Simultaneous transarterial chemoembolization and radiofrequency ablation for large hepatocellular carcinoma
Feng Duan, Yan-Hua Bai, Li Cui, Xiao-Hui Li, Jie-Yu Yan, Mao-Qiang Wang
Feng Duan, Yan-Hua Bai, Li Cui, Xiao-Hui Li, Jie-Yu Yan, Mao-Qiang Wang, Department of Interventional Radiology, General Hospital of Chinese People’s Liberation Army, Beijing 100853, China
Abstract
Key words: Chemoembolization; Radiofrequency ablation; Hepatocellular carcinoma;Simultaneous treatment; Transcatheter arterial chemoembolization; Radiofrequency ablation
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common cancer and a leading cause of tumorrelated death[1,2]. Patients who have large HCC (≥ 8 cm) are generally at advanced stages and have poor prognosis[3,4]. Hepatectomy may not suitable for patients who have large HCC or dysfunction of liver reserve and few patients are suitable for surgery. Besides, the postoperative recurrence is high[5].
Transcatheter arterial chemoembolization (TACE) and radiofrequency ablation(RFA) is commonly used for liver cancer. For TACE, the best candidates are patients with no symptoms and well-preserved liver function, as well as multifocal tumors with no vascular invasion or extrahepatic spread. However, TACE alone only leads to partial tumor necrosis. For small liver cancers (< 3 cm), RFA and surgery are comparable when it comes to therapeutic efficacy[6,7], but for tumors > 3 cm, RFA has poor local tumor control[8,9]. Therefore, combination of TACE and RFA may improve therapeutic efficacy and extend survival time.
In the present study, we evaluated the efficacy of combined TACE and RFA for large HCC. We retrospectively followed up 46 patients who received the combination treatment from March 2010 to November 2013 and assessed mid-term efficacy of the combination treatment modality as a novel strategy.
MATERIALS AND METHODS
Patient data
A total of 46 consecutively identified patients with large HCCs (at least one lesion diameter ≥ 8 cm) were enrolled. The baseline characteristics of these patients were as follows: (1) 42 men and four women; (2) Median age: 53.5 years (range 36-70 years);and (3) According to the Barcelona Clinic Liver Cancer (BCLC) staging classification,advanced HCC was classified as B/C (42/4); liver function: Child-Pugh class A (n=45) and class B (n= 1). The mean tumor size was 8.17 cm (range 8.0-14.0 cm) (Tables 1 and 2). Sex, age, tumor stage, tumor size, number of tumors, Child-Pugh score,vascular invasion (tumor thrombus in the first branch or trunk of the portal vein) and pseudocapsule were taken into consideration as factors for subgroup analysis. This study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital, and patients’ informed consent was obtained. The diagnosis of HCC was based on imaging fi ndings and/or α-fetoprotein (AFP) levels. Tumor stage was classified according to the BCLC classification system. The patients were surgically unsuitable, and without arteriovenous fistula or ascites.
Treatment protocol
After routine preoperative preparation, TACE was performed first, under sterile conditions and general anesthesia[10]. The right femoral artery was cannulated by a 4F vascular sheath (Radifocus Introducer II; Terumo Corp., Japan) and Seldinger’s technique. Selective celiac artery and superior mesenteric artery angiography was performed by 4F hepatic artery catheter (HA; Terumo), which was through thevascular sheath. Maximum catheter selectivity of the hepatic artery was achieved using a microcatheter (Progreat; Terumo), with administration of an embolic agent into the tumor feeding arteries. Drug dose varied from 15 to 20 mL lipiodol (Guerbet Corp., France) each procedure, 30-50 mg doxorubicin (Pfizer Pharmaceuticals Ltd.,United States), 100-150 mg oxaliplatin (Sanofi Pharmaceuticals Co. Ltd., France),depending on the tumor size, patient’s weight and laboratory results. Lipiodol chemotherapeutic agents were injected until stasis to minimize reflux into nontarget vessels. Administration of agents continued until quiescence, and was observed in the arteries that directly fed the tumor (i.e., the control column was fully cleared in five heart beats). After administration of 20 mL lipiodol, gelatin sponge, which served as a supplement, was injected if stasis was not achieved. If the inferior phrenic, internal thoracic artery branches and omental branches fed the tumor, these collateral arteries were embolized accordingly.

