Existence Criterion of Three-Dimensional Regular Copper-1, 3, 5-Phenyltricarboxylate (Cu-BTC) Microparticles
YANG Chengfei(楊成飛), GAO Dongjie (高東杰), HE Jihuan (何吉歡), 3*
1 National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, China 2 School of Mathematics and Statistics, Heze University, Heze 274015, China 3 School of Science, Xi’an University of Architecture and Technology, Xi’an 710055, China
Abstract: Nano or micro-scale particles are considered as a zero-dimensional material, and their shape is difficult to control. Here the crystal-like microparticles are formed, and the existence criterion of crystal-like low dimensional materials is established. The hydrothermal method is used to fabricate copper-1,3,5-phenyltricarboxylate (Cu-BTC) microparticles, which have a regular geometric shape with regular triangle, quadrangle and hexagon. The benzene ring and carboxyls (—COOH) attached to the benzene ring form two basic molecular structures of [Cu3(BTC)2]n, which play an essential role in the construction of a three-dimensional crystal-like structure.
Key words:hydrothermal synthesis; copper-1, 3, 5-phenyltricarboxylate (Cu-BTC); fractal; metal-organic framework
Introduction
Low dimension materials,e.g., graphene[1-2], nanofibers[3-11], and nanoparticles[12-14], have caught much attention recently due to extensive applications in almost all advanced fields. The theory of Peierls-Landau instability has been adopted to explain low-dimensional crystalline lattices[15-17]. There are many technologies to fabricate various nanoparticles or microparticles, among which electrospinning[12]and the hydrothermal method[18]are commonly used. Generally, nanoparticles or microparticles are irregular, and their morphology is difficult to control. Here we suggest an existence criterion for three-dimensional regular micro or nanoparticles for metal-organic frameworks (MOF) materials, among which copper-1,3,5-phenyltricarboxylate (Cu-BTC) has caught wide attention. It is first synthesized by Chuietal.[19], and its specific surface area is up to 1 500 m2/g. The pore structure is orderly, and the chemical properties are stable. These unique advantages make Cu-BTC widely used in the fields of gas storage, separation, sensing, and catalysis. For example, Cu-BTC is supported on SiO2to achieve the purpose of improving thermal stability and reusability. The prepared SiO2-Cu-BTC can be used in the catalytic oxidation reaction of active methylene compounds to obtain higher yield and selectivity. Cu-BTC microparticles can also be added to the polyvinyl alcohol (PVA) solution in proportion, and Cu-BTC/PVA films can be prepared by using an electrostatic spinning experiment method. The prepared Cu-BTC/PVA film has good sterilization effect[20-23].
Gimeno-Fabraetal.[24]reported an impressive high-speed method for the synthesis of Cu-BTC, which was achieved by continuously impinging on a jet reactor under high pressure to uniformly mix reaction fluid. Carné-Sánchezetal.[25]used a special nozzle to spray the precursor solution, resulting in atomized droplets, which immediately reacted to form nanocrystals. In this research paper, three-dimensional regular micro-nano particles were synthesized by the hydrothermal synthesis in a polytetrafluoroethylene (PTFE) reactor at high temperature. Through careful analysis of particle morphology, the existence criteria of three-dimensional regular micro-nano particles were established. The properties of Cu-BTC microparticles can be further analyzed on a theoretical basis to broaden the practical application field of Cu-BTC microparticles.
1 Experimental Design
In this research paper, Cu-BTC microparticles were prepared by hydrothermal synthesis[18-26]. The preparation process is shown in Fig.1. 30 mL deionized water was added to 2.0 g (8.2 mmol) Cu(NO3)2·3H2O, and 30 mL anhydrous ethanol (C2H5OH) was added to 1.0 g (4.7 mmol) trimesic acid (H3BTC) to make two solutions which were then mixed and magnetically stirred for 5 min and ultrasonically stirred for 15 min at room temperature, respectively. The resultant solution was transferred to a high-pressure reactor with a PTFE reactor, and then it was placed in a drying chamber at 120 ℃ for 15 h.
