Changing patterns and survival improvements of young breast cancer in China and SEER database, 1999-2017
Rong Guo, Jing Si, Jingyan Xue, Yonghui Su, Miao Mo, Benlong Yang, Qi Zhang,Weiru Chi, Yayun Chi, Jiong Wu,4
1Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 3Department of Cancer Prevention, Fudan University Shanghai Cancer Center, Shanghai 200032, China; 4Collaborative Innovation Center for Cancer Medicine, Shanghai 200032, China
Abstract Objective: Breast cancer in young females was usually considered more aggressive and requires aggressive therapy. We investigated whether early detection and improved treatments changed the patterns of characteristics,management and outcomes of young breast cancer patients over time.Methods: Females under 40 years of age diagnosed with breast cancer during the periods 1999-2017 and 1999-2015 were identified in the Fudan University Shanghai Cancer Center (FUSCC) and the population-based Surveillance, Epidemiology, and End Results (SEER) registry, respectively. Clinicopathologic characteristics and treatment information were collected. Patients diagnosed before 2013 were followed up.Results: The proportions of young breast cancer patients were 15.0% and 5.3% in the FUSCC and SEER cohorts, respectively. In the FUSCC cohort, there was a significant increase in the proportion of ductal carcinoma in situ (DCIS) (from 8.8% to 16.9%; P<0.0001) and it remained stable in SEER cohort. The proportion of T1-stage tumors increased dramatically in the FUSCC cohort (from 35.3% to 41.9%; P=0.008), whereas it decreased in SEER cohort (from 42.4% to 33.0%; P<0.0001). The percentage of estrogen receptor (ER)-positive cancers was consistently increased in both the invasive ductal carcinoma (IDC) and DCIS patients in the two cohorts. Breastconserving surgery and immediate implant reconstruction after mastectomy both exhibited increased use over time in the FUSCC cohort. Both the FUSCC and SEER cohorts showed a significantly better prognosis in the recent time period.Conclusions: With the increased early-stage and ER-positive diseases in young patients as well as better systemic treatment strategies, improved survival has been observed in recent years. There has been a substantial deescalation in surgical therapies in young breast cancer patients.
Keywords: Breast cancer; young age; tumor pathology; survival
Introduction
Breast cancer is still the most common cancer incidence and mortality worldwide among 20-39-year-old women(1). The incidence and mortality of breast cancer in young patients have experienced an increase these years. In 2012,the incidence rate was 30.2% and the mortality rate was 25.1% in all the young patients diagnosed as cancer (1).
In China, over 50,000 women under the age of 40 are diagnosed as breast cancer each year. Breast cancer is the most common cancer diagnosed in young adults (2,3). In the 20- to 39-year-old age group, breast cancer is also the leading cause of death in China (2). Young breast cancer has become a major public health problem.
Younger patients with breast cancer seem to exhibit a more aggressive tumor biology than breast cancers in older women, such as a higher grade, higher proportion of nodepositive disease, and higher frequency of hormone receptor(HR)-negative and human epidermal growth factor 2(HER2)-positive tumors (4-6). In addition, young patients usually have a worse prognosis, even in multivariate models(5-7). Young age was previously considered a justification for the use of more aggressive surgical approaches to prevent local recurrence (LR). In 2012, the National Comprehensive Cancer Network (NCCN) guidelines, for the first time, removed age younger than 35 years old from the relative contraindications for breast conserving surgery(BCS).
However, with developments in the diagnosis and treatment of breast cancer, the characteristics, management and outcomes of young breast cancer patients have changed remarkably. The aim of this study was to report and analyze the changing patterns in young breast cancer patients, including their age distribution, clinical characteristics, management and prognosis. We analyzed data from the Fudan University Shanghai Cancer Center(FUSCC) in mainland China during 1999 and 2017 and data from the population-based Surveillance,Epidemiology, and End Results (SEER) registry during 1999 and 2015.
Materials and methods
Patients
Women with newly diagnosed primary breast cancer with a definite pathological diagnosis who received surgery before the age of 40 years between January 1999 and July 2017 at FUSCC were retrospectively reviewed. Overall, a total of 34,959 breast cancer patients underwent surgical treatment in the FUSCC breast surgery department. Of these patients, 5,227 (14.95%) patients diagnosed under the age of 40 years old were included in this study.
The SEER cohort was derived from the SEER database(November 2015 submission) by using SEER*Stat software provided by the National Cancer Institute (NCI). All incident cases of breast cancer patients under the age of 40 from 1999 to 2015 were identified (8). A total of 50,354 patients were included. After excluding patients with distant metastasis at diagnosis, a total of 46,857 patients were included into analysis.