Table 1 Baseline characteristics of patients before treatment
Percutaneous RFA was immediately performed after TACE. It was under general anesthesia and with the guidance of digital subtraction angiography (DSA) combined with cone-beam computed tomography (CBCT)[11]. One multipolar RF probe (RITA Co., Crystal Lake, IL, USA) with 5-7 cm maximum ablation diameter and 10-15 cm length was used during RFA. Guided by fluoroscopy, the RF probe was inserted into the center of the tumor. During puncture, both the lateral and postural views were obtained. CBCT was then performed to confirm the position of the RF probe (Figure 1). Ablation began when the target position was reached. The operation parameters were power, 150-200 W; and ablation time, 15 min when temperature rose until 105°C. According to tumor size and maximum ablation diameter, RFA was performed 2-5 times. Puncture tract ablation was carried out to avoid bleeding and tumor seeding.
Patient follow-up and clinical data collection
Enhanced magnetic resonance imaging was used for follow-up every 1-2 mo during the first year, and every 2-4 months afterwards. Tumor recurrence or metastasis was recognized as disease progression. Comprehensive treatment including TACE, RFA,radiotherapy, and sorafenib was performed on patients with disease progression. Two independent authors followed up all the clinical data and follow-up outcomes.
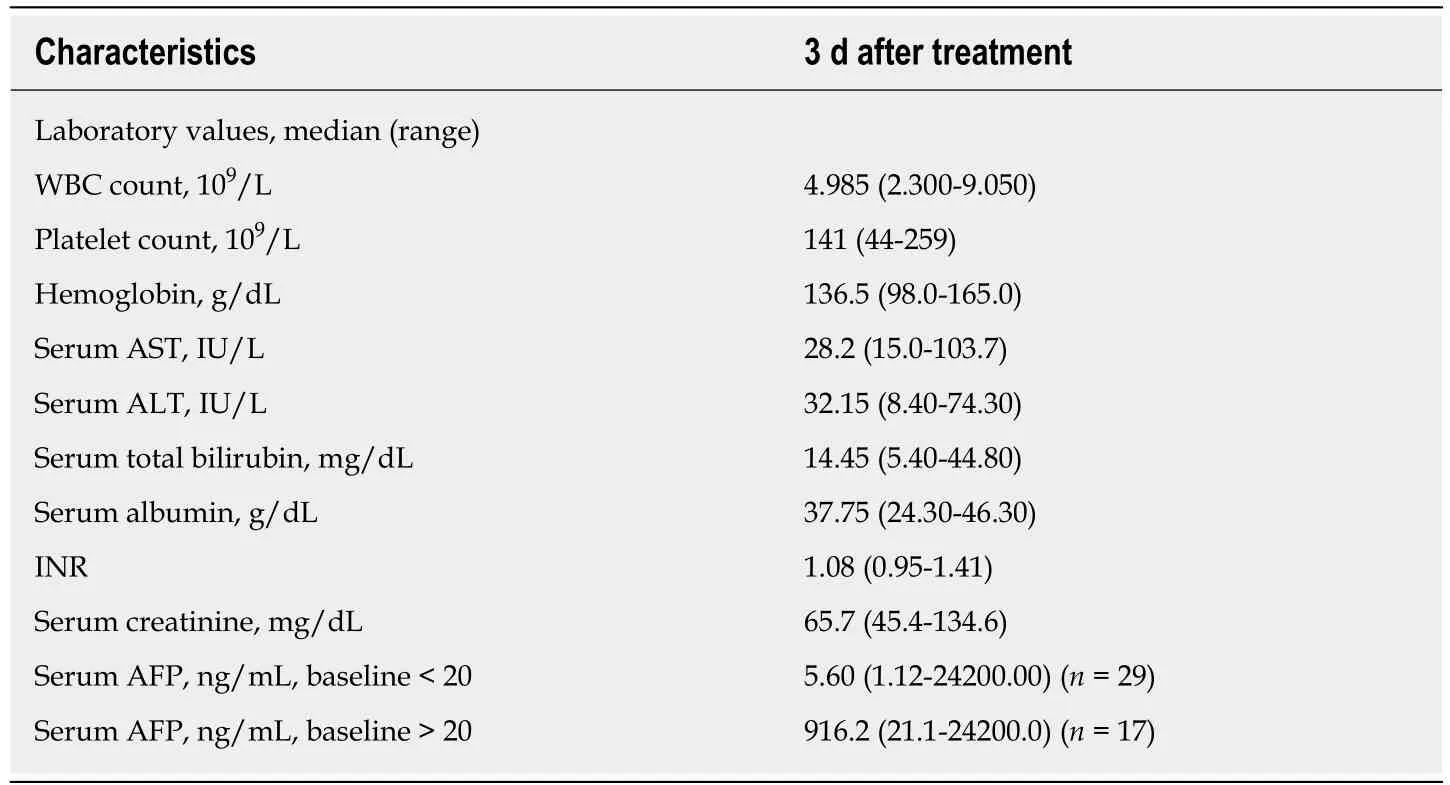
Table 2 Baseline characteristics of patients after treatment
Statistical analysis
SPSS for Windows (SPSS Inc., Chicago, IL, United States) was used for analyzing data.The estimated local tumor progression and overall survival (OS) rates were compared by the Kaplan-Meier method. Cox proportional hazards model was used to fit survival time for each variable.P< 0.05 was considered to be a significant difference.
RESULTS
Treatment response