After reaction, the reactor was cooled at room temperature until the temperature dropped to room temperature. After cooling, the reaction products were transferred to the centrifugal tube, and centrifuged for 10 min at a speed of 9 000 r/min, and then the upper layer fluid was discarded to obtain the Cu-BTC microparticles, which were washed three times by a mixed solvent of dimethylformamide (DMF) and anhydrous ethanol. Finally the precipitation was placed in a drying box at 130 ℃ for 10 h to obtain the dark blue Cu-BTC microparticles.
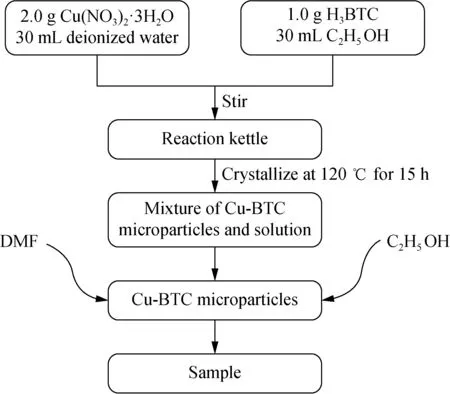
Fig. 1 Preparation process of Cu-BTC
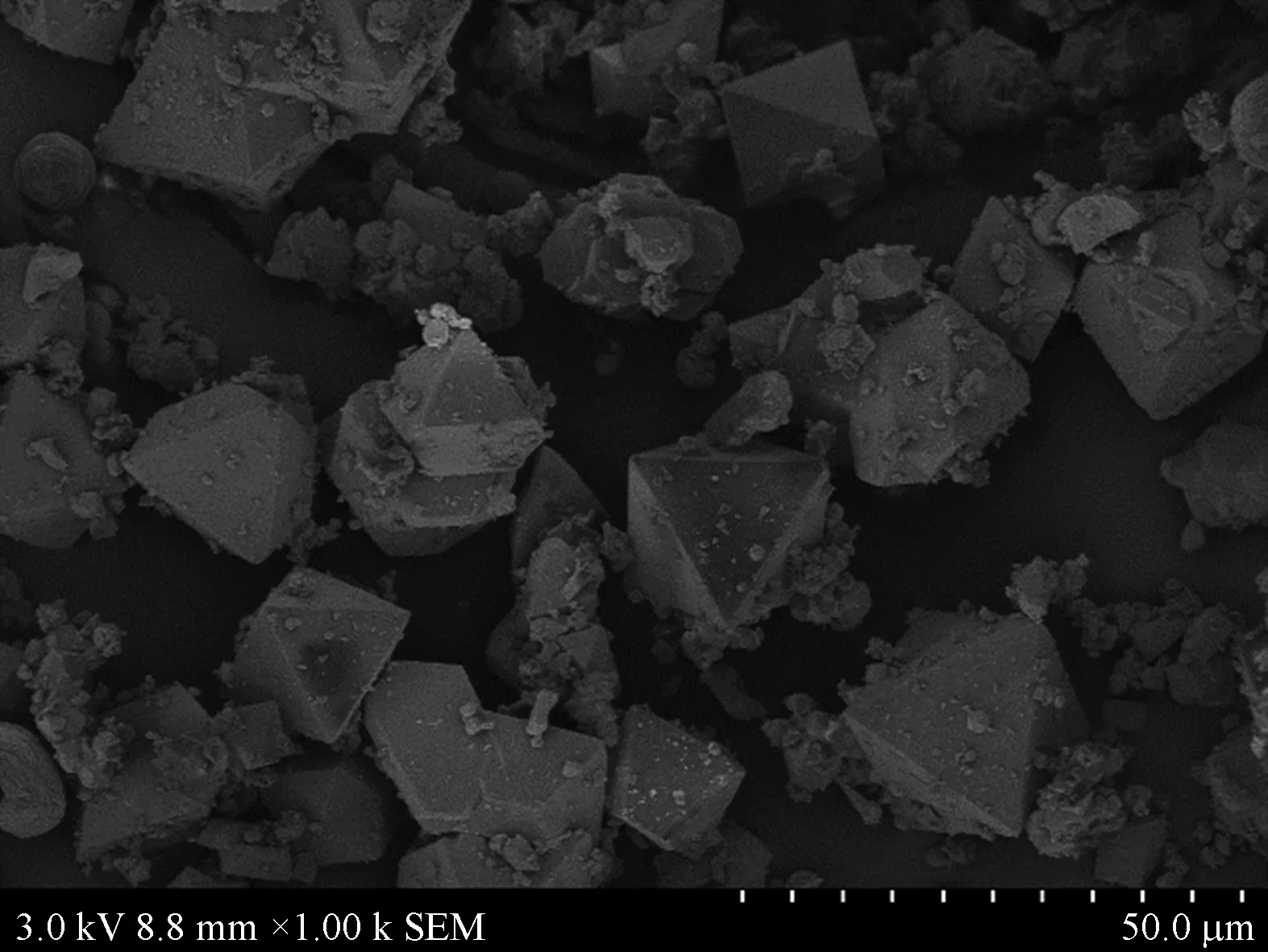
