Biodegradability and Tribological Properties of Mineral Base Oil Enhanced by Caprylic Methyl Diethanolamine Phosphate Ester
Ding Jianhua; Fang Jianhua; Chen Boshui; Liu Ping; Fan Xingyu; Chen Ran
(1. Department of Oil, Army Logistics University, Chongqing 401311;2. Military Representative Office in PetroChina Dalian Petrochemical Company, Dalian 116033)
Abstract: The influence of synthetic caprylic methyl diethanolamine phosphate ester (abbreviated as MDEACP) on biodegradability and tribological properties of 400SN mineral base oil was studied. The biodegradability of the neat base oil and the oil doped with MDEACP was determined on a biodegradation tester. The tribological properties of the neat base oil and the oil doped with MDEACP were evaluated on a four-ball tester. Moreover, the worn surfaces were investigated by scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). The results revealed that MDEACP significantly promoted the biodegradation of the mineral base oil. The improvement in biodegradability was attributed to the enhanced growth and quantity of microbes by MDEACP. Furthermore, MDEACP enhanced the anti-wear properties, the friction-reducing properties, and the extreme pressure properties of the base oil. It was mainly attributed to the formation of the complex boundary lubrication film resulted from the adsorption and tribochemical reactions of MDEACP on the friction surface.
Key words: additive; biodegradability; friction; wear; extreme pressure properties
1 Introduction
Mineral lubricant are among the most important and the most widely used lubricants, but they possess inherent eco-toxicity and low-biodegradable nature, because studies show that the biodegradation rate of most mineral lubricants is no more than 40%[1-3]. Environment is seriously polluted by mineral lubricants because of leakage, spillages and careless disposal, which would directly restrict the development of mineral lubricating oil[4-5]. Currently, the environmentally friendly lubricant has become the mainstream of lubricant development,and good biodegradability is one of the most important characteristics of environmentally friendly lubricants[6-8].Nowadays, the researches on environmentally friendly lubricants mainly focus on two aspects, viz.: the biodegradable lubricant base oils such as vegetable oils and synthetic esters and the environmentally friendly additives[9-12]. However, mineral lubricants are going to play a great role in the future lubrication applications.Unfortunately, it is difficult for mineral lubricants to meet the performance requirements for environmentally friendly lubricants on account of their low-biodegradable nature[13-14]. Hence, improved biodegradability of mineral lubricants is of great significance for reducing the pollution caused by mineral lubricants and promoting the development of environmentally friendly lubricants.
Researches on environmental microbiology make clear that many compounds containing N and P elements can enhance the microbial growth, improve microbial activity and promote biodegradation of petroleum hydrocarbons[15-17]. Therefore, promoting biodegradation of mineral lubricants by additives containing N and P elements is evidentially possible. In the present paper, caprylic methyl diethanolamine phosphate ester was prepared. Its influence on biodegradability and tribological properties of mineral base oil was studied.
2 Experimental
2.1 Materials
The AR grade experimental reagents (methyl diethanolamine,caprylic acid, toluene, phosphorus pentoxide and toluenesulfonic acid monohydrate) were purchased from the Chengdu Kelong Chemical Reagent Company. A non-polarized paraffinic lubricating base oil (400SN),the kinematic viscosity of which at 40 °C was equal to 84.93 mm2/s, was provided by the Shenzhen Lubricating Oil Industry Company.
2.2 Preparation of additive
Figure 1 gives the synthetic pathways and molecular structure of caprylic methyl diethanolamine phosphate ester (denoted as MDEACP). The brief synthetic process is demonstrated as follows: 30 mL of toluene serving as solvent and water extracting agent, 0.05 mol of methyl diethanolamine, 0.05 mol of caprylic acid, and catalyst were added into a 500-mL three-necked flask equipped with a thermometer, a reflux condenser, a Dean-Stark trap for water separation, and a stirrer. The reaction was carried out under refluxing and stirring at 160 °C until no obvious water production could be observed. Then the reactor was cooled down to about 40 °C and P2O5in batches was added into the reactor. The mixture was heated to 80 °C with the reaction taking place under stirring for 4 h, then a moderate amount of water was added into the reactor with the reaction carried out under stirring for 2 h in order to hydrolyze a small amount of polyphosphoester produced in reaction. After being cooled down, the mixture was subject to distillation to remove toluene under reduced pressure. MDEACP was then obtained.
The product was characterized by the Fourier transform infrared spectroscopy (FT-IR). Figure 2 presents the FT-IR spectrum of MDEACP. The significant features of MDEACP are bands corresponding to the CH3/CH2stretching vibration at the wavenumbers of 2 926.4 cm-1and 2 855.8 cm-1, with the C=O in ester group identified at 1 737.1 cm-1, the C-O-C in ester group found at 1 163.8 cm-1, the P=O identified at 1 224.5 cm-1, and the P-O-C verified at 1 073 cm-1. The results of FT-IR spectroscopic analysis suggest that MDEACP is synthesized thereby.