Clinical data collection
A retrospective review of medical records and pathology reports was conducted. Staging was performed according to the American Joint Committee on Cancer (AJCC)guidelines (9,10). We used a cutoff of 14% for Ki67, which was recommended by 2011 St Gallen consensus panel (11).
Patients at the FUSCC were told to have an examination according to the guidelines of the breast cancer center and were followed up by telephone. Date of progression metastasis, date of relapse and date cause of death were collected. The date of survival and systemic treatment was collected for patients diagnosed before 2013.
Statistical methods
The IBM SPSS Statistics (Version 23.0; IBM Corp., New York, USA) and GraphPad Prism (Version 6.0; GraphPad software, Inc., LaJolla, CA, USA) were used for statistical analysis. A linear regression analysis was used to determine trends in each parameter over time. Disease-free survival(DFS) was measured from the date of surgery to the date of disease relapse or last follow-up. We applied the Kaplan-Meier method to estimate DFS and breast cancer-specific survival (BCSS) and compared the patients diagnosed during different time ranges using the Log-rank test. For complete 5-year follow-up, the FUSCC and SEER cohorts were truncated in 2008 and 2007 (year of diagnosis),respectively. Statistical significance was set at a P<0.05(95% level of confidence).
Results
Patients and age distributions
Between 1999 and 2017, a total of 34,959 females received surgical treatment in the FUSCC breast surgery department. Among them, 5,227 (15.0%) patients were diagnosed under the age of 40 years (Figure 1A). The proportion of newly diagnosed breast cancer patients below 40 years old in the FUSCC cohort increased continuously over the last decade. Compared with 11.4% of patients diagnosed under the age of 40 years in 1999, it was 16.4%in 2017 (43.9% rise; R2=0.908, P<0.0001) (Figure 1A).
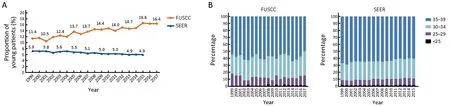
Figure 1 Time trends of newly diagnosed young breast cancer patients. (A) Proportion of patients diagnosed under the age of 40 years old among all breast cancer newly diagnosed in Fudan University Shanghai Cancer Center (FUSCC) and population-based Surveillance,Epidemiology, and End Results (SEER) cohorts; (B) Age distributions of young breast cancer patients in FUSCC and SEER cohorts.
In the SEER dataset, a total of 954,885 patients were diagnosed with breast cancer between 1999 and 2015,among which 50,354 (5.3%) were under the age of 40 years. The proportion of young patients in SEER dataset experienced a slight decrease from 5.9% in 1999 to 4.9% in 2015 (16.9% decrease; R2=0.833, P<0.0001)(Figure 1A).
In the FUSCC cohort, the proportions of very early onset breast cancer were stable in recent years. Among young patients, the age range with the largest number of newly diagnosed cases was 35-39 years of age (2,987 patients, 57.1%), followed by 30-34 years of age (1,476 patients, 28.2%) (Figure 1B). Only 676 (12.9%) and 88(1.7%) patients were diagnosed between 25 and 29 years old and under the age of 25 years, respectively (Figure 1B,Table 1). However, the proportion of patients with extreme young age, defined as under the age of 35 years old was higher in the FUSCC cohort than SEER cohort (Figure 1B, Table 1). There were no significant changes during these years in both cohorts.
Staging and pathology
In FUSCC, the proportion of young patients with ductal carcinoma in situ (DCIS) increased dramatically from 8.8%of all cases in 1999 to 16.9% in 2017 (92.0% rise;R2=0.809, P<0.0001) (Figure 2A). However, it remained stable in SEER cohort at approximately 13% (R2=0.066,P=0.270) (Figure 2B). An increase in the proportion of T1-stage patients was observed in FUSCC cohort, which exhibit a rise from 35.3% in 1999 to 41.9% in 2017 (18.7%rise; R2=0.346, P=0.008) (Figure 2A). In contrast, T1-stage disease experienced a decrease in SEER cohort from 42.4%in 1999 to 33.0% in 2015 (22.2% decrease; R2=0.931,P<0.0001) (Figure 2B).
In FUSCC, the proportion of young patients with negative axillary lymph nodes increased substantially over the study period from 50.0% to 68.7% (37.4% increase;R2=0.786, P<0.0001) (Supplementary Figure S1C). There was no significant change in the SEER cohort(Supplementary Figure S1D).