Figure 2 shows the X-ray diffraction (XRD) spectra of Cu-BTC prepared by the hydrothermal synthesis. The XRD spectrum of powder Cu-BTC coincides with those reported in Ref. [27]. It shows that we have successfully synthesized Cu-BTC powders. Figure 3 shows scanning electron microscopic (SEM) images of Cu-BTC microparticles at different multiples. We can see that the synthesized Cu-BTC has a regular shape, showing a perfect three-dimensional structure.
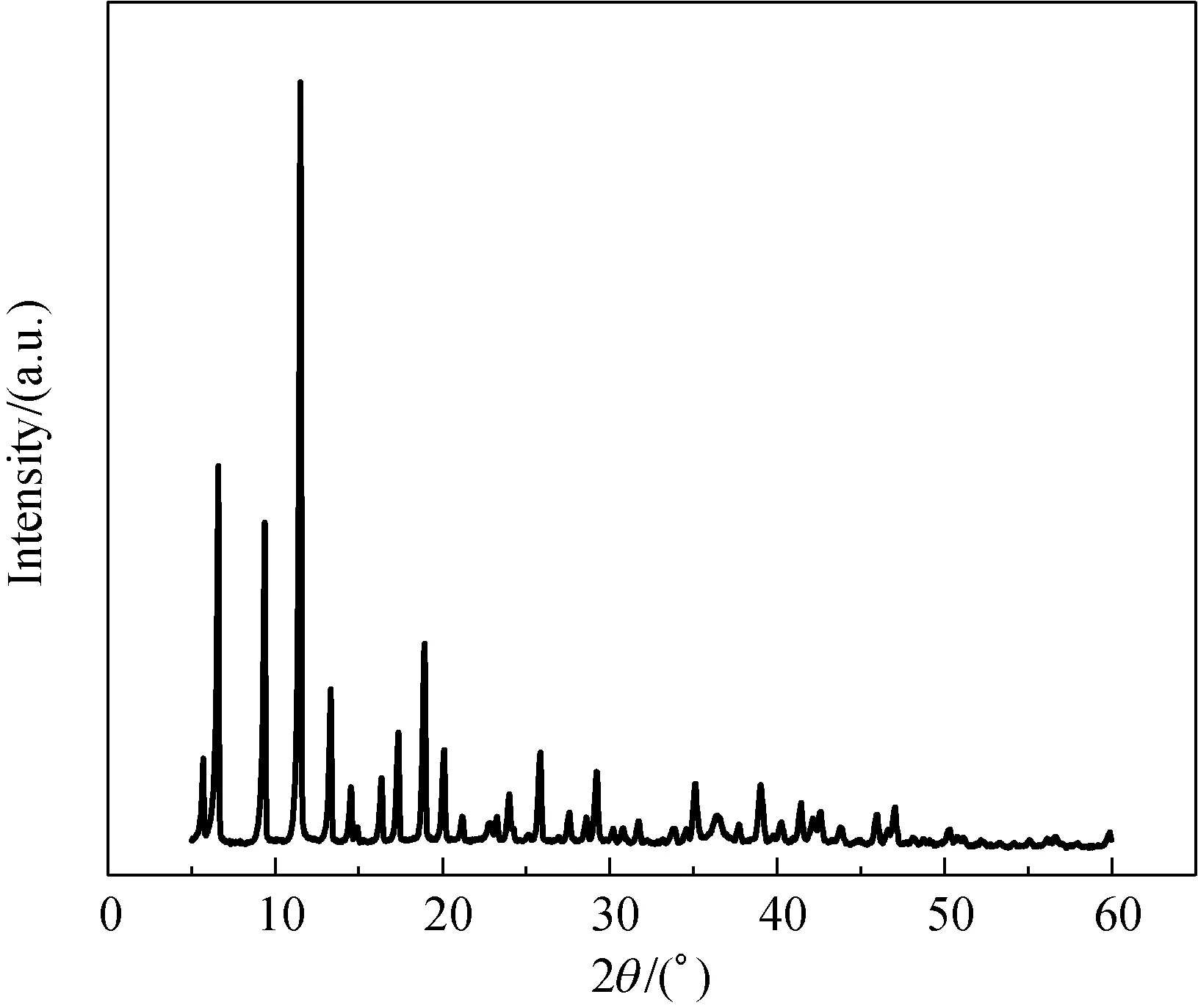
Fig. 2 XRD spectrum of Cu-BTC



Fig. 3 SEM images of Cu-BTC microparticles
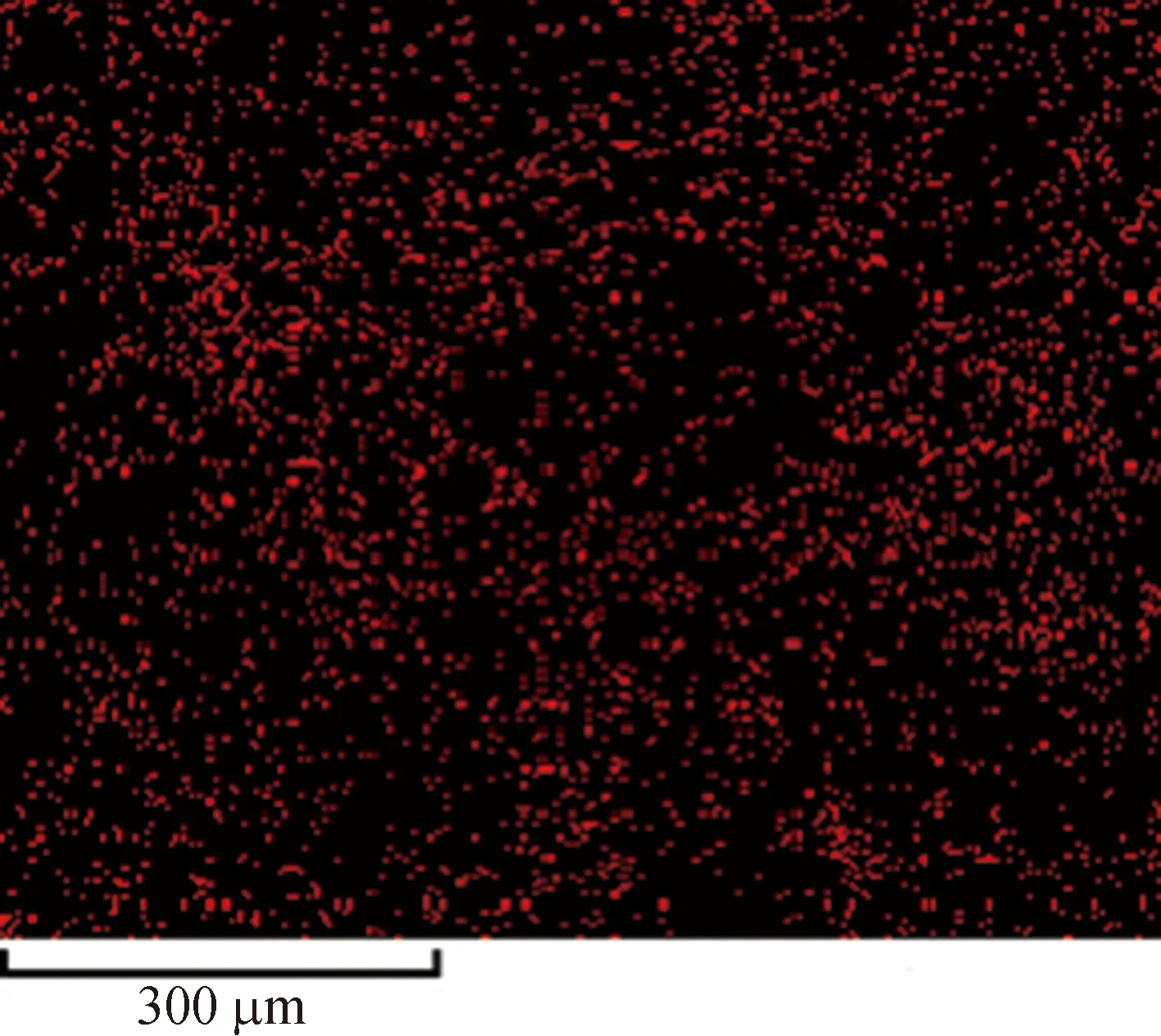
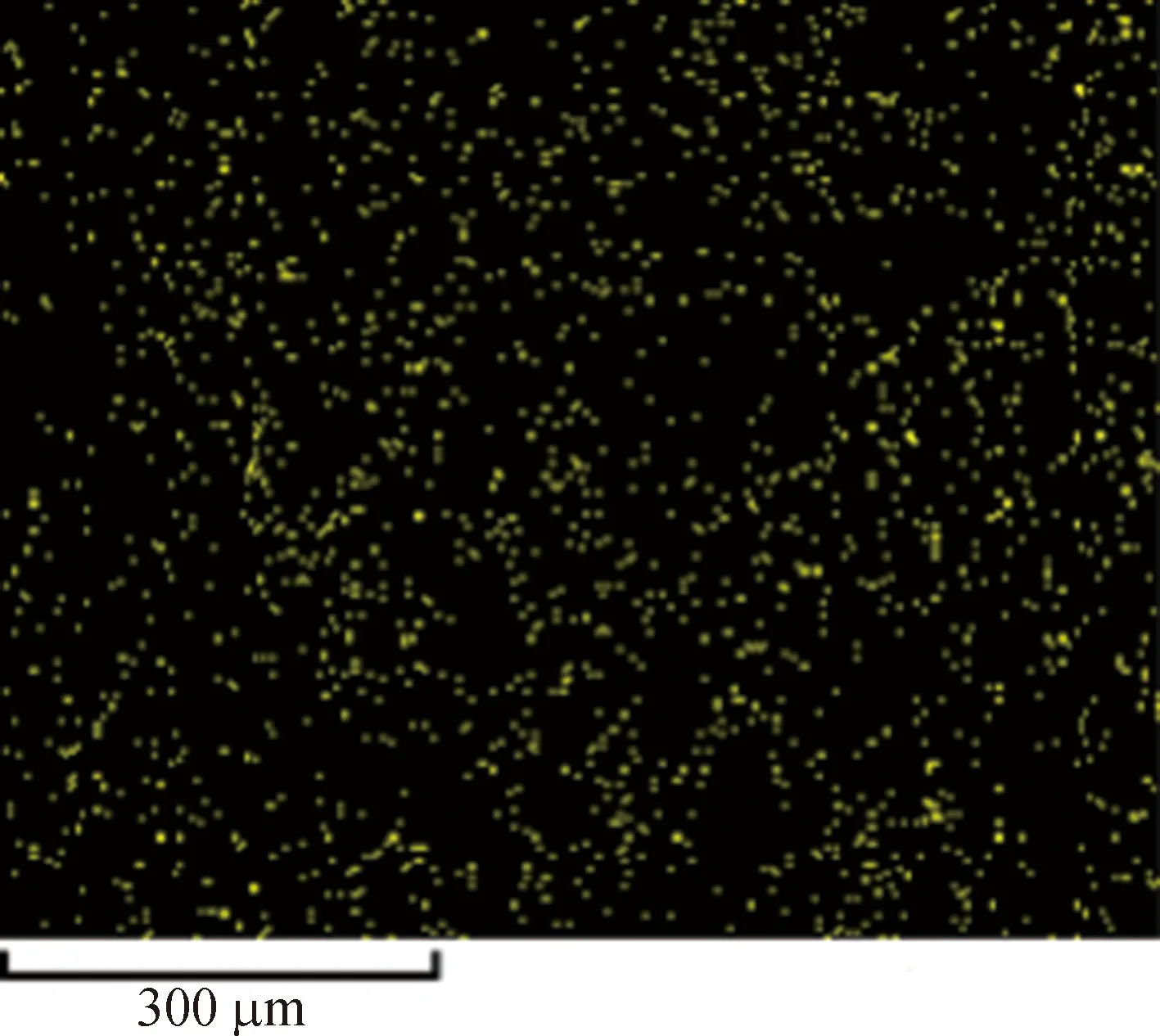