Figure 2 shows a represe ntative condition after TACE and RFA combination treatment. As shown in Tables 1 and 2, there were no significant differences between laboratory results before and 3 d after treatment. After 2 years, OS was 18.43 ± 1.34 mo and progression-free survival (PFS) was 9.40 ± 1.31 mo; however, after 3 years, OS was 26.44 ± 2.26 mo and PFS was 10.21 ± 1.58 mo. Figure 3 shows the OS and PFS results.
Subset analysis showed similar OS and PFS (Table 3). Among these subsets, four groups showed different results, which were the vascular invasion group, nonvascular invasion group, male group, and female group (marked as A, B, C and D,respectively). OS in the A and D groups was shorter than in the B and C groups. ThePvalues for B and C were 0.019 and 0.031, respectively.
Adverse effects and complications
Clinical adverse events included fever, pain, nausea, fatigue, transient reduction in blood counts and transient elevations of aspartate aminotransferase and alanine aminotransferase levels, but were mostly limited to grade 1 and 2 (Table 2). No severe complications associated with our treatment protocol were noted.
DISCUSSION
HCC is a leading cause of liver-disease-related mortality. Although rapid progress in treatment for large liver cancer has been made in the past few years, neither the prognosis nor postoperative outcomes are satisfactory.
According to a previous report, the combination of TACE and RFA has a synergistic effect on HCC inactivation[12]. The combination improves treatment efficacy, prolongs survival, and reduces recurrence rate. Thus, the combined treatment is superior to TACE or RFA alone[5,13]. So far, treatments are generally launched separately in practice. The time interval between the two modalities was 1 d to 4 wk. Because of the possible collateral formation and elimination of lipiodol chemotherapeutic agents after embolism, the effects of TACE or RFA alone are not synergistic[6,14,15]. Therefore, evaluating the effect of the combined treatment is necessary.
