platelet aggregation and activation[28,29].Therefore, we measured the expression of CD62P and CD41 surface molecules, which are markers of platelet activation and aggregation, respectively, in experiments where T cells were either cultured alone, or co-cultured with ASC, in the presence of PHA and G-HSA, or HSA.Whereas markers of platelet aggregation and activation increased in activated ASC/MNC co-cultures, no difference was observed whether G-HSA or HSA was present.ICAM-1 expression, which has also been shown to increase in endothelial cells under the influence of RAGE activation[30]and in co-cultures of obese ASC with T cells[31], did not increase in the presence of G-HSA.
Therefore, these results suggested a specific effect of G-HSA on RAGE and HLA-DR expression in co-cultured cells.
Anti-RAGE mAb inhibits RAGE and HLA-DR expression in co-cultured cells
To better define the effects of G-HSA on RAGE and HLA-DR expression, we then added anti-RAGE mAb during co-cultures of lean ASC with MNC for 48 h, and measured the expression of RAGE, HLA-DR, CD41, CD62P and ICAM-1.As expected, RAGE expression decreased.Among the other molecules that were analyzed, only HLA-DR expression decreased down to the levels of cells co-cultured in the presence of HSA.
Specific inhibitory effects of anti-RAGE mAb on ASC-mediated IL-17A production
Because the anti-RAGE antibody was able to inhibit RAGE and HLA-DR expression, we then investigated whether anti-RAGE mAb could inhibit IL-17A production.Therefore, co-cultures of PHA-activated ASC/MNC cells were performed in the presence or absence of anti-RAGE mAb.Results showed that IL-17A secretion significantly decreased in the presence of anti-RAGE mAb (P =0.0402 in pairedttests), but not IFNγ, nor TNFα, although a trend was observed for the latter.Therefore, our results suggested that RAGE might be specifically implicated in lean ASC-mediated IL-17A production, but not in IFNγ or TNFα secretion.
DISCUSSION
IL-17A/F are pro-inflammatory cytokines known to play an important role in AT-low grade inflammation in obese individuals, possibly leading to T2D[25,32-36].Interestingly, IL-17A/F cytokines have also been involved in the pathogenicity of T1D[37], notably through their peri-pancreatic fat location[38,39].Indeed, deletion of sentrin-specific protease 1 (SENP1), a SUMO-specific protease in AT, resulted in activating NFKB and pro-inflammatory cytokine/chemokine secretion in peri-pancreatic AT, ultimately leading to the recruitment of immune cells, including Th17 cells[38].Subsequent to induced beta cell death and pancreatic disruption, spontaneous development of T1D was further observed in these SENP1-invalidated mice[39].Strengthening the potential role of pancreatic fat as a pathogenic factor leading to beta cell dysfunction is the demonstration that pancreatic fat has been negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance[40].Moreover in this study, pancreatic fat was found to be a stronger determinant of impaired insulin secretion than visceral fat[40].In the present study, we investigated whether AGE could be involved in the dysfunction of lean AT, through increase of IL-17A production by T cells interacting with adipocyte progenitors.To address this question, we used the co-culture model that we have previously reported to lead to Th17 cell activation by ASC[25], and added low concentrations of HSA or G-HSA.When using obese ASC, we observed only a weak, but not significant effect of G-HSA on increased IL-17A production, suggesting other mechanisms than AGE in obese-ASCmediated IL-17A secretion (Figure 1).However, when lean ASC/MNC co-cultures were incubated in the presence of 1% G-HSA, a significant increase of IL-17A production was observed, together with increased IFNγ and TNFα production.This increase was probably related to specific activation of T cells by G-HSA, as neither IL-1β nor IL-6 significantly increase (Figure 2).

Figure 1 Glycated human serum albumin increases the levels of interleukin 17A promoted by obese adipose-derived stem cells, at suboptimal conditions.