Figure 4 shows the element analysis of the Cu-BTC microparticles by the energy dispersive spectrometer (EDS). It is obvious that the contents of carbon and copper elements are predominated, followed by the oxygen element. At the same time, the distributions of the three elements are very uniform, indicating that the elements are distributed regularly, and the metal-organic framework is geometrically constructed[28-33]. The results also show that the impurities in the synthesized Cu-BTC powders are less, and the synthesis rate of the crystals is higher.
(a)

(b)
(c)
(d)
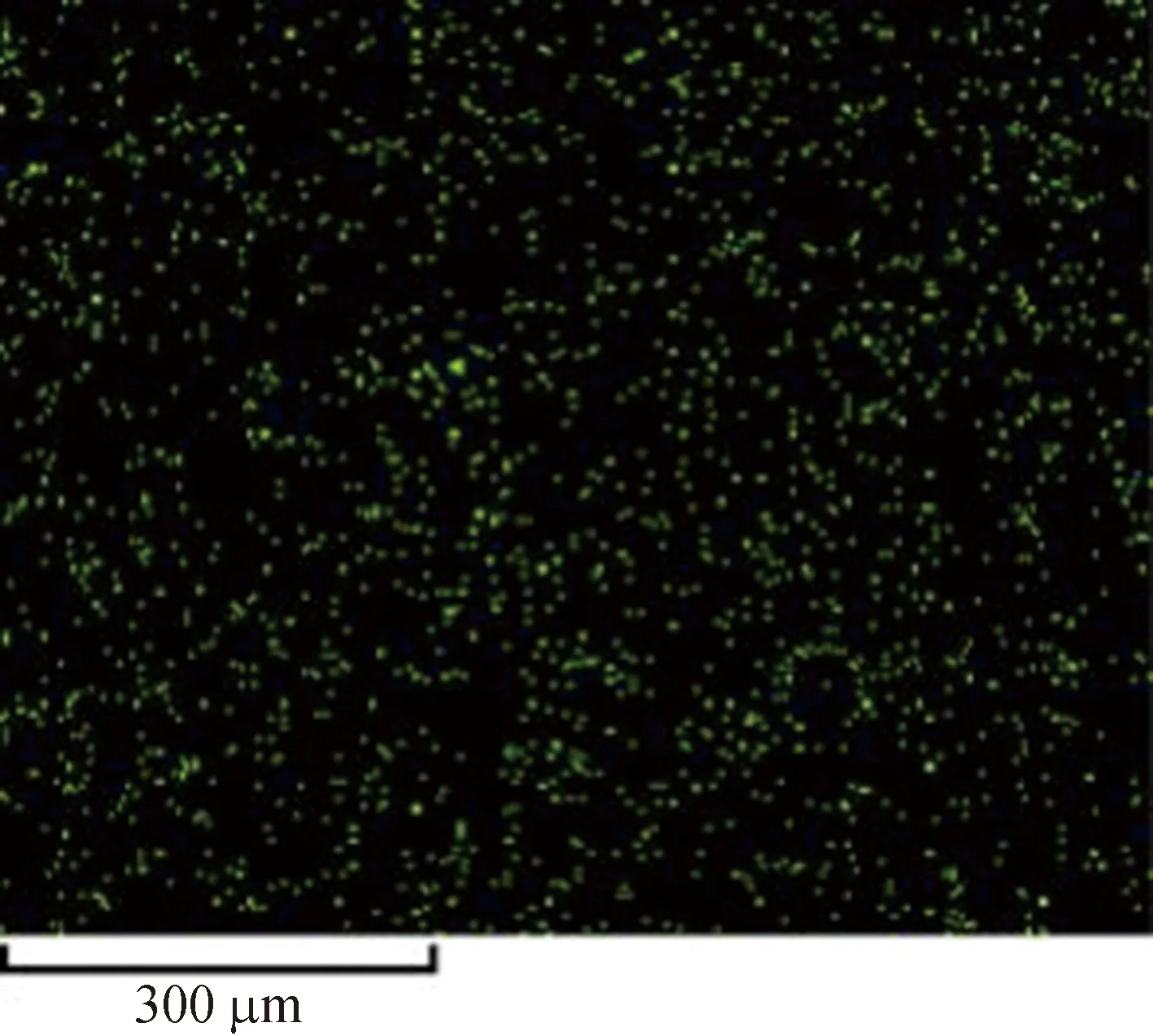
