Transcriptome analysis suggests mechanisms for a novel flowering type: Cleistogamous wheat
Ciguo Tng, Mingho Li, Minghui Co, Ruiju Lu,Huiln Zhng,Chenghong Liu,Shengwei Hung, Pingping Zhng,Ho Hu, Weiwei Zho, Lifng Wu,e,*
aKey Laboratory of High Magnetic Field and Ion Beam Physical Biology,Hefei Institutes of Physical Science,Chinese Academy of Sciences,Hefei 230031,Anhui, China
bSchool of Life Sciences,University of Science and Technology of China,Hefei 230027,Anhui,China
cBiotech Research Institute, Shanghai Academy of Agricultural Sciences,Shanghai 201106, China
dSchool of Life Sciences,Anhui University, Hefei 230601,Anhui,China
eTaihe Experimental Station,Hefei Institutes of Physical Science,Chinese Academy of Sciences,Taihe 236626,Anhui, China
A B S T R A C T
1. Introduction
Wheat (Triticum aestivum L.) is one of the most widely grown crops worldwide. It is a major source of protein, starch, and minerals for human consumption and is used in animal feed,industrial raw materials and biofuels [1]. Wheat Fusarium head blight (FHB), also called scab, is caused mainly by Fusarium graminearum and is one of the most destructive fungal diseases of wheat worldwide [2,3]. Infected wheat kernels contain deoxynivalenol (DON, also called vomitoxin),which is harmful to human and animal health [2,4,5]. FHB infection is more prominent in warm, humid, and subhumid areas,particularly during flowering and early stages of kernel development in wheat, and the frequency and range of FHB occurrence continue to increase [6,7]. In China, FHB occurs mainly in winter wheat areas in the middle and lower reaches of the Yangtze River, winter wheat areas in southern China,and spring wheat areas in northeastern China [8,9], with an average annual FHB area coverage of >5.33 Mha [8,10]. The annual FHB-affected area in Jiangsu province (about 1.2 Mha)exceeded 50% of the total area under wheat cultivation in 2012-2015 [8,11]. In recent years, owing to global warming,FHB-affected areas have expanded to the northern wheat areas of China. The wheat scab-affected area in the Henan province was most extensive in 2012,reaching 3.40 Mha,but it decreased to 1.74 Mha in 2016 [8,12]. For decades, the application of chemical insecticides and fungicides used for controlling pests and diseases has been harmful to agriculture, the ecological environment, and human health [13,14].The need to find eco-friendly and cost-effective strategies and develop new scab-resistant wheat cultivars is thus urgent[15].
Cultivating cleistogamous wheat may be a new strategy for controlling FHB, given that the inner surfaces of lemmae and paleae are the main sites prone to FHB infection [16]; in particular,the anthers provide the initial path for FHB infection during the flowering stages[3,17-19].Kubo et al.[20]have shown that a cleistogamous wheat cultivar (U24) had a lower FHB infection rate than a chasmogamous cultivar (Saikai 165).Cleistogamous barley (Hordeum vulgare L.) varieties, which selffertilize within permanently closed flowers [21], also showed greater resistance to FHB infection than chasmogamous barley varieties, which have open flowers [21,22]. Thus, cleistogamous phenotypes could be very important for disease-resistance breeding programs, providing structural barriers for diseases of FHB appearing during flowering time [23]. The trait of cleistogamy has theoretical research and other application value. For instance,cleistogamy,as a novel flowering type in wheat,could provide a model material for general biological study of wheat flower development [24] (cited in [25]). Second, cleistogamous plants could also be important for better control of gene flow in plants [26,27]. Finally, the cleistogamous phenotype offers a time-saving and cost-saving way to purify breeding lines.
In physiology and morphology, changes in lodicule size,morphology, and content are dynamic processes that occur during the flowering stage [28-30]. The reason the lodicules expand is that osmotic regulation substances accumulate in the lodicule cellule,consisting mainly of total soluble sugar,calcium ions,and potassium ions[28,30-34].Phytohormones also participate in the osmotic regulation process of lodicules[34,35].
The genetic mechanism of cleistogamy has been studied in grasses. In barley, cleistogamy is genetically determined by the dominant allele Cly2 and the recessive allele cly1[36];and the presence or absence of a miRNA172 target site determines the function of the cly1 gene[37-39].In rice,a single recessive gene, ld(t), which cosegregates with a sequence-tagged site marker,S01181a,controls cleistogamy,leading to the absence of lodicules in the mutant [40,41]. Another cleistogamyassociated gene in a rice mutant, cl7(t), encodes a truncated DEP2 protein and leads to weak swelling ability of the lodicules [26]. Lodicule development in rice was shown to be regulated by the superwoman 1 (spw1) gene, which has two mutant alleles, spw1-cls (temperature-sensitive) [42,43] and spw1-cls2 (temperature-stable) [44]. A common feature of these mutants is that the lodicules lose the ability to push the lemmae and paleae apart in the flowering stage.In wheat,a gene,TaAP2,that is homologous to the cleistogamous barley cly1 gene,was cloned[45],and it was suggested that this gene might generate a cleistogamous type of wheat with improved resistance to FHB. A novel mechanism of cleistogamous wheat was that a swelling ovary could push the lemmae and paleae apart to open the florets in wheat flowers [46].However, anther extrusion is a complex trait in wheat[47].
After the cleistogamous individual line,ZK001,was identified in 2008, kernels taken from a single spike with complete cleistogamy were planted repeatedly until the cleistogamous trait of this inbred line was completely stable.In this study,the lodicules of a static magnetic field (SMF)-treated wheat mutant(ZK001) and its wild-type cultivar (Yumai 18, YM18, a northern cultivar of China) were investigated. Resistance to FHB was assessed in YM18, ZK001, and Quanmai 725 (QM725, a widely grown wheat cultivar in Anhui province, China). To further investigate the possible mechanisms of cleistogamy in ZK001,RNA-Seq was performed on the spikelets of YM18 and ZK001 at the green anther stage (GAS). We attempted to identify genetic changes associated with the cleistogamous phenotype in wheat,with a particular focus on lodicule-expansion mechanisms.
2. Materials and methods
2.1. Plant material
ZK001 (Zhongke 0101, CNA20150325.7), the cleistogamy mutant line, was isolated from a mutagenized population of the wheat cultivar YM18 using an SMF of 7 T for 5 h in 2008 according to the protocol of Zhang et al. [48]. The seeds of YM18 and ZK001 were stored at the Key Laboratory of High Magnetic Field and Ion Beam Physical Biology,Hefei Institutes of Physical Science, Chinese Academy of Sciences (CASHIPS),Anhui, China. YM18 and ZK001 were grown in a greenhouse(31°54′N,117°10′E)at CASHIPS.QM725 and ZK001 were grown in an experimental field at the Taihe Experimental Station,CASHIPS (33°15′N, 115°37′E) at Taihe, Anhui, China. Fertilizer and weed management followed methods used in wheat breeding[49].The spikelets and lodicules of YM18 and ZK001,in three biological replicates,were harvested during the white anther stage (WAS), GAS, yellow anther stage (YAS), and anthesis stage (AS) [50-52]. These samples were frozen in liquid nitrogen and stored at -80°C.