With the decrease in the proportion of patients with positive axillary lymph nodes and the increased proportion of Tis- and T1-stage patients, patients classified as stage 0 were increased steadily with time in FUSCC(Supplementary Figure S1E). The proportion of young patients diagnosed with stage 0 disease increased from 9.4% to 19.2% (104.3% increase; R2=0.798, P<0.0001). In contrast, the percentage of patients with stage II cancers decreased from 46.9% to 39.6% (15.6% decrease;R2=0.466, P=0.001), and the proportion of stage III cancers decreased from 18.8% to 13.5% (28.2% decrease;R2=0.687, P<0.0001) (Supplementary Figure S1E). The trends were different in the SEER cohort, which exhibited an increase in the proportion of stage II patients from 39.4% in 1999 to 48.8% in 2015 (23.9% rise; R2=0.827,P<0.0001) (Supplementary Figure S1F).
The proportion of patients with a positive estrogen receptor (ER) status was much higher in patients with DCIS than those with invasive ductal carcinoma (IDC) in both cohorts. For the patients with DCIS in FUSCC cohort, the percentage of tumors with positive ER expression increased from 66.7% in 1999 to 80.0% in 2017(19.9% rise; R2=0.785, P<0.0001) (Figure 2C). Similar trends were observed in SEER cohort, which exhibited an even higher increase to 91.0% in 2015 (Figure 2D). In addition, the proportion of ER-positive tumors increased with time in IDC patients in FUSCC cohort, rising from 38.7% to 69.3% (79.1% rise; R2=0.736, P<0.0001) (Figure 2C). Similar results were found in IDC patients in the SEER cohort (Figure 2D).
HER2 and Ki-67 data were available for FUSCC cohort.For the patients with DCIS, the percentage of tumors with positive HER2 expression was 31.25%, which was relatively higher than the percentage of positive tumors among the IDC patients, and no significant changes were observed over these years (Figure 2E). Of the 4,060 patients with IDC, HER2 positive rate was 25.01% and shown a rising trend over the time period (Figure 2E). With respect to Ki-67 index, 55.78% of DCIS patients were positive in 2009 and 2013, and the proportion increased to 60.22% in 2014 and 2017 (Figure 2E). In addition, the proportion of Ki-67-positive tumors increased with time among IDC patients,rising from 64.95% to 83.67% (Figure 2E).