(e)Fig. 4 Distribution of C, O and Cu in Cu-BTC powders by the EDS: (a) Cu-BTC microparticles;(b) element content; element distributions for C (c), O(d) and Cu(e)
2 Existence Criterion of Three-Dimensional Microparticles
As shown in Fig. 3, the microparticles have regular shapes of regular triangle, quadrangle and hexagon. The molecular structure of Cu-BTC microparticles is the benzene ring with three carboxyls (shown in Fig. 5), which is obtained through the following reaction (shown in Fig. 6).
3[Cu(NO3)2·3H2O]+2H3BTC →Cu3(BTC)2·3H2O+6HNO3+6H2O
(1)
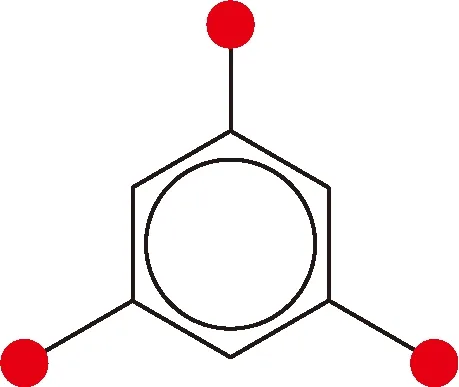
Fig. 5 1,3,5-Benzenetricarboxylic acid (BTC, C9H6O6) (the solid red circle represents —COOH)