2.2. Calcium, potassium, and sodium content
Twenty pairs of lodicules from YM18 and ZK001 were sampled in triplicate and snap-frozen in liquid nitrogen between 13:30 and 15:00 at the four flower-development stages. A steel ball(diameter 2 mm) was placed in a 2.0-mL EP tube and used to grind these samples using a Tissuelyser-24 instrument(Shanghai Jingxin Industrial Development Co.,Ltd.,Shanghai,China)for 45 s at 50 Hz.
After the ground sample was weighed, it was treated with 400 μL of hydrogen peroxide and 3 mL of nitric acid at room temperature until the liquid became transparent. After filtering and 20× and dilution, the solution was prepared for inductively coupled plasma atomic spectroscopy(ICP-AS),and a Thermo Scientific iCAP 7400 duo ICP-OES (Thermo Fisher Scientific, Inc., Waltham, MA, USA) instrument was used to determine the content of calcium (λ = 315.887 nm) and potassium(λ= 766.490 nm)in lodicules.
2.3. Observation of spikes and lodicules
Spikes images of YM18 and ZK001 were captured with a D90 camera (Nikon Corporation, Tokyo, Japan) at GAS, YAS, and AS, and spikelet, partial floret, and lodicule images of YM18 and ZK001 were observed with an SZX10 upright fluorescence stereomicroscope (Olympus Corporation, Tokyo, Japan) and DP72 photographed(Olympus Corporation,Tokyo,Japan).The lodicules of YM18 and ZK001 were separated from the central young spikes in triplicate during GAS and were cultured on Murashige and Skoog (MS) medium containing graphite.These lodicules were observed every 10 min with an SZX10 upright fluorescence stereomicroscope and DP72 photographed. The lodicule width (the horizontal length between the two sides of lodicule), effective length (the vertical length that effectively absorbs water and expands of lodicule) and total length (the vertical length from top to bottom of lodicule) of YM18 and ZK001 was measured in triplicate using ImageJ (1.50i, https://imagej.nih.gov/ij)software.
For transmission electron microscopy(TEM)observation of lodicule ultrastructures, lodicules of YM18 and ZK001 at late YAS were fixed in 4.0% (v/v) glutaraldehyde, vacuuminfiltrated until the material sank, and left overnight at 4 °C.The samples were then rinsed with 0.1-mol L-1phosphate buffer (pH 7.2) and postfixed with 1% osmium tetroxide for 8 h at 4 °C. After fixation, the samples were serially dehydrated with graded alcohol and propylene oxide, embedded in resin,and polymerized in a thermostatic chamber at 37°C(12 h),45°C(12 h)and 60°C(48 h).Ultrathin sections(70-90 nm in thickness) were prepared with a Lycra UC6 ultramicrotome(Leica Microsystems,Weztlar,Germany)and stained with 2.0% uranyl acetate for 30 min and then with lead citrate for 15 min. These sections were examined on a Hitachi H7500 TEM (HITACHI, Tokyo, Japan) instrument at 100 kV[53].
2.4. FHB resistance testing
FHB resistance was tested in both 2013-2014 and 2014-2015 during the flowering stage of YM18 and ZK001 in the greenhouse by spraying spores of the FHB strain Chi-2 (F.graminearum Schw.cv.Chi-2,which carries the GFP gene).The inoculum(50 μL at 105 spores per milliliter) was deposited by spraying all sides of spikes. Green signals of the GFP which were enriched in the FHB hyphae would be detected on spikelets,flowers,and stalks using the SZX10 upright fluorescence stereomicroscope and DP72 photographed. Under natural conditions, diseased kernels of QM725 and ZK001 were counted in the plot at Taihe,and DON content in QM725 and ZK001 was quantified at Microspectrum Technology Co.,Ltd.(Shanghai,China)in 2018.
2.5. RNA isolation, quantification, and quality control
Total RNA of spikelets and lodicules of YM18 and ZK001 at GAS and YAS was isolated with a Plant RNA Kit (Omega,R6827) according to the manufacturer's instructions. The quality of each RNA sample was checked on 1% agarose gel.RNA concentrations were measured with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham,MA, USA). Three biological replicates were used in RNA-Seq experiments and for quantitative real-time polymerase chain reaction (qRT-PCR)validations.
2.6. Library construction and sequencing
Transcriptome sequencing of spikelets at GAS of YM18 and ZK001 were performed by BGI Tech Company (Shenzhen,China).After total RNA extraction,DNase I(10 U)was used to purify the RNA at 37°C for 30 min.Then magnetic beads with oligo(dT) were used to isolate the mRNA. The mRNA was cleaved into short (approximately 90 nt) fragments with a Thermomixer instrument. Complementary DNAs (cDNAs)were then synthesized using the mRNA fragments as templates. Short fragments were purified and resolved with elution buffer (EB) for end repair and single-nucleotide adenine addition. The short fragments were ligated with adapters. After agarose gel electrophoresis, fragments which were suitable for sequencing were selected as templates for PCR amplification. An Agilent 2100 Bioanalyzer and ABI StepOnePlus RT-PCR system were used to assess the quantification and qualification of the sample library. The library was then sequenced with an Illumina HiSeq 2000 sequencing system.
2.7. Bioinformatics analyses for RNA-Seq
The quality of 90 paired-end reads was assessed with FastQC(http://www.bioinformatics.babraham.ac.uk/projects/fastqc/)[54]. The paired-end raw reads were trimmed using the Trimmomatic 0.33 pipeline to remove low-quality base calls and adaptor sequences [55]. The cleaned reads were mapped to reference sequences of T. aestivum downloaded from the Chinese Spring (CS) database (ftp://ftp.ensemblgenomes.org/pub/release-39/plants/fasta/triticum_aestivum) using TopHat 2.09 [56] with default parameters. Differential expression between YM18 and ZK001 (each with three biological replicates) was evaluated with the DESeq R package [57]. For each gene, the adjusted P-value and log2fold change were computed using DESeq, and those with an adjusted P-value< 0.05 and a log2fold change absolute value ≥1 were considered to be differentially expressed. Gene functions were annotated by BLAST alignment against reference protein coding sequences in the CS database, Gene Ontology (http://www.geneontology.org/, GO) [58] and the Kyoto Encyclopedia of Genes and Genomes (http://www.genome.jp/kegg/, KEGG)[59]. Fisher's exact test was used to evaluate enrichment of functional terms(P <0.05).