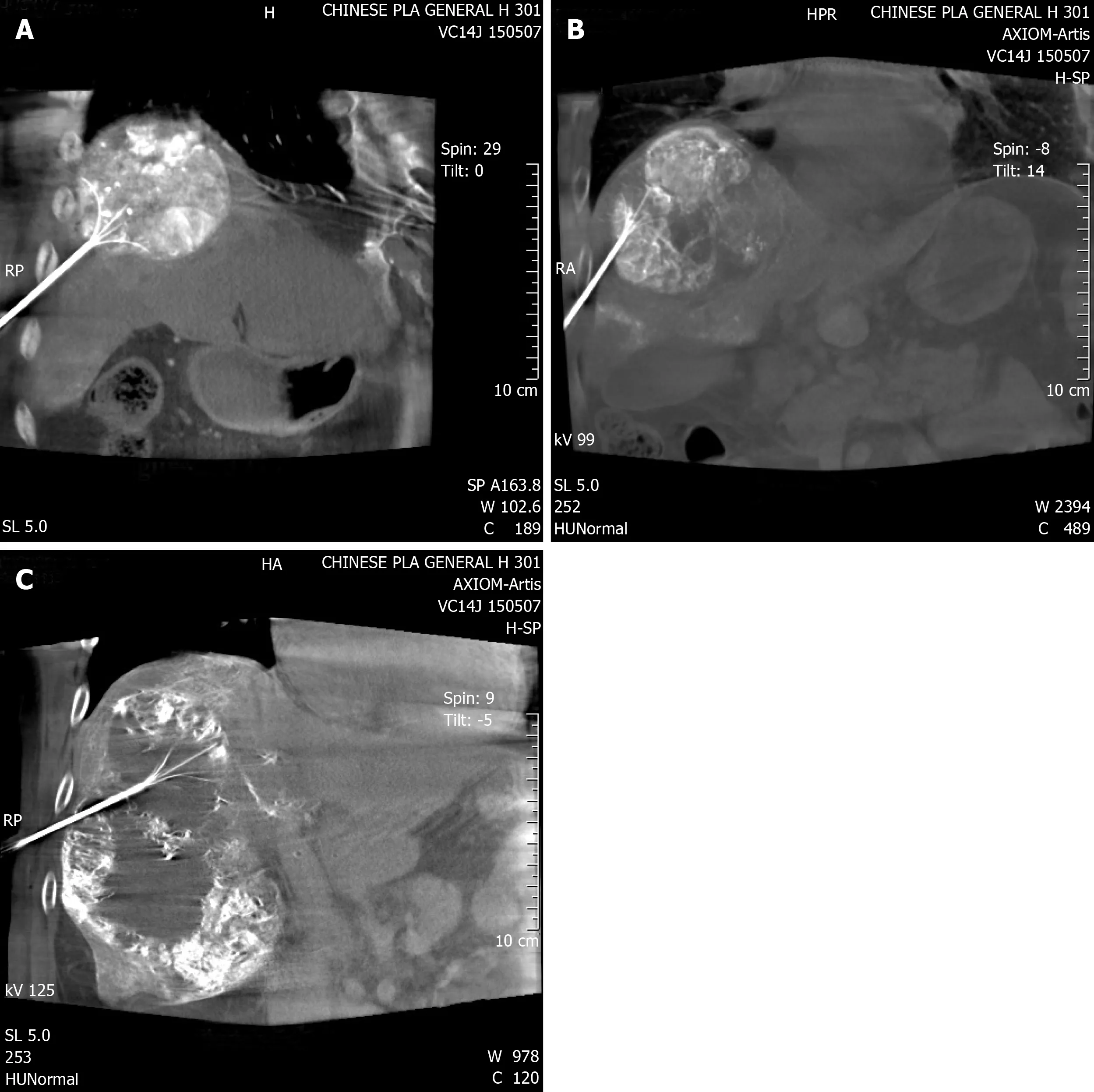
Figure 1 Cone-beam computed tomography image confirmed the position of the radiofrequency probe.