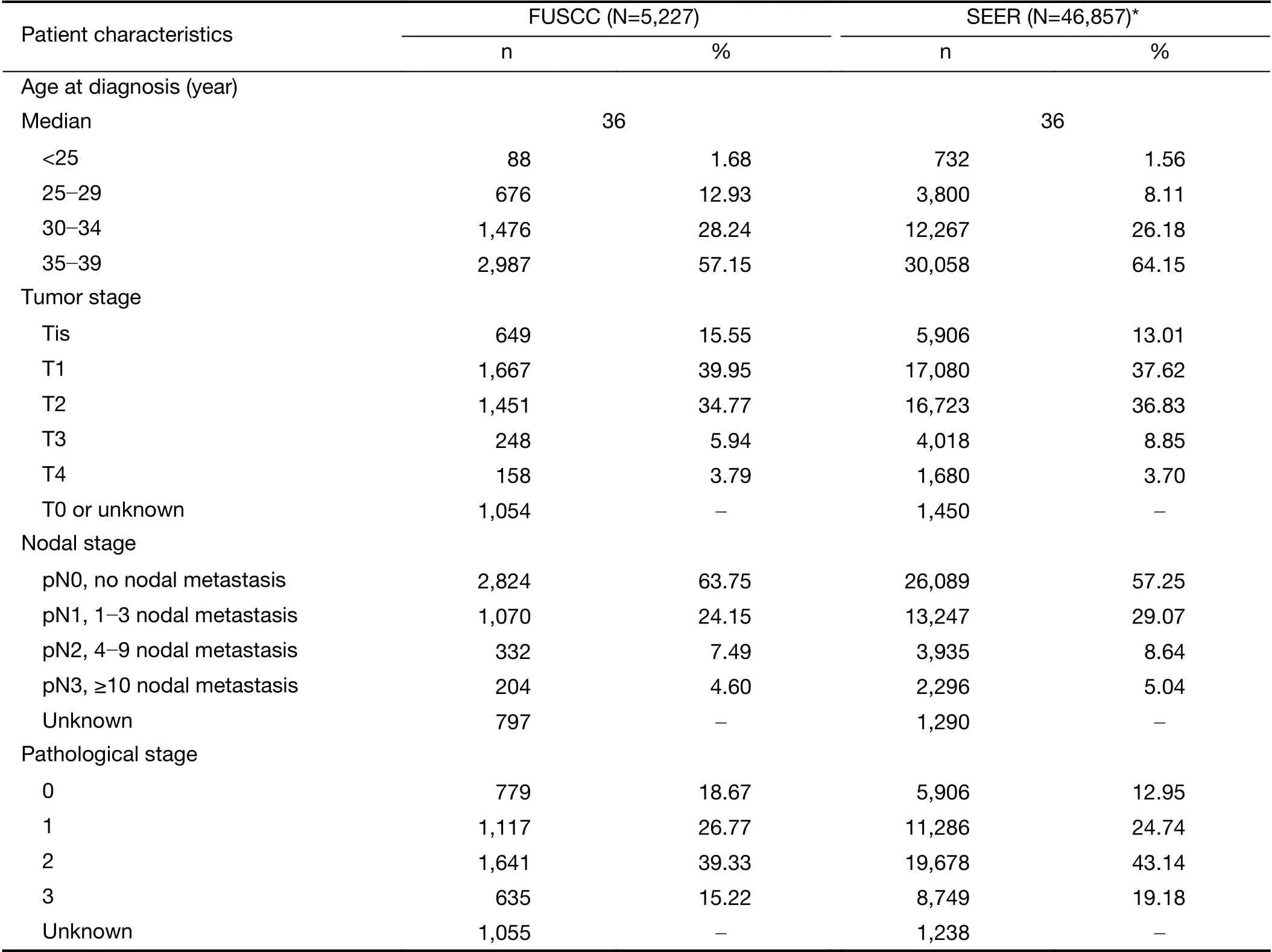
Table 1 Clinicopathologic characteristics of young breast cancer patients in FUSCC and SEER cohorts
We also analyzed breast cancer phenotype of FUSCC cohort. With the increased proportion of luminal-B/HER2-negative patients, rising from 8.46% in 2009 to 38.37% in 2017 (353.5% rise; R2=0.827, P=0.007), this subtype has become the most common phenotype of young patients in recent years (Supplementary Figure S1G). In contrast, the percentage of patients with luminal-A and luminal-B/HER2-positive cancers decreased (P=0.006 and P=0.006, respectively) (Supplementary Figure S1G). There were no significant changes of the proportion of HER2-positive and triple negative breast cancer (TNBC) patients during the studied years (Supplementary Figure S1G).
The proportion of patients with lymphatic vessel invasion (LVI) decreased from 48.5% to 20.4% (57.9%decrease; R2=0.885, P<0.0001) in FUSCC cohort (Figure 2F).
Surgery and systemic therapy
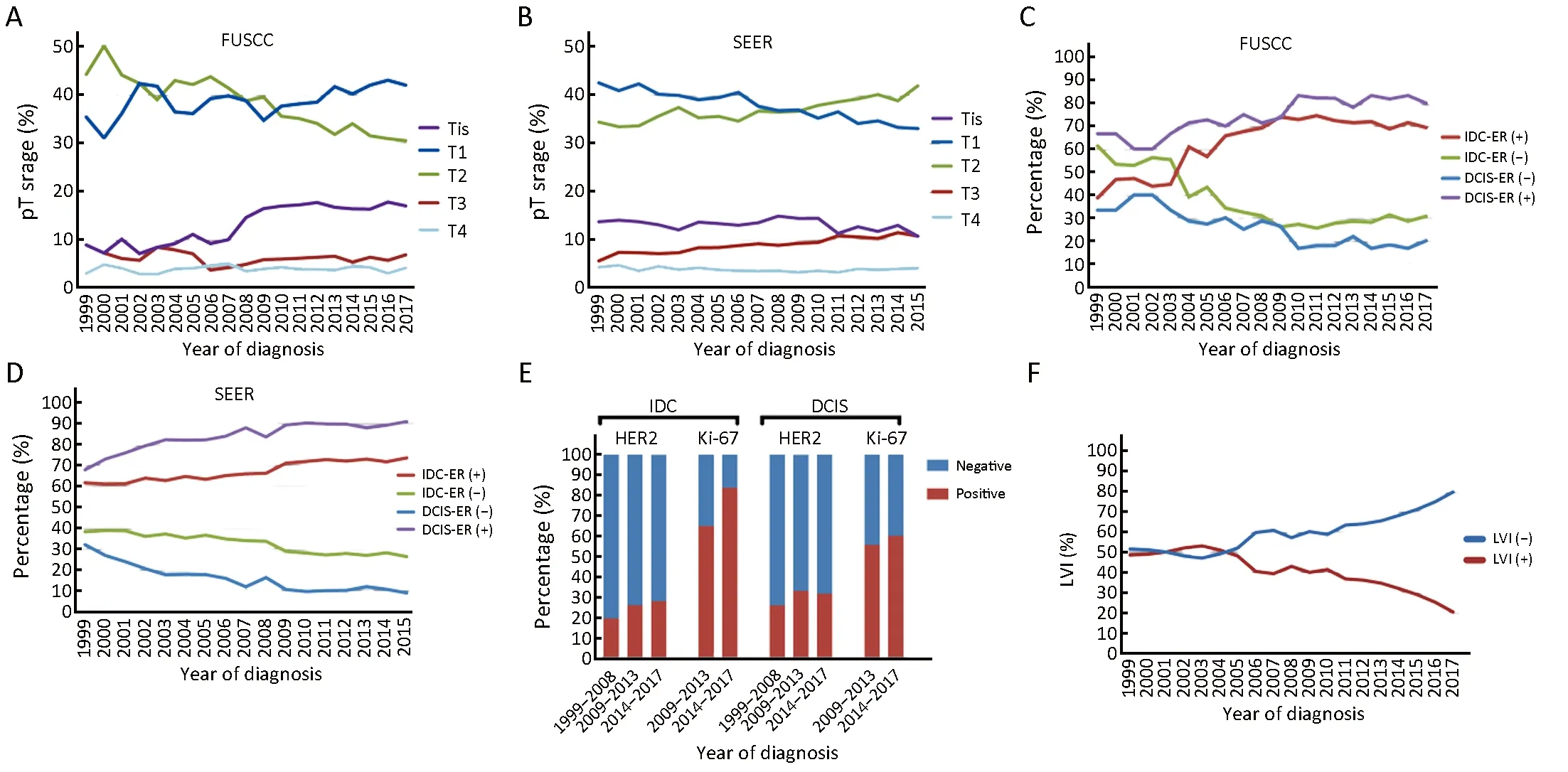
Figure 2 Tends in staging and pathology of young breast cancer patients during 1999-2017 and 1999-2015 in Fudan University Shanghai Cancer Center (FUSCC) and population-based Surveillance, Epidemiology, and End Results (SEER) cohorts. (A,B) Pathological tumor size stage according to American Joint Committee on Cancer (AJCC) system; (C,D) Estrogen receptor (ER) status during time in young patients with ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC); (E) Human epidermal growth factor 2 (HER2) and Ki-67 status during different time range in young patients with IDC and DCIS in FUSCC; (F) Lymphatic vessel invasion (LVI) involvement proportion in FUSCC.