Sequences of differentially expressed genes (DEGs) extracted from the T. aestivum database were also annotated using the Mercator sequence annotation pipeline (http://mapman.gabipd.org/home)after being analyzed by Mercator4 Fasta Validator, then final analysis were performed with MapMan version as 3.6.0RC1, including metabolism overview mapping and phytohormone mapping[60].
2.8. q-RT-PCR validation
Total RNAs were reverse-transcribed into cDNA using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, AT311) according to the manufacturer's protocol.qRT-PCR was used to measure the transcript levels of DEGs of interest. Each experiment was performed in triplicate with three biological replicates. Target gene-specific primers (Table S1) were designed using the online software Primer 3 version 4.1.0 (http://primer3.ut.ee/http://bioinfo.ut.ee/primer3-0.4.0/primer3/) [61,62]. RT-PCR was performed with a Roche LightCycler 96 instrument according to the manufacturer's instructions for the SYBR Premix Ex Taq II(Tli RNaseH Plus)(TaKaRa, RR820). The glyceraldehyde-3-phosphate dehydrogenase gene from T.aestivum(TaGAPDH,GI:7579063)was used as an internal control and the relative expression of target genes was calculated by the 2-ΔΔCTmethod[63].To verify the reliability of DEGs, qRT-PCR was employed to study the relative expression levels of arbitrarily selected genes and 20 selected genes.
3. Results
3.1. FHB resistance testing
More hyphae of FHB with green signals were enriched on florets and stalks of YM18(Fig.1-B,D)than in that of ZK001(Fig.1-F,H).In 2018, Fusarium-damaged kernels (FDKs) of QM725, YM18, and ZK001 were counted in a plot of 0.13 ha without fungicide treatment. Table 1 shows that the diseased kernel proportions of ZK001 were 2.67- and 7.81-fold lower than those of QM725 and YM18(Table 1,Fig.S1).The DON content of ZK001 was 0.20 mg kg-1, significantly lower than in QM725 and YM18 (Table 1). Thus, the cleistogamous wheat showed lower FHB infection than the chasmogamous wheat.
3.2. Comparison of phenotypic differences between YM18 and ZK001
There were significant differences in phenotype between YM18 and ZK001(Fig.S2).For instance,the maturation period of ZK001 was a week longer than that of YM18.Compared with YM18, ZK001 was about 15 cm taller (Fig. S2-A, P <0.001).Effective tillering (which can produce spikes of wheat and thus contribute to yield) number, spike length, and kernel width in YM18 were similar to those of ZK001(Fig.S2-B,C, F).The number of spikelets in ZK001 was 1.65 greater than that of YM18(Fig.S2-B),whereas the number of kernels in ZK001 was 8.15 fewer than that of YM18(Fig.S2-B).The thousand-kernel weight of ZK001 was 1.09-fold that of YM18 (Fig. S2-D). The kernel thickness of ZK001 was 95.3% that of YM18 and its kernel length was 118%that of YM18(Fig.S2-E,G).
The anthers of YM18 were being extruded from the paleae and lemmae at flowering(Fig.2-A),whereas all anthers of ZK001 were retained inside the paleae and lemmae at all flowering stages(Fig.2-B). The lodicule width of YM18 and ZK001 was significantly increased from GAS(Fig.2-E,H)to YAS(Fig.2-K,N)and from YAS(Fig. 2-K, N) to AS (Fig. 2-Q, T), while that of YM18 was dramatically greater than that of ZK001 at YAS (Fig. 2-Q, T, Fig.S4,Table 2).Total length(TL)and effective length(EL)were both increased from GAS to AS in YM18,whereas TL showed almost no change from GAS to AS in ZK001 and EL decreased(Table 2,Fig.S4).The ratio EL/TL was essentially the same from GAS to AS in YM18,but decreased from GAS to AS in ZK001 (Table 2, Fig. S4).The size of YM18 lodicules,which were harvested at the GAS and cultivated on MS media was gradually increased; while that of ZK001 lodicules was small,thin and barely changed(Fig.S3).
Lodicule ultrastructures were clearly visible in TEM images in both YM18 and ZK001 (Fig. 3-A). However, the mean size of lodicule cells was greater in ZK001(Fig.3A-e-h)than in YM18(Fig.3A-a-d).There were fewer cells per unit area in ZK001(Fig.3A-eh) than in YM18 (Fig. 3A-a-d). Many circle-like structures containing almost no organelles were detected in the lodicule cells of both YM18(white arrow in Fig.3A-a,d)and ZK001(white arrow in Fig. 3A-e, h). These structures were speculated to be a nutrient substance. Exorheic nuclear matter of the irregular nucleus and abnormal structure of the mitochondria were detected in lodicule cells of YM18 (black arrow in Fig. 3A-c). In contrast,the nucleus and mitochondria were normal and intact in the lodicule cells of ZK001(Fig.3A-e-h).These results indicated that the deforming of the nuclear staining and mitochondria occurred in the flower development stage of YAS in lodicule cells of YM18 and was the result of water absorption.