The present study involved 3-year follow-up of the efficacy of simultaneous TACE and RFA in patients with large HCC. This combination treatment may have the following advantages. First, DSA or CBCT can clearly show blood vessels. Both imaging modalities allow successful puncture of the liver and can verify treatment efficacy during the treatment in real time[16,17]. Second, during combination treatment,iodine oil precipitates around the lesions. Thus, it can be used as a heat-transmitting medium to improve ablation efficiency and make the surrounding HCC microenvironment inactive[18]. This can reduce tumor recurrence by improving ablation efficacy. Third, TACE can block blood flow into the tumor, thereby reducing heat loss during RFA[19]. Fourth, after TACE, ablation can be performed immediately,which may also localize damage such as liquefaction necrosis as well as coagulation sclerosis. Moreover, the immediate combination procedure is considered to reduce the side effects of TACE[20]. Finally, in one session of treatment, combination of TACE and RFA can be performed, which may reduce financial burden for the patient. In general,TACE with simultaneous RFA leads to synergistic effects of thermal ablation and chemotherapy. No significant adverse effects were observed in our study. In short, for the efficacy and survival of patients with large HCCs, TACE with simultaneous RFA may be a useful and novel tactic.
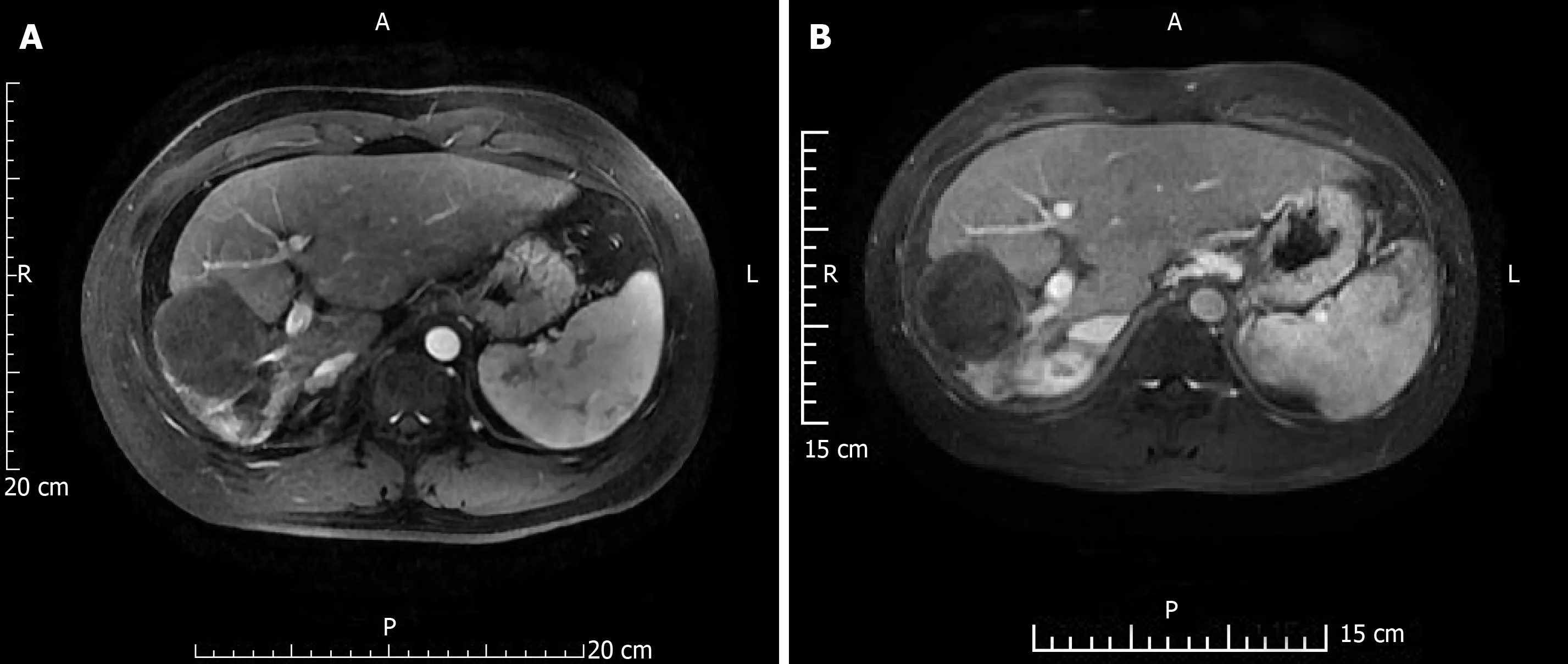
Figure 2 A 41-year-old male patient re-examined 2 and 4 years after combination therapy.