RAGE is one of the AGE receptors and has been widely implicated in most of the pro-inflammatory mechanisms mediated by AGE and leading to chronic inflammation disorders.They are constitutively expressed in T cells from diabetic patients, and are known to activate the NFKB pathway leading to inflammatory cytokine production[15].However, not all T cells are regulated by RAGE, as shown by Chenet al[20]who demonstrated a differential effect of RAGE blockade on splenic T cells but not on fully activated T cells in a transfer model of diabetes.Supporting these results, we also demonstrated herein that RAGE was involved in Th17 cell, but not Th1 cell activation, since only IL-17A secretion was inhibited by anti-RAGE mAb (Figure 5).A similar differential effect of RAGE on IL-17A and TNFα production was also observed in T1D, where RAGE positive T cells were found to express higher levels of IL-17A but not TNFα nor IFNγ, as compared with RAGE negative cells in the same patients.This demonstrated thus a potentiating effect of RAGE signaling pathway on IL-17A production[22].Confirming the implication of RAGE in ASC-mediated Tcell activation, we observed increased RAGE expression, together with HLA-DR expression when GHSA was added to the co-cultures, and an abolition of this effect in the presence of RAGE mAb which concomitantly resulted in inhibition of IL-17A production (Figures 4 and 5).Finally, although glycated albumin has been shown to increase platelet aggregation[28,29],we did not find its involvement in AGE-mediated activation of T cell secretion.Indeed, up-regulation of CD41 and CD62P expression, two markers of platelet aggregation and activation respectively, did not further increase in the presence of G-HSA (Figure 3).Moreover, RAGE mAb did not inhibit the expression of these two markers, either (Figure 4).ICAM-1 expression, which has been shown to be up-regulated by AGE in other cell models[41], did not increase in the presence of AGE, and was not inhibited by RAGE mAb (Figures 3 and 4).Therefore, we concluded that in our model platelet aggregation and ICAM-1 were not involved in the potentiation of Th17 cytokines production by G-HSA.

Figure 2 Lean adipose-derived stem cells increase the levels of interleukin 17A, tumor necrosis factor alpha, and interferon gamma secretion by mononuclear cells, in the presence of glycated human serum albumin.L
In conclusion, we have shown herein that the presence of G-HSA enhances lean ASC-mediated IL-17A production through a mechanism requiring RAGE signaling.Moreover, our study suggests a new mechanism by which ASC could contribute to inflammatory processes through AGE-mediated IL-17A production in AT of lean individuals.This could be potentially of importance in the context of T1D pathophysiology.
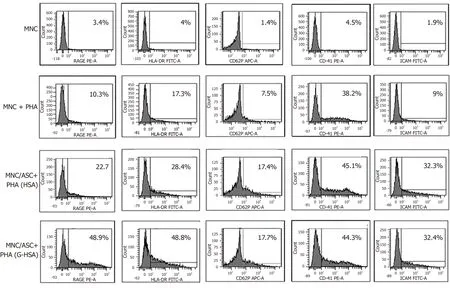
Figure 3 Glycated human serum albumin increases receptor for advanced glycated end products and human leukocyte antigen-DR expression in adipose-derived stem cell / mononuclear cell co-cultures.
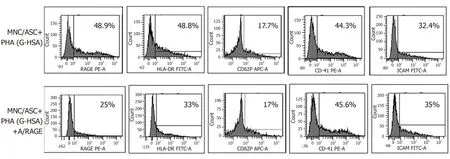
Figure 4 Anti- receptor for advanced glycated end products monoclonal antibody inhibits receptor for advanced glycated end products and human leukocyte antigen-DR expression.