3.3. Physiological characteristics of lodicules in YM18 and ZK001
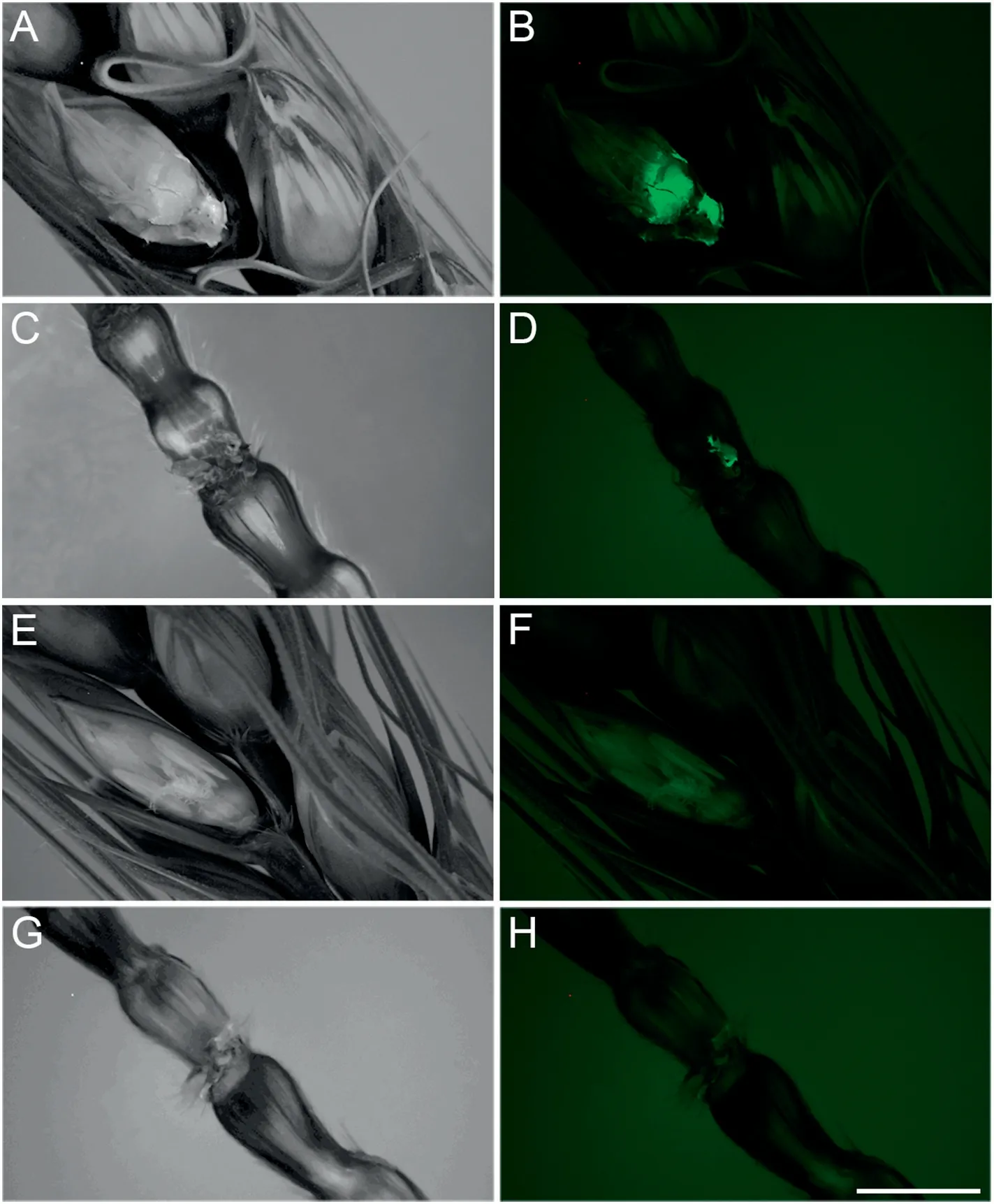
Fig.1-Infection type comparison of spikes of YM18(A to D)and ZK001(E to H).Signals of FHB on flowers(B and D)and stalks(F and H)comparison between ZK001 and YM18,respectively.A,C,E,and G show bright-field images of B,D,F,and H,respectively.Bar,5 mm.
Lodicule potassium ion(Fig.3-B,Table S2)content showed an overall increase from the WAS to the AS for YM18 and ZK001.No significant differences in potassium ion content in the lodicules were observed between YM18 and ZK001 during GAS. Potassium ion content in ZK001 during WAS and YAS significantly decreased 1.25- and 1.73-fold, respectively (both P < 0.05), but significantly increased 1.47-fold during AS(P <0.05)compared with those in YM18(Fig.3-B,Table S2).
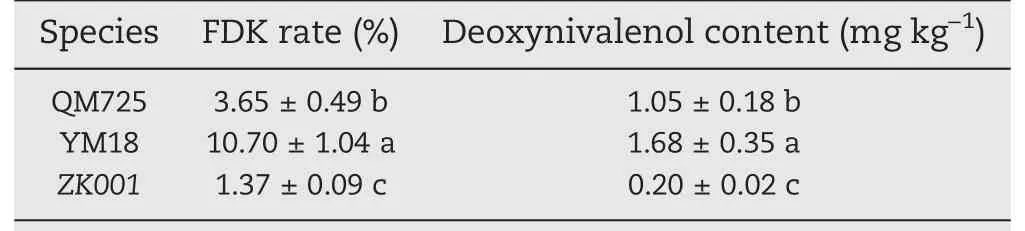
Table 1-Infection type in adult plants tested in 2017-2018.
At WAS, the lodicule calcium content was 7.00-fold(P <0.05)higher in YM18 than in ZK001, detected in each pair of lodicules (Fig. 3-C, Table S2). There were no significant differences between GAS and YAS in this respect; however,lodicule calcium content decreased dramatically by 2.38-fold(P <0.05) from GAS to YAS in YM18 (Fig. 3-C, Table S2). In contrast, there was no significant difference in lodicule calcium content from WAS to YAS or from GAS to AS in ZK001(Fig.3-C,Table S2).
3.4. RNA-Seq and differential gene expression analysis

Fig.2- Comparison of phenotypic characters between YM18 and ZK001.A.Characteristics of flowering types of YM18(bar,1 cm).B.Characteristics of flowering types of ZK001(bar,1 cm).C-E,I-K,and O-Q show spikelet,partial floret,and lodicules in YM18 at GAS,YAS,and AS(bar,2 mm).F-H,L-N,and R-T show spikelet,partial floret,and lodicules in ZK001 at GAS,YAS,and AS(bar,2 mm).
In the present study, the lodicules of YM18 began to absorb water and expand at GAS, whereas the sizes of the ZK001 lodicule at GAS,YAS,and AS retained their status at WAS.We accordingly hypothesized that gene expression profiles would differ between YM18 and ZK001 at GAS. Agarose gel electrophoresis of total RNAs extracted from YM18 and ZK001 with three biological replicates(YM18-1,2 and 3 and ZK001-1,2 and 3)showed that these RNAs were of sufficient quality for RNAseq experiments(Fig.S5).RNA-seq reads were mapped to the reference sequences of the wheat genome with 122,576,887 raw paired-end 90-bp reads of all samples (Table 3). Unique reads were determined following Mayer et al. [64]. A cluster dendrogram and boxplots analysis showed that gene expression levels were highly correlated between replicate samples,with correlation values >0.98 (R2>0.98, Fig. 4-A, B; Fig. S6),except for ZK001-3. Among the expressed genes, 13,523(11.04%) genes were assigned as DEGs with thresholds of an adjusted P <0.05 and a log2fold change absolute value ≥1(Fig.4-C).Among the DEGs,6583(5.38%)underexpressed and 4703(3.84%) overexpressed genes were detected in ZK001 by comparison with YM18 (Fig. 4-D, Fig. S7). In YM18 and ZK001,respectively 1499 (1.22%) and 738 (0.60%) specific expressed genes were detected(Fig.S7).The qRT-PCR expression profiles of selected genes were found to be essentially consistent with the transcriptomic profiles revealed by RNA-Seq, except for AA1174460, AA0292870, AA1277850, AA0556860, and AA1734440 (Figs. S8, S9). This finding indicated that the relative expression levels from RNA-seq were reliable and suitable for further analysis.

Table 2-Comparison of lodicule size between YM18 and ZK001 at GAS,YAS,and AS.