During our follow-up, the incidence of intrahepatic and extrahepatic metastases was higher in patients with vascular invasion than in those without vascular invasion.This indicates that tumor thrombus exhibits poor response to treatment. Multivariate Cox proportional hazard analysis also demonstrated that vascular invasion was an important prognostic factor. In addition, the male and female patients showed significant differences in OS and PFS. The cause of the false-positive result may be the small size of the female group. The pseudocapsule group showed better treatment efficacy mainly because the pseudocapsule may enhance the thermal aggregation effect of ablation, resulting in greater tumor inactivation. However, it was not significant, possibly because of the small sample size. The other subgroups did not show significant differences.
The main limitation of our study was that it was retrospective. Thus, a multicenter prospective study, with a large sample size should be conducted to evaluate further the outcome of TACE and RFA combination treatment in patients with large HCCs.
In conclusion, these preliminary data show that the simultaneous TACE and RFA is a safe, effective and valuable strategy for large HCC, as it improves efficacy and prognosis.
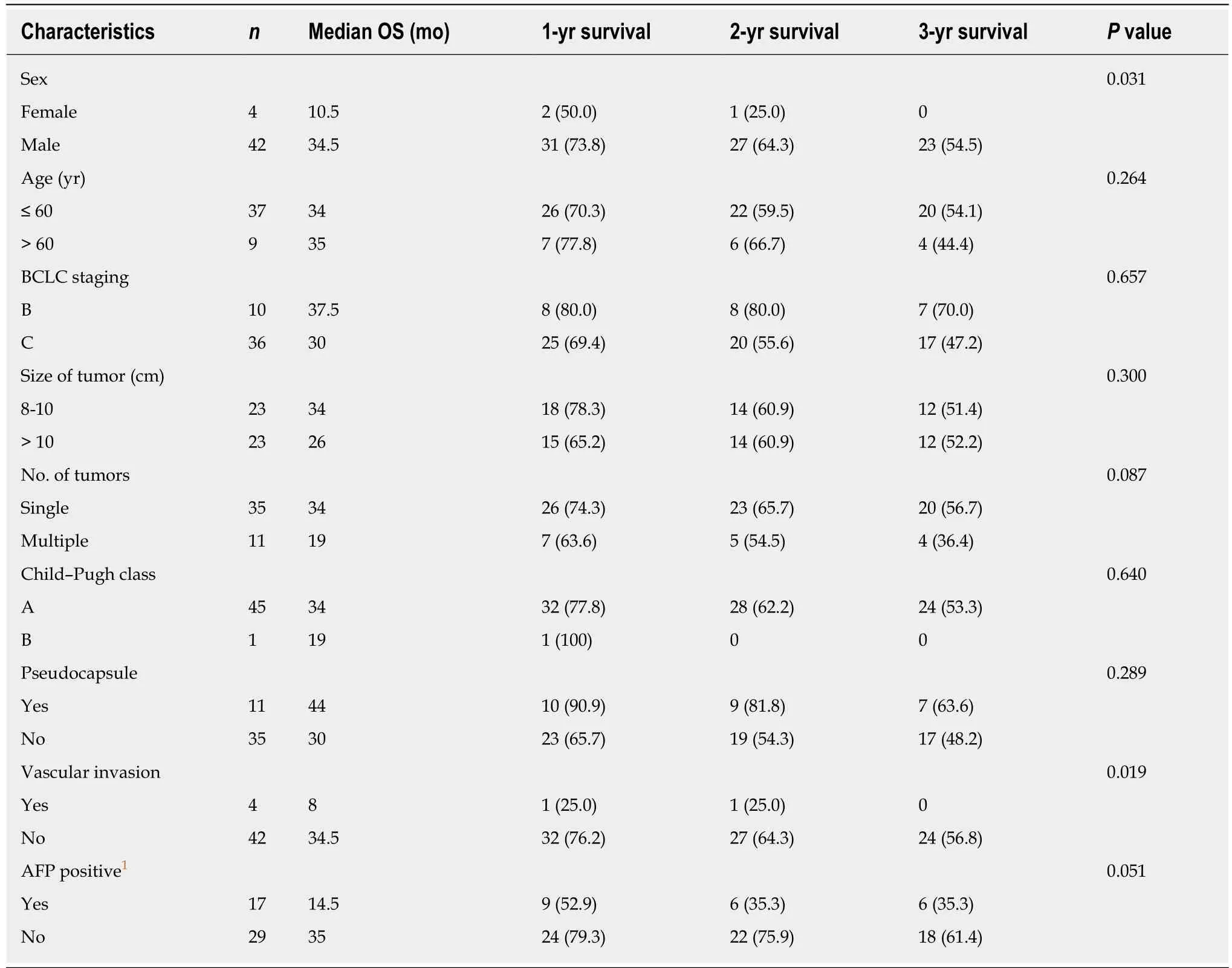
Table 3 Multivariate analysis using Cox proportional hazard model, n (%)
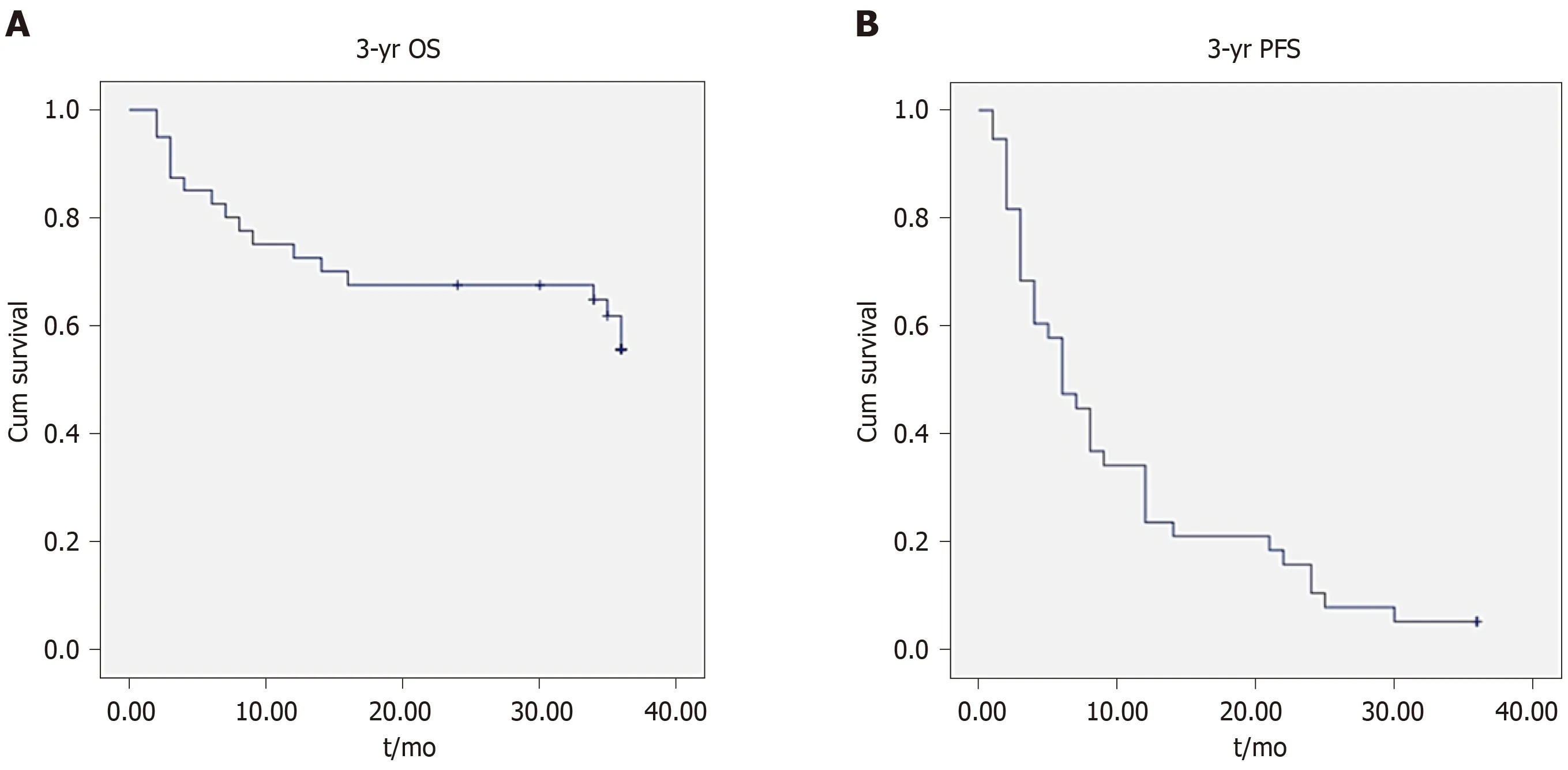