Among the 5,227 young patients in FUSCC cohort,mastectomy was still most frequently performed; However,the use of BCS increased rapidly during the studied years.A steady decrease in the proportion of patients underwent mastectomy from more than 80.0% before 2002 to 68.0%in 2017 (15.0% decrease; R2=0.610, P<0.0001) was noted,whereas the percentage of patients underwent BCS accordingly increased from less than 20.0% to 32.0%(60.0% rise; R2=0.610, P<0.0001) (Figure 3A). Breast reconstruction has begun to perform in young patients in 2003, and the proportion of young patients who received breast reconstruction surgery after mastectomy increased from 6.02% in 2003 to 31.78% in 2017(427.9% rise;R2=0.716, P<0.001) (Figure 3A).
The surgical treatment pattern in SEER cohort was quite different from that in FUSCC cohort. In SEER cohort, BCS was the dominant surgery type in young patients; however, after 2012, mastectomy plus reconstruction became the most common surgery used by young breast cancer patients (Figure 3B).
Implant-based breast reconstruction (IBBR) gradually became the most common type of reconstruction young patients received after mastectomy in both the FUSCC and SEER cohorts (Figure 3C,D). In the more recent years, the proportion of patients underwent IBBR was approximately 80% of all the patients who received reconstruction in FUSCC cohort; however, approximately 30% of patients still received autologous flap reconstruction in SEER cohort (Figure 3C,D).
The pattern of systemic therapy for young patients with IDC also changed dramatically in FUSCC and could generally be divided into two periods. From 1999 to 2005,with the increased use of hormonal therapy, the proportion of patients who received chemotherapy, but not hormonal therapy decreased markedly from 58.8% in 1999 to 27% in 2005 (Figure 3E). However, none of the patients with IDC received hormonal therapy alone without chemotherapy before 2005. After 2005, the proportion of patients who received hormonal therapy alone increased steadily until 2013 and the proportion of hormonal therapy alone was 1.4% in 2005 and 8.1% in 2013 (478.6% rise; R2=0.848,P<0.001) (Figure 3E).