3.5. Function and enrichment analysis of DEGs
The 13,523 transcripts were functionally annotated by GO analysis (Figs. S10, S11) and KEGG (Fig. 5). The GO terms of all identified DEGs were enriched mainly in metabolic processes,cellular processes,and single-organism processes for biological processes,catalytic activity,or binding and transporter activity for molecular function and cell,membrane,cell part,membrane part, and organelle for cellular components (Fig. S10). The GO terms of overexperssed, underexperssed, YM18-specific, and ZK001-specific genes were consistent with those of all DEGs in biological processes, molecular functions, and cellular components (Figs. S10, S11). The KEGG function assignments of all DEGs were enriched mainly in carbohydrate metabolism, lipid metabolism,and cell motility,including regulation of the actin cytoskeleton,pentose and glucuronate interconversions,starch and sucrose metabolism, and cutin and suberine and wax biosynthesis(Fig.5).
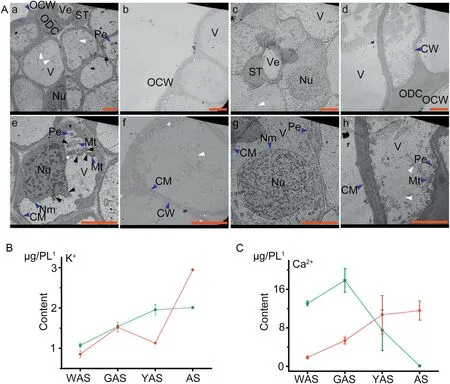
Fig.3- Comparison of ultrastructural and physiological characters between YM18 and ZK001.A.Transmission electron microscope(TEM)images of lodicule ultrastructures of YM18 and ZK001.a-d,YM18;e-h,ZK001.OCW,outer cell wall;CW,cell wall;Ve,vessel;ST,sieve tube;Pe,peroxysome;Nu,nucleus;V,vacuole;CM,cytomembrane;Mt.,mitochondrion;ODC,outer degenerated cell. All bars represent 5 μm.B. Comparison of potassium content change tendency in lodicules between YM18(green line)and ZK001(red line).C.Comparison of calcium content change tendency in lodicules between YM18 and ZK001. 1,pair of lodicules(PL).Values are the means of three independent experiments.Error bars,standard deviation.
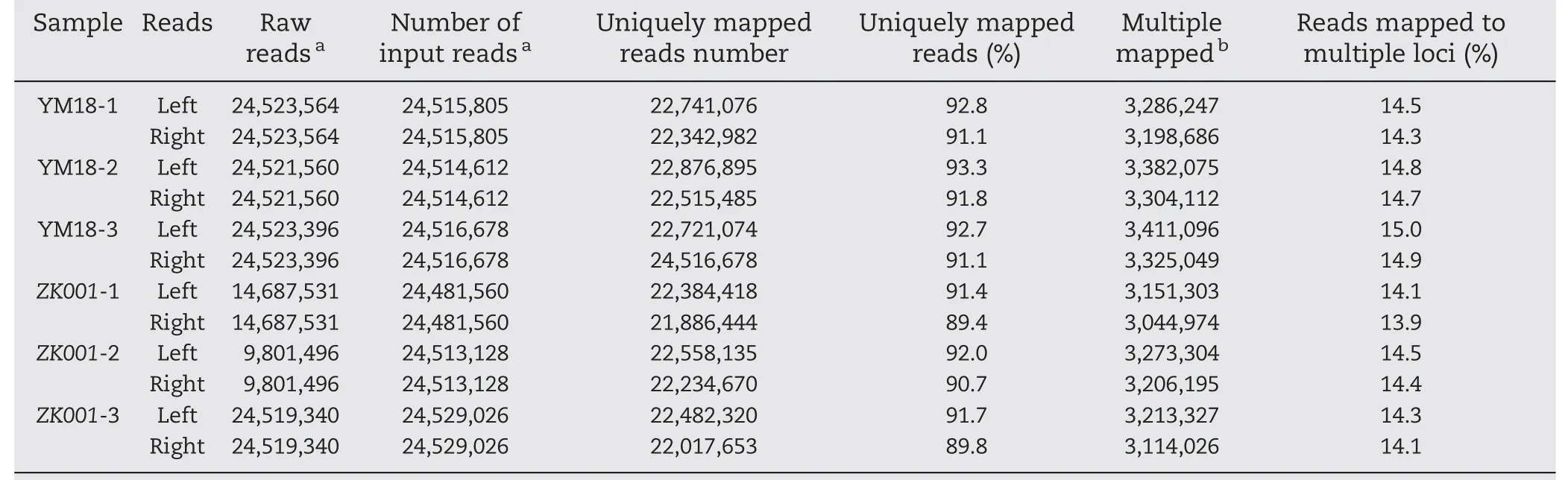
Table 3-Statistics of RNA-Seq read mapping results.
3.6. DEGs associated with cell wall and carbohydrate metabolism
According to the mapping results using the metabolism overview and hormone pathways in MapMan, these genes were concentrated mainly in the cell wall, lipid and carbohydrate metabolic pathways(Fig.6).
To investigate the expression profiles in lodicules of YM18 and ZK001, DEGs with adjusted P <0.001 were selected for further analysis using qRT-PCR. Many of the DEGs were associated with cell wall metabolism(Fig.6).In this pathway,the gene encoding the cell wall degradation enzyme pectinesterase (AA1174460) was selected to compare the gene expression profile in the lodicules of YM18 and ZK001(Fig.7).DEGs that involve in regulation the lodicule cell wall were detected in YM18,including an arabinogalactan-protein(AGP)gene (AA0292870), pectinesterase genes (AA1277850 and AA0556860), and a precursor synthesis gene (AA2028090)(Fig. S12). Compared to genes of YM18 lodicules, these genes were all downregulated in ZK001 from GAS to YAS,except for AA2028090 (Fig. 7-A-E). However, the gene expression of AA2028090 was downregulated in the lodicules of YM18 from GAS to YAS(Fig.7-E).
In previous studies(unpublished)in our laboratory,starch and total soluble sugar content were lower in ZK001 than in YM18 during YAS. After mapping in MapMan, sugar transmembrane transporter activity genes(AA1914070 and AA0095680)and starch degradation genes(AA0614380 and AA1237310)were selected to compare the expression profile in the lodicules of YM18 and ZK001.Compared with YM18,the gene expression of AA0095680 was upregulated in ZK001 at GAS and YAS, whereas that of AA1914070 was upregulated in ZK001 only at GAS(Fig.7-F,G).The expression of the starch degradation genes AA0614380 and AA1237310 was upregulated in ZK001 at GAS and YAS, respectively (Fig. 7-H, I). The glycolysis gene encoding UDP-glucose pyrophosphorylase (UGPase) (AA1637150) and the tricarboxylic acid(TCA)cycle genes AA0799240 and AA0289880 were differentially expressed in YM18 and ZK001. According to the qRT-PCR results, the expressions of AA1637150 and AA0289880 were upregulated in ZK001 at YAS and GAS, respectively; further,AA0799240 was upregulated in ZK001 at GAS and YAS(Fig.7-J-L).