Figure 3 Kaplan-Meier analysis of overall survival and progression-free survival.
ARTICLE HIGHLIGHTS
Research background
Hepatocellular carcinoma (HCC) is a common cancer and a leading cause of tumor-related death. Patients who have large HCC (≥ 8 cm) are at advanced stages and have poor prognosis.Transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) is commonly used for patients with large HCC, however, both treatments has their own limitation.Recently study showed that combination of TACE and RFA may improve therapeutic efficacy,but how to combine these two treatment modalities is still a controversial topic.
Research motivation
The combination of TACE and RFA has a synergistic effect on HCC inactivation; however, most treatments are generally launched separately in practice, the effects of TACE or RFA alone are not synergistic very well. Therefore, evaluating the effect of the simultaneous combined treatment is necessary.
Research objectives
In the present study, we evaluated the efficacy and safety of simultaneous combined TACE and RFA for large HCC, to figure out how to combine these two treatment modalities.
Research methods
A retrospective study was conducted. From 2010 to 2013, 46 consecutive patients with large HCC were treated with simultaneous TACE and RFA. Thirty-five of 46 patients had a single tumor.Progression-free survival (PFS) and overall survival (OS) were analyzed at 2 years and 3 years,respectively.
Research results
Forty-six patients treated by simultaneous TACE and RFA had no significant complications and treatment was successful. After 3 years, median PFS and OS were 10.21 ± 1.58 and 26.44 ± 2.26 mo, retrospectively. The survival rate was 67.5% after 2 years and 55.67% after 3 years.
Research conclusions
These preliminary data show that simultaneous TACE and RFA are safe and effective for large HCC.
Research perspectives
With the simultaneous combination of TACE and RFA, it is expected to bring us a better treatment for large HCC.
 World Journal of Gastrointestinal Oncology2020年1期
World Journal of Gastrointestinal Oncology2020年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Validity of studies suggesting preoperative chemotherapy for resectable thoracic esophageal cancer: A critical appraisal of randomized trials
- Adenosquamous carcinoma may have an inferior prognosis to signet ring cell carcinoma in patients with stages l and ll gastric cancer
- Multi-institutional retrospective analysis of FOLFlRl in patients with advanced biliary tract cancers
- Laparoscopic dissection of the hepatic node: The trans lesser omentum approach
- Abnormal CD44 activation of hepatocytes with nonalcoholic fatty accumulation in rat hepatocarcinogenesis
- ldentification of candidate biomarkers correlated with pathogenesis of postoperative peritoneal adhesion by using microarray analysis