Survival
From 1999 to 2013, follow-up was performed in 2,331 patients in FUSCC cohort. We divided these patients into two groups according to their date of diagnosis. After a median follow-up time of 32.9 months, the patients diagnosed after 2009 had significantly longer DFS than the patients diagnosed between 1999 and 2008 [hazard ratio(HR)=0.664, 95% confidence interval (95% CI),0.478-0.813, P=0.006] (Figure 4A). Patients with ER+/HER2- or ER-/HER2+ disease exhibited the most significant improvements in DFS over time (HR=0.633,95% CI, 0.385-0.885, P=0.021 and HR=0.477, 95% CI,0.200-0.928, P=0.042, respectively) (Figure 4B,D).Conversely, in ER+/HER2+ and ER-/HER2- subgroups,the year of breast cancer diagnosis had no impact on DFS(HR=0.484, 95% CI, 0.156-1.108, P=0.082 and HR=0.946,95% CI, 0.519-1.705, P=0.848, respectively) (Figure 4C,E).
Long-term survival improvements were also observed in SEER cohort. A total of 46,857 young breast cancer patients with complete BCSS data were included in the analysis. We found that patients diagnosed after 2008 had significantly longer BCSS than patients diagnosed before 2008 (HR=0.826, 95% CI, 0.780-0.876, P<0.0001)(Figure 4F).
Discussion
In the present study of breast cancer patients diagnosed between 1999 and 2017, we observed that approximately 15% of breast cancers were diagnosed under 40 years and that proportion exhibited a slight increase over time in FUSCC. We also analyzed the proportion of newly diagnosed breast cancer patients under the age of 40 years in Shanghai, a regional area in China, between 2001 and 2014. Data were provided by the Shanghai Cancer Report of Shanghai Municipal Center for Disease Control and Prevention (12-14). The proportion of young breast cancer patients under 40 years old in Shanghai was stable at 5%which was much lower than that in FUSCC cohort. In addition, there were no significant changes during the studied years, and only a slight increase from 5.2% in 2001 to 6.5% in 2014 was observed (25% rise; R2=0.063,P=0.385) (Supplementary Figure S1A). Thus, the age distribution of breast cancer patients differed between a region in China and a single cancer center, and FUSCC cohort contained a relatively larger number of young patients. The data from Shanghai cohort were similar to the data of population-based SEER cohort and the data published by Western countries, that only 5%-7% of patients diagnosed under the age of 40 years in developed countries (15,16).
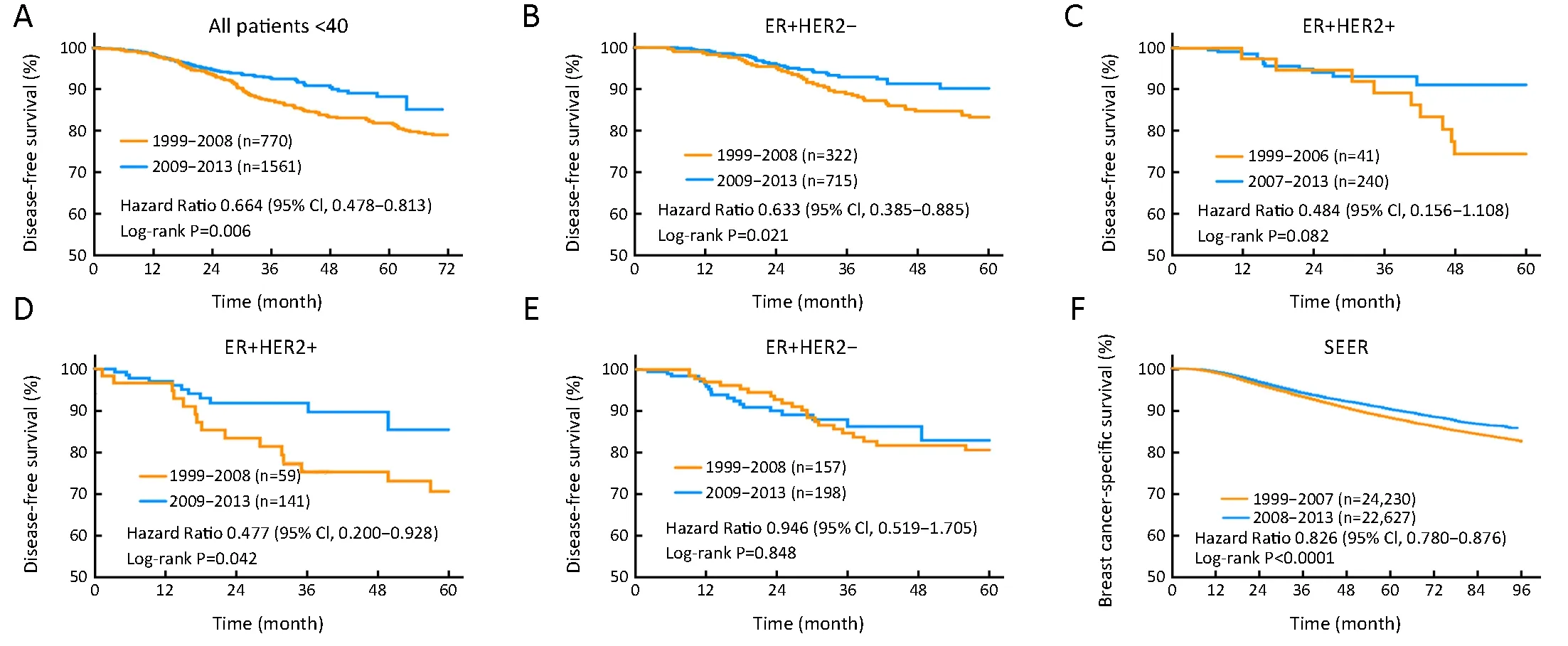
Figure 4 Long-term survival of young patients during different time range. (A) Kaplan-Meier estimates disease-free survival (DFS) of all patients under age of 40 years old diagnosed between 1999-2008 and 2009-2013 in Fudan University Shanghai Cancer Center (FUSCC)cohort; (B-E) Kaplan-Meier estimates DFS of young patients with different estrogen receptor (ER) and human epidermal growth factor 2(HER2) expression status diagnosed between 1999-2008 and 2009-2013 in FUSCC cohort; (F) Kaplan-Meier estimates breast cancerspecific survival of young patients in population-based Surveillance, Epidemiology, and End Results (SEER) cohort diagnosed between 1999-2007 and 2008-2013. HR, hazard ratio; 95% CI, 95% confidence interval.