3.7. DEGs associated with hormone metabolism
Forty-two DEGs were enriched in the primary functional category hormone metabolism, including GA, ABA, and JA(Fig. S13). Of these, three differentially expressed genes that were involved in ABA (AA1260570), GA (AA1873370), and JA(AA1558340) metabolism were selected for investigation of their expression profiles by qRT-PCR. Compared with YM18,the gene expression of AA1260570 was upregulated in lodicules of ZK001 at both GAS and YAS, and the gene expression of AA1260570 was upregulated from GAS to YAS(Fig. 7-M). In contrast, there were no changes in the gene expression of AA1260570 between GAS and YAS in the lodicules of YM18 (Fig. 7-M). The gene expression of AA1873370 was downregulated in the lodicules of both YM18 and ZK001 from the GAS to YAS(Fig.7-N).The gene expression of AA1558340 was upregulated in the lodicules of YM18 from GAS to YAS, with both being lower than the expression in ZK001(Fig.7-O).
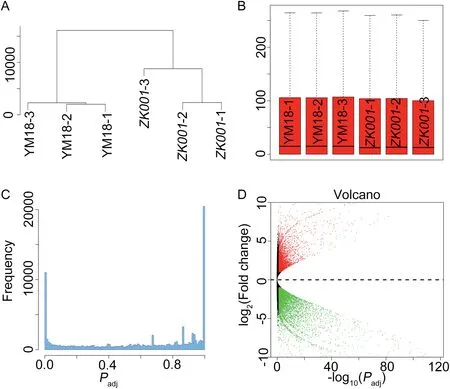
Fig.4- Gene expression profiles in spikelets at green anther stage of YM18 and ZK001. A.Cluster dendrogram of gene expression profiles between biological replicates and between YM18 and ZK001.B. Boxplots indicate the quality of all replicates of YM18 and ZK001.C.Frequency distribution of all mapped genes.D.Volcano plot showing the distribution of all genes detected.
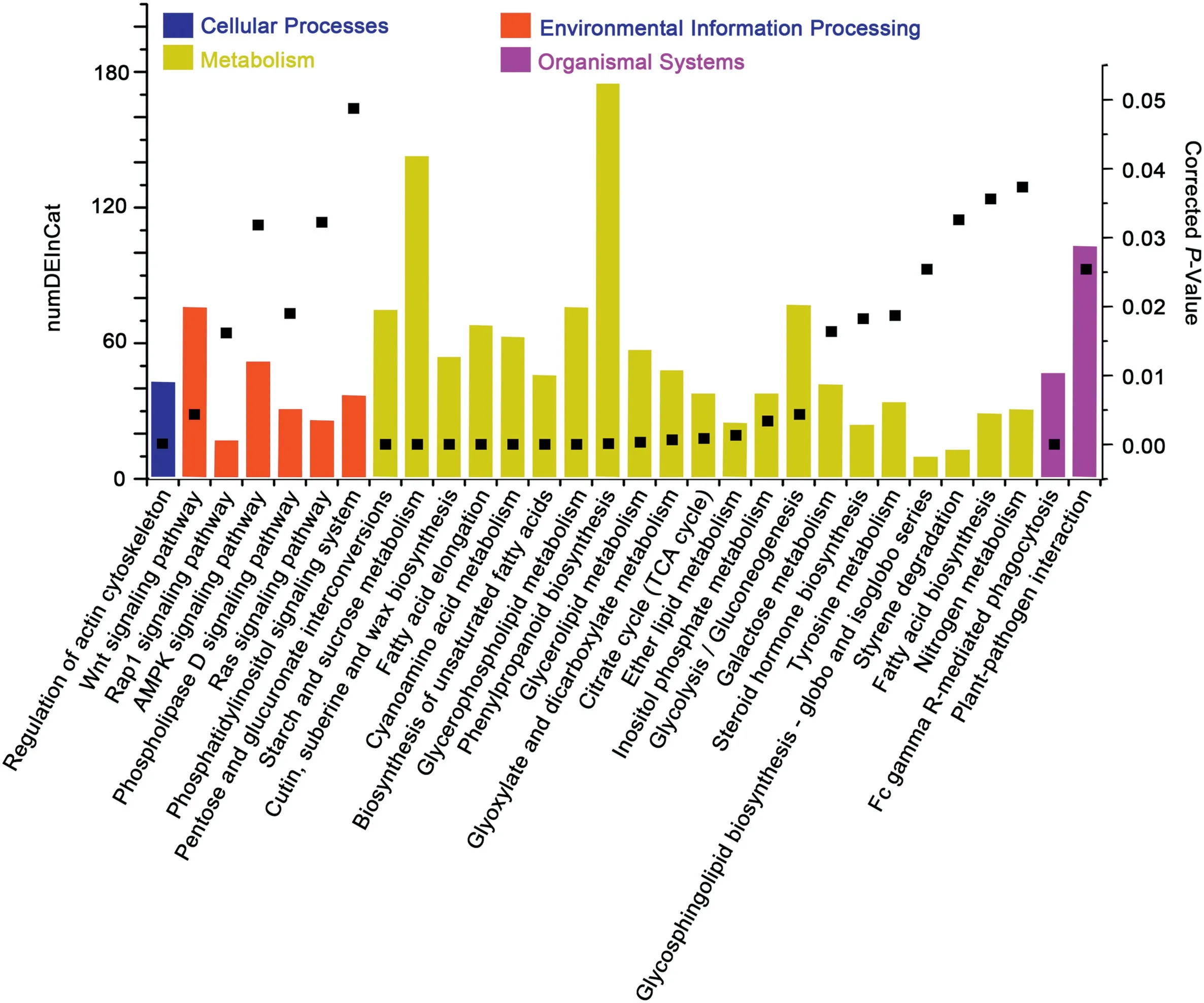
Fig. 5-KEGG classification of all identified DEGs of YM18 and ZK001.
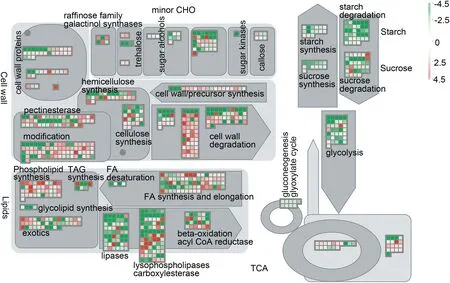
Fig.6-MapMan metabolism overview maps showing differences in transcript levels between YM18 and ZK001 at green anther stage.log2 fold change ratios for average transcript abundance were calculated based on three replicates of YM18 and ZK001.The resulting file was loaded into the MapMan Image Annotator module to generate the metabolism overview map.On the logarithmic color scale,green represents downregulated and red represents upregulated transcripts.