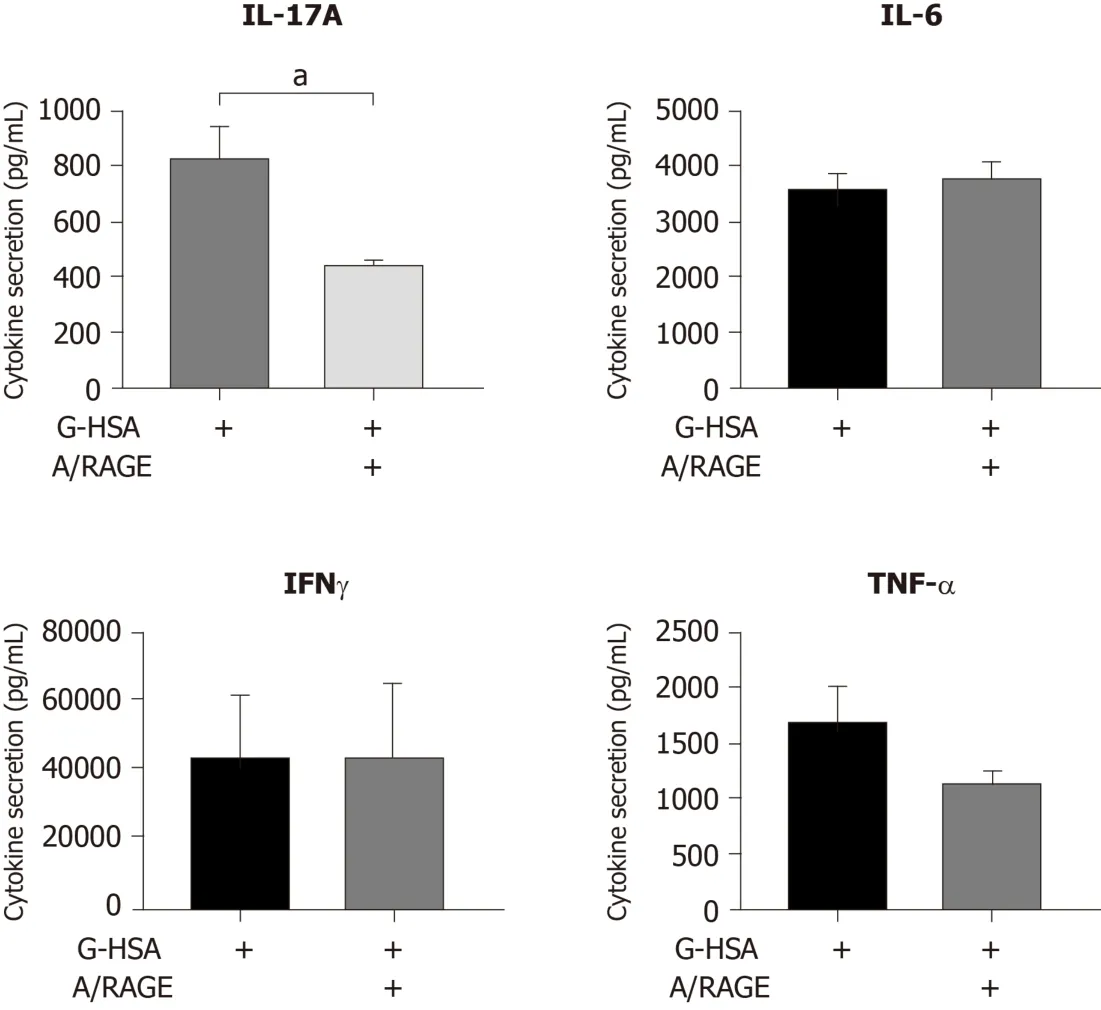
Figure 5 Inhibitory effects of anti-receptor for advanced glycated end products monoclonal antibody on interleukin 17A production.
ARTICLE HIGHLIGHTS
Research background
Advanced glycation end products (AGE) are involved in type 1 diabetes (T1D)through reduction of glucose uptake and attenuation of insulin sensitivity.Moreover,AGE are known to promote interleukin (IL)-17A secreting T cells.
Research motivation
Adipose Tissue (AT), and especially pancreatic AT is a pathogenic factor leading to beta cell destruction partly due to IL-17A secreting T helper (Th17) cell recruitment; IL-17A/F are pro-inflammatory cytokines known to play an important role in AT-low grade inflammation and propagation of inflammation outside AT.
Research objectives
We have previously shown that adipose-derived stem cells (ASC) promote Th17 cells in obese AT, but not or less in lean AT.Here, we investigated whether AGE could improve lean ASC ability to promote IL-17A production by T cells.
Research methods
With this aim, we cocultured ASC from lean AT with mononuclear cells in the presence of glycated human serum albumin (G-HSA) or human serum albumin.We then analyzed the influence of AGE by blocking their ability to bind to receptor of advanced glycated end products (RAGE).IL-17A and other pro-inflammatory cytokine secretions were measured, together with surface expression of RAGE, and other relevant molecules.
Research results
We have demonstrated herein that G-HSA enhances IL-17A, interferon gamma and tumor necrosis factor alpha secretion by MNC in the presence of ASC harvested from lean individuals.This effect involves the RAGE/AGE axis as assessed by anti-RAGE blocking monoclonal antibodies (mAb) and is associated with increased expression of RAGE and human leukocyte antigen-DR molecules.
Research conclusions
Thus, our results demonstrate that G-HSA is able to improve lean ASC-mediated IL-17A production in AT, suggesting a new mechanism by which AGE could contribute to T1D pathophysiology.
Research perspectives
Here we propose a mechanism by which AT can lead to the recruitment of Th17 cells in lean individuals through activation of the AGE/RAGE axis.Because pancreatic fat has been involved in the pathogenicity of T1D, this model deserves to be validated in animal studies, in order to evaluate the efficacy of RAGE blocking mAb as a therapeutic tool.
ACKNOWLEDGEMENTS
We wish to thank Yassine Corbin, for his help in English editing corrections.
 World Journal of Stem Cells2020年7期
World Journal of Stem Cells2020年7期
- World Journal of Stem Cells的其它文章
- Potential of transposon-mediated cellular reprogramming towards cell-based therapies
- Approaches to promoting bone marrow mesenchymal stem cell osteogenesis on orthopedic implant surface
- Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species
- Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration
- Vitamin D and calcium signaling in epidermal stem cells and their regeneration
- Adipose-derived stem cell therapy shows promising results for secondary lymphedema