FUSCC is the largest cancer center in Shanghai, and young patients who living in urban areas or patients with better financial ability are inclined to seek treatment at FUSCC center. This preference creates a highly selected cohort that differ from Shanghai cohort and populationbased SEER cohort. This is the main reason that the FUSCC cohort has a higher proportion of young patients and patients with early-stage diseases. Based on this, the main purpose of this study is not to make a comparison of the two cohorts, but to investigate the changing patterns of patients' characteristics and clinical practice as well as survival improvements during nearly two decades in both cohorts. However, those trends of changes shared some similarities which reflected the changing patterns of young breast cancer. Meanwhile, with the changes in the economic level of patients' place of residence, the impact of selection bias changes. In addition, because of the aging population of China, the increasing incidence of young breast cancer patients may be more significant than the changes of proportion.
Another reason for the difference between those cohorts was that all of the patients we included in FUSCC cohort received surgery, which excluded the patients who were older or had comorbidities and hard to have a surgery.However, data from Shanghai cohort included all the newly diagnosed breast cancer patients. For more reasonable results, we also excluded patients in the SEER cohort who have distant metastases at diagnosis and have not received surgery.
Overall, the clinical and pathological characteristics of young patients in FUSCC cohort exhibited a downstage from 1999 to 2017. The proportion of young patients with DCIS increased by approximately 2-fold during the 19-year-period. Furthermore, increasing number of young breast cancer patients were being diagnosed at an early stage at the end of the time period, with about approximately 40% of T1-stage patients and 63.8% of N0-stage patients. The widespread utilization of screening mammography and ultrasound has produced a shift in the stage of breast cancer in young patients (17,18). These findings indicate that public awareness and increased access to screening programs have been a successful to some extent.
However, the patients in the SEER cohort experienced an upstage in some extent. One of the reasons may be that a relatively large proportion of patients were diagnosed with early-stage disease in baseline 1999. The development of screening and diagnosis of breast cancer in China was far behind that of developed countries.
A notable increase in the proportion of patients with ERpositive breast cancer was observed in both DCIS and IDC subgroups. A similar trend was found in SEER cohort, and similar results have been reported in other studies as well(19,20). One of the reasons for this increase might be the change in the cutoff level of ER positivity, which was altered from 10% to 1% (21). However, the impact might be limited, since only a small number of patients have an ER expression between 1% and 9%. Additionally, the use of screening mammography has been associated with an increased risk of developing early-stage breast cancer, and early-stage and low-grade breast cancers have a larger proportion of ER-positive tumors (22). In addition, dietary and reproductive factors, such as higher body mass index,early age at menarche, delay in marriage, having fewer children and changes in infant feeding patterns, may have contributed to the trends we observed (23). At the same time, the collection of ER status data become more complete with time in both of the cohorts. This may be one of the reasons why the proportion of ER positive patients stabilized after 2010.