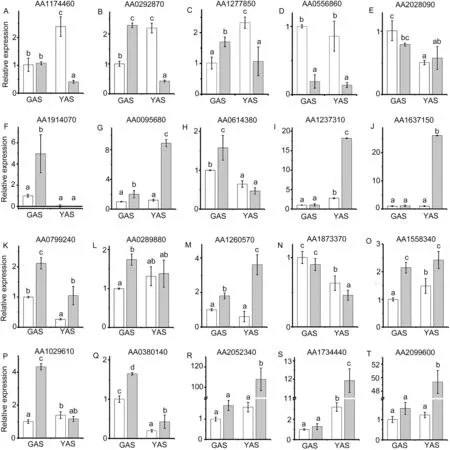
Fig.7-Relative expression profiles of selected differentially expressed genes in lodicules of YM18(white column)and ZK001(gray column).Values are the means of three independent experiments.Error bars,standard deviation.Different lowercase letters indicate significant difference at P <0.05.
3.8. DEGs associated with water channel, ion binding,transport, signaling, and homeostasis
Ions play an important role in the development and metabolism of cellules.According to the biological process of GO annotation,five ion-associated genes were selected for investigation of their expression profiles of the lodicules in YM18 and ZK001 using qRTPCR, including potassium ion-related genes (AA1029610 and AA0380140), calmodulin binding-related genes (AA2052340 and AA1734440) and a calcium-mediated signaling-related gene(AA2099600) (Fig. 7-P-T). The qRT-PCR expression of the twopotassium ion-related genes was almost always upregulated in the lodicules of ZK001 at GAS and YAS (Fig. 7-P, Q). In addition,unlike in the lodicules of YM18, the expressions of calmodulin binding-related genes and the calcium-mediated signalingrelated gene were upregulated in the lodicules of ZK001 at YAS(Fig.7-R-T).
4. Discussion
4.1. Cleistogamy as a new way of reducing susceptibility to FHB
The result showed in Fig. 1 agrees with a finding in barley[21,22] of a lower FHB infection rate in cleistogamous than in chasmogamous cultivars. Although the FHB infection rate is strongly influenced by environmental factors, cleistogamous cultivars may have a lower risk of FHB infection than chasmogamous cultivars [18,20]. Table 1 shows that the FDK rate and DON content were significantly lower in ZK001 than in QM725 and YM18 in the absence of a fungicide.Thus,using cleistogamous varieties might reduce Fusarium incidence[18,20]. We propose cultivating cleistogamous wheat as a new strategy for controlling FHB. This cleistogamous mutant could be used as a basis for further genetic improvement via conventional crossing.
4.2. Lodicule swelling may be the beginning of nuclear degradation and organelle autolysis
Lodicules, which are considered equivalent to eudicot petals,are grass-specific floral organs with scale-like shapes that play an important role in the flower opening process [65]. In wheat, the pair of lodicules situated at the base of the ovary and adjacent to the palea and lemma of the floret swell and force the lemma away from the palea, induces the wheat floret to open at anthesis [66] (cited in [28]). A few minutes later and presumably in response to a stimulus associated with pollination, the lodicules collapse and the floret closes[28].Evidently,the nuclear matter starts deforming and flows out and the structure of the mitochondria start deforming in the lodicule cell of YM18 at late YAS(black arrow in Fig.3A-c).The mean lodicule cell size was larger in ZK001 (Fig. 3A-e-h)than in YM18(Fig.3A-a-d),and there were fewer cells per unit area in ZK001(Fig.3A-e-h)than in YM18(Fig.3A-a-d).But the nucleus and mitochondria were normal and intact in the lodicule cell of ZK001 in late YAS(Fig.3A-e-h),suggesting that lodicules absorb water and expand normally in YM18. Craig and Brien [29] showed that the dorso-ventral plane of the lodicule increased in size from two weeks to five days before anthesis that the lodicule was almost spherical 45 min after the glumes had opened and that at that stage nuclear staining clearly deformed the parenchyma cells of the lodicules in wheat. We accordingly speculate that the water absorption and expansion of a normal lodicule is a determinant of nuclear degradation and organelle autolysis and that the thin lodicules of the mutant line ZK001 are unable to absorb water and expand. We speculate that GAS is the key period for lodicule development in wheat.
4.3.Carbohydrates and ions are key factors in lodicule swelling after water absorption
Liu et al.[31]suggested that the lodicule expansion caused by reduced water accumulation was directly related to the accumulation of osmotic regulation substances, and that total soluble sugar was the main osmotic adjustment substance in absorption of the water of lodicules [32,34].Interestingly, the total soluble sugar content in the lodicules of YM18 increased from GAS to YAS[67],permitting the water uptake required by lodicules [28,30,31]. Many circle-like structures were detected in the lodicule cells of YM18 and ZK001(white arrows in Fig.3A-a,d,e,h),which we suggest are nutrient substances, such as sugar, starch and other carbohydrates. Sugar transmembrane transporter activity genes(AA1914070 and AA0095680) and starch degradation genes(AA0614380 and AA1237310) were upregulated in lodicules of ZK001 (Fig. 7-F-I). However, the expression levels of a glycolysis gene (AA1637150) and TCA cycle-related genes(AA0799240 and AA0289880) were upregulated in ZK001 (Fig.7-J-L). We accordingly speculate that the starch and total soluble sugar content in the lodicules of ZK001 were metabolized via glycolysis and the TCA cycle pathway,respectively.In contrast, starch and total soluble sugar content were enriched in the lodicules of YM18 and provided the main osmotic adjustment substance for absorbing the water of lodicules.
qRT-PCR showed that the expression of the potassium ion import gene AA0380140 was downregulated in the lodicules of both YM18 and ZK001 from GAS to YAS.However,in lodicules of ZK001, it was upregulated in both GAS and YAS (Fig. 7-Q).The expression of the potassium ion homeostasis gene AA1029610 was upregulated in lodicules of YM18 from GAS to YAS but downregulated in lodicules of ZK001 from GAS to YAS(Fig.7-P).The potassium ion content rapidly increased in the lodicules of YM18 from GAS to YAS but decreased in ZK001(Fig. 3-B, Table S2). We speculate that potassium ion flux is a crucial factor in the closure of flowers in ZK001,given that the potassium ion content was significantly lower in the lodicules of ZK001 than in those of YM18 during the flowering process.This speculation is in accord with a finding in rice [30]. The failure of osjar1 mutant florets to close was likely associated with potassium ion flux and homeostasis in its lodicules[33].