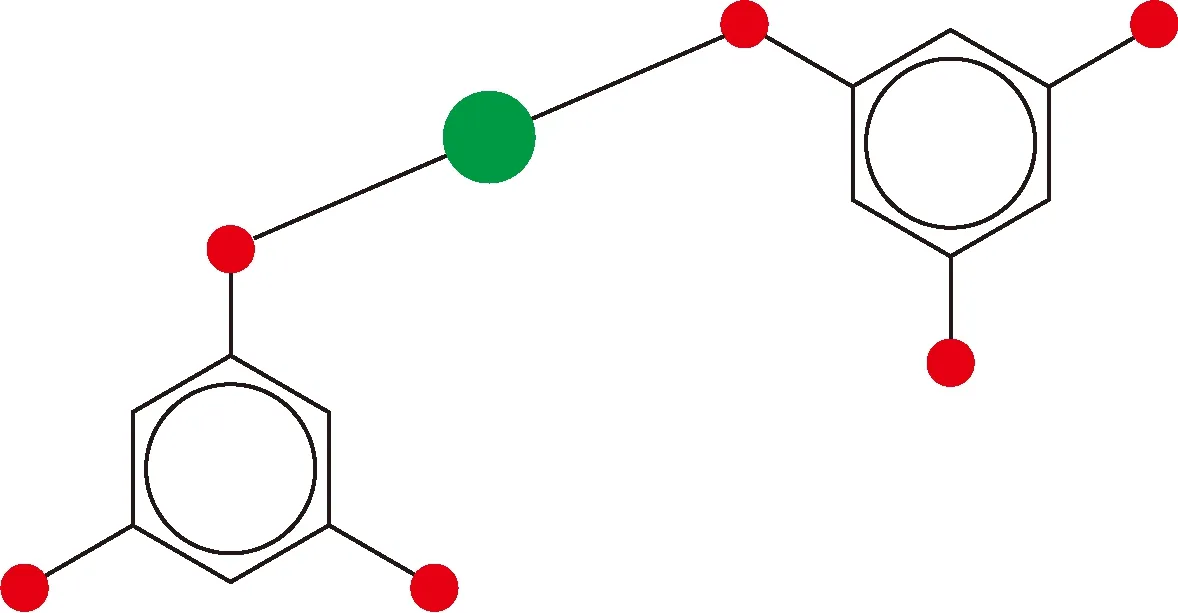
Fig. 6 Reaction mechanism (the solid green circle represents Cu2+)
The three carboxyls of the molecular structure, which are considered to be the lowest cascade to form a crystal-like structure, endow the Cu-BTC microparticle with a regular triangle boundary, as shown in Fig. 7(a). Similarly, the benzene ring of the molecular structure endows the particle with a hexagon surface, as shown in Fig. 7(b). A quadrangle boundary can be formed as a transitional one between the two molecular surfaces, as shown in Fig. 3.

(a)
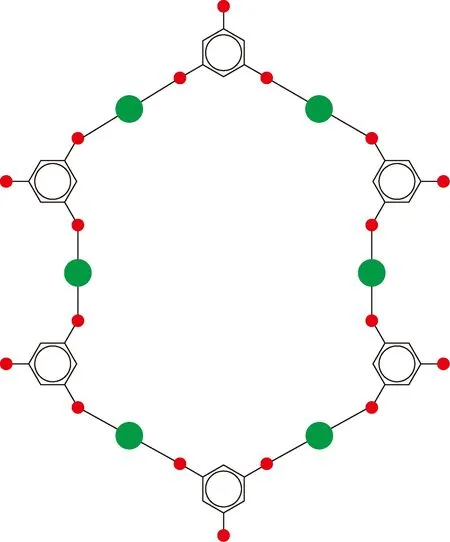
(b)Fig. 7 Molecular elements for (a) regular triangle boundary;(b) hexagon surface
3 Conclusions
The hexagonal benzene ring and the three carboxyls attached to the benzene ring form two basic molecular structures for hexagon and regular triangle surfaces, respectively. A lower number of benzene rings can form a large fractal-like hexagonal surface, and thus nanoparticles or microparticles with a hexagonal boundary are obtained. A higher number of benzene rings can form a larger fractal-like positive triangle surface to obtain nanoparticles or microparticles with a positive triangle boundary.
 Journal of Donghua University(English Edition)2020年2期
Journal of Donghua University(English Edition)2020年2期
- Journal of Donghua University(English Edition)的其它文章
- Acoustic Performance of Green Composites for Chinese Traditional Percussion Drums
- Fabrication and Characterization of Polypyrrole/Polyurethane/Polyamide/Polyamide Yarn-Based Strain Sensor
- Friction and Wear Behaviors of C/C-SiC Composites under Water Lubricated Conditions
- Performance Analysis of Cushioned Sport Soles with Plantar Pressure Test
- Combining User-Driven Social Marketing with System-Driven Personalized Recommendation for Student Finding
- Generative Adversarial Network with Separate Learning Rule for Image Generation