HER2 positive rate in the young patients with invasive carcinoma was approximately 25%, which was obviously higher than that in the whole breast cancer cohort(approximately 10%-20%) (24,25). The proportion of patients with positive Ki-67 index in young breast cancer with DCIS (57.4%) or IDC (76.2%) was much higher than that previously reported for the whole breast cancer cohort(26,27). Ki67 has been reported to be highly expressed in approximately 40% of DCIS tumors and 50%-60% of IDC tumors (27,28). In the setting of those molecular markers, our data again reflected the more aggressive biological behavior of breast cancer in young patients.
Although mastectomy is still the most frequently performed surgical intervention, the frequencies of BCS and mastectomy with reconstruction have shown increasing trends in recent years. The frequency of BCS increased to more than 30% in recent years in FUSCC cohort. Early detection and neoadjuvant chemotherapy may have contributed to the increase in the number of patients with operable breast cancer, as these patients are the ones who are candidates for BCS. The increased BCS rate is also in accordance with the guidelines used after 2012, which state that young age is not a relative contraindication for BCS(29). However, the proportion is still largely lower than that in Western countries. In SEER cohort, breast conserving rate is higher than 50% in the young patients,even though it has decreased in recent years. This result is in concordance with the results of a previous report, which showed that because of the worry about LR and the development of breast reconstruction techniques, a rapid decrease in the rate of BCS has been observed in American patients (30). The number of breast reconstructions after mastectomy increased rapidly in the FUSCC cohort, rising from 6.02% in 2003 to 31.78% in 2017 (427.9% rise;R2=0.716, P<0.001). Among the young patients who received breast reconstruction, an overwhelming number of patients selected implant/expander reconstructions, with almost 80% of young patients with breast reconstruction selecting IBBR in recent years.
The use of adjuvant therapy has also experienced a deescalation, with the use of chemotherapy in particular exhibiting a dramatic decrease. In contrast, the use of hormonal therapy has increased. Although more patients received BCS and fewer patients received chemotherapy,DFS showed improvement in recent years. The improvement in survival can be partially explained by the increased proportion of patients with early-stage and ERpositive breast cancer. Improved survival was more obvious in the ER+/HER2- and ER-/HER2+ patients. While no significant improvement could be evidenced among patients with the ER+/HER2+ or triple-negative subtypes(ER-/HER2-) subtypes. Thus, early diagnosis and detection are not the only factors to improving prognosis of young breast cancer patients. Improved therapies also contribute to prognostic improvements. The use of effective HER2-targeted therapy and endocrine therapy during the observation period contributes substantially(31,32). In contrast, these findings also suggest that BCS is not contraindicated for all the young breast cancer patients,and not all the young patients need adjuvant chemotherapy.
There are some limitations to this study. The data from FUSCC cohort are based on a single cancer center; these data may therefore be slightly different from that of the National Cancer Registry System. Additionally, the presence of missing data and limited follow-up time can be considered weaknesses of this study. Other limitations include the lack of systemic treatment data and data on HER2 and Ki-67 statuses in SEER cohort, which limited the analysis of their influence on patients' survival improvements.
Conclusions
A down stage trend of young breast cancer patients during study period was found in cohort of FUSCC. The percentage of ER-positive cancers was increased consistently in both the FUSCC and SEER cohorts. BCS and immediate implant reconstruction after mastectomy have been increased during time. Both FUSCC and SEER cohorts showed a significantly better prognosis in the recent time period. Taking the above into consideration,with the changing patterns of young breast cancer characteristics and treatment, young age alone should not be a reason to prescribe more aggressive therapy. We also expect better survival as well as long-term quality of life in young patients in the future.
Acknowledgements
This study was supported in part by grants from the Shenkang center city hospital emerging frontier technology joint research project (No. SHDC12015119) and National Key R&D Program of China (No. 2017YFC1311004).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2019年4期
Chinese Journal of Cancer Research2019年4期
- Chinese Journal of Cancer Research的其它文章
- Identification of cancer patients using claims data from health insurance systems: A real-world comparative study
- An enrichment model using regular health examination data for early detection of colorectal cancer
- Evaluation of COC183B2 antibody targeting ovarian cancer by near-infrared fluorescence imaging
- Distribution of high-risk human papillomavirus genotype prevalence and attribution to cervical precancerous lesions in rural North China
- Radiomics-based predictive risk score: A scoring system for preoperatively predicting risk of lymph node metastasis in patients with resectable non-small cell lung cancer
- A 18FDG PET/CT-based volume parameter is a predictor of overall survival in patients with local advanced gastric cancer