Calcium ions are an important intracellular secondary messenger in plants and play important roles in plants that encounter unfavorable environmental conditions [68,69]. The calcium ion content was significantly lower in lodicules of ZK001 than in those of YM18 from GAS to YAS(Fig.3-C,Table S2). We infer that GAS is a critical period for lodicules to absorb water.Qin et al.[34]described the dynamic changes in calcium ion content in lodicules during the flowering stage in rice. Calcium ions are gradually absorbed by the cytoplasm and nucleolus of lodicule cells from one day to 4 h before AS.A large amount of flocculent precipitate accumulates in the vacuoles at the beginning of anthesis,and the amount of this precipitate gradually decreases until it completely disappears.In the present study, the total calcium ion content in YM18 was higher than that in ZK001 from WAS to YAS and there was no significant difference in content between YM18 and ZK001 during YAS. The difference in calcium ion content in the lodicules was widened between YM18 and ZK001 by the dramatic reduction in calcium content in YM18 lodicules(Fig.3-C, Table S2). In lodicules of ZK001, the expressions of calmodulin binding and calcium-mediated signaling genes were upregulated from GAS to YAS (Fig. 7-R-T). These genes are involved mainly in osmotic adjustment and signal transduction processes of plasma cells, and we speculate that they are closely associated with water absorption and play an important role in promoting the expansion of lodicules.
4.4. Metabolism of phytohormone and cell walls ensures lodicule swelling
9-cis-epoxycarotenoid dioxygenase(NCED)is a key enzyme in the biosynthesis of abscisic acid (ABA), which is associated with osmotic regulation in plants [35]. Gibberellins (GAs),which are endogenous hormones, are necessary for normal plant growth [70].The GA3oxidase (GA3ox) gene functions in the late stages of GA biosynthesis and catabolic pathways,which are crucial regulatory steps in GA balance[71,72].In the present study, the expression level of AA1260570 was upregulated in lodicules of ZK001 from GAS to YAS to a higher level than in YM18 (Fig. 7-M). The expression of AA1873370 was downregulated in the lodicules of both YM18 and ZK001 from GAS to YAS (Fig. 7-N). However, whether ABA or GA are involved in regulation of the opening of wheat spikelets and whether AA1873370 participates in flowering time regulation by regulating the biosynthesis of ABA and GA in lodicules await further study.
Allene oxide synthase (AOS), a cytochrome P-450 (CYP74A),plays an important role in catalyzing the first step in the conversion of 13-hydroperoxy-linoleic acid to jasmonic acid(JA)and related signaling molecules in plants[73].According to the expression level of a JA-related gene, allene oxidase synthase(AA1558340)is upregulated in the lodicules of YM18 from GAS to YAS (Fig. 7-O). Qin et al. [34] reported that rice florets treated with methyl jasmonate were induced to open within 10-30 min.We accordingly speculate that endogenous JA participates in the regulation of water absorption by lodicules.
The plant cell wall is a key factor in morphogenesis,growth,physical strength,transport of intercellular materials,and signal transduction. To complete the water absorption and swelling of lodicules in rice, the cell walls of lodicules must relax and thus the osmotic potential in lodicules should be low [30].Xyloglucan cross-links with cellulose, pectin, and certain extensions(such as arabinogalactan proteins)to form the main bearing structure of the primary cell wall in plants[74,75].In the present study,the expressions of pectinesterase genes (AA1174460, AA1277850, and AA0556860) were sharply downregulated in lodicules of ZK001 from GAS to YAS; in contrast, the expression of the precursor synthesis gene(AA2028090) was downregulated in the lodicules of both YM18 and ZK001 (Fig. 7-A, C-E). This finding suggests that these genes are involved in floret expansion in wheat by reducing the strength of the cell wall and increasing its ductility. Arabinogalactan-proteins (AGPs) are presumably involved in molecular interactions and cellular signaling at the cell surface [76], and the AGP gene (AA0292870) may play an important role in the part of the lodicule that senses external changes and absorbs water(Fig.7-B).
5. Conclusions
The wheat mutant ZK001, with a cleistogamous phenotype,shows a lower FDK and DON content than YM18 and QM725.The thin lodicules in its florets have lost the ability to push the lemma and palea apart during the flowering stages,leading to the cleistogamous phenotype. Comparative transcriptome analysis revealed that the main differentially expressed genes identified in the spikelets of YM18 and ZK001 at GAS were associated with cell walls, carbohydrates, phytohormones, water channel, and ion binding, transport, and homeostasis. These DEGs may play an important role in regulating cellular homeostasis, osmotic pressure, and lodicule development. Genes that regulate potassium and calcium ions are involved mainly in the osmotic adjustment and signal transduction processes of plasma cells, and these processed are closely associated with water absorption and play an important role in promoting the expansion of lodicules. Analysis of the transcriptome invites further research on lodicule development mechanisms in wheat.
Accession numbersThe raw read data for this study have been submitted to the NCBI Sequence Read Archive (SRA; http://www.ncbi.nlm.nih.gov/sra)with accession number PRJNA491844.Declaration of competing interestAuthors declare that there are no conflicts of interest.AcknowledgmentsThis work was supported by the National Key Research and Development Program of China, China (2018YFD0300901), the Science and Technology Service Program of Chinese Academy of Sciences, China (KFJ-STS-ZDTP-054), the Key Program of 13th Five-Year Plan, Hefei Institutes of Physical Science, Chinese Academy of Sciences,China(No.kp-2017-21),Shanghai Agriculture Applied Technology Development Program, China(G2015060104), and the Opening Fund of State Key Laboratory of Crop Genetics and Germplasm Enhancement, China(ZW2013003).We thank Dr.Guomin Han,School of Life Sciences,Anhui Agricultural University,for data processing and analysis.We thank Prof. Xiu'e Wang, College of Agriculture, Nanjing Agricultural University,for providing FHB strain Chi-2.We thank Prof.Jianlai Wang,Institute of Crop Research,Anhui Academy of Agricultural Sciences, for providing seed of QM725. We thank Lingzhi Wei, Modern Experimental Technology Center, Anhui University, for ICP-AS analysis. We also thank Shiliang Li,Shengqun Zheng,and Xiangfei Ruan for field management.Appendix A.Supplementary dataSupplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2019.08.009.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Boosting proso millet yield by altering canopy light distribution in proso millet/mung bean intercropping systems
- Changes in leaflet shape and seeds per pod modify crop growth parameters,canopy light environment,and yield components in soybean
- Genome-wide association study of vitamin E in sweet corn kernels
- Strip rotary tillage with subsoiling increases winter wheat yield by alleviating leaf senescence and increasing grain filling
- Vacuolar invertase genes SbVIN1 and SbVIN2 are differently associated with stem and grain traits in sorghum(Sorghum bicolor)