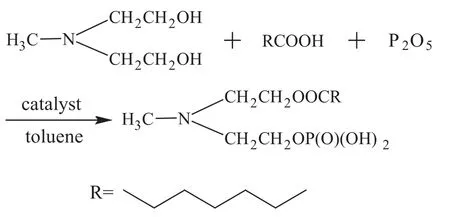
Figure 1 Pathway for synthesis of MDEACP
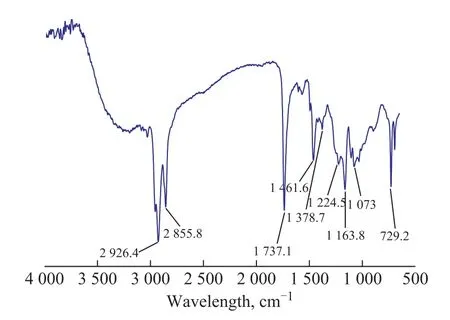
Figure 2 FT-IR spectrum of MDEACP
2.3 Biodegradation test
To evaluate the in fluence of MDAECP on biodegradation of mineral base oil, different mass fraction of MDEACP,viz. 0, 0.5%, 1.0%, 1.5% and 2.0%, respectively, was added into the 400SN base oil. The biodegradability of the formulated oils was tested by a fast method created by our research group and mentioned in the reference[18]. In brief, the effect of lubricant biodegradability was determined by the reference substance oleic acid and the parallel biodegradation reactions of the lubricant. The carbon dioxide created by the test sample was measured every two days under formulated conditions, and after 10 days of biodegradation, the accumulated amount of CO2generated from oleic acid (abbreviated asMO) and the tested lubricant (abbreviated asML) was measured, respectively.Biodegradability index (BDI), a comparative parameter of the percentage ratio of the amount of CO2produced by single tested lubricant versus that produced by oleic acid,was used to determine the biodegradability of the lubricant. BDI was calculated by the following equation:
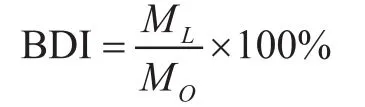
The higher the BDI value was, the better the biodegradability of the lubricant would be. Figure 3 displays the diagrammatic sketch of biodegradation test. The air flow was controlled in the range of 180—200 mL/min by an air flow meter. The CO2degasser and the absorber contained NaOH solution and Ba(OH)2solution,respectively. The CO2examiner contained Ba(OH)2solution. The bioreactor contained the test sample and the microbes.
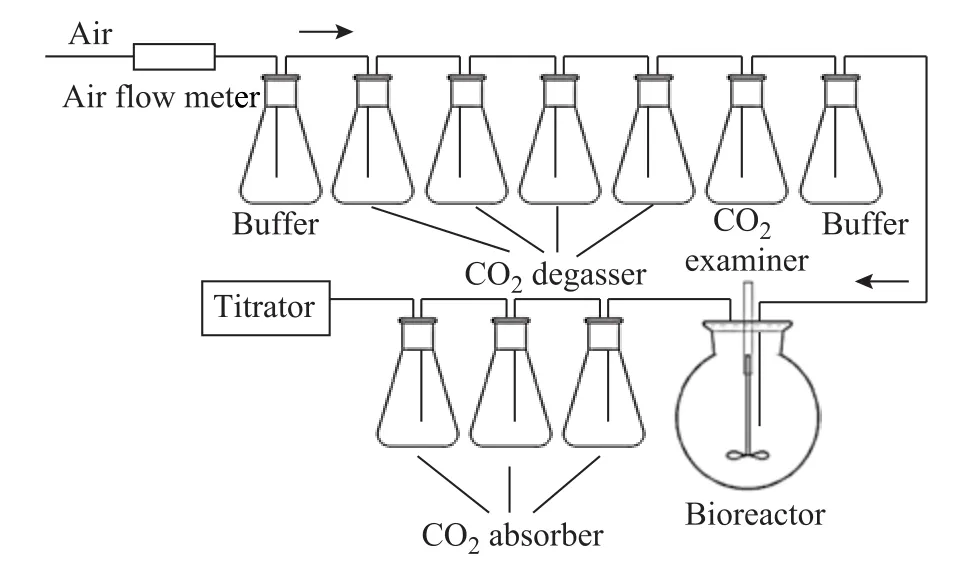
Figure 3 Diagrammatic sketch of biodegradation test
The optical density of microbial culture medium at a wavelength of 600 nm (abbreviated as OD600) was measured by an ultraviolet spectrophotometer every two days. The OD600value represented the situation of microbes' growth. The higher the OD600value was, the more the microbial quantity would be.
2.4 Tribological properties
The tribological properties of MDEACP in 400SN mineral base oil were evaluated on a four-ball tribotester following the procedures specified in the national standard
GB/T 3142—1982, which was a Chinese standard method for determining friction and wear properties of lubricants.The four-ball tribotester was composed of a rotating ball that glided on three fixed balls under chosen loads. The steel balls used in the present test were made of GCr15 standard steel with a diameter of 12.7 mm, a hardness of 59—61 HRC, and a surface roughness Raof 0.040 μm.The wear scar diameters (WSD), the friction coefficients,the maximum non-seizure loads (PB) and the welding
loads (PD) were evaluated in the present test. The WSD and friction coefficient were determined under a load of 392 N and at a rotary speed of 1 500 r/min for 30 min.The duration of testing PBand PDwas 10 seconds under a selected load and a rotary speed of 1 450 r/min.
2.5 Surface analysis
The steel balls, which were lubricated by 400SN base oil with 1.0% of MDEACP, were subjected to a load of 392 N and a rotary speed of 1 500 r/min for 30 min. Then they were ultrasonically cleansed with petroleum ether for 10 min. The surface morphology of the steel balls was observed by a scanning electron microscope (SEM).The chemical features of the typical elements on the worn surfaces were analyzed by an ESCALab250 X-ray photoelectron spectroscope (XPS).
3 Results and Discussion
3.1 Biodegradable properties
Figure 4 gives BDI which varies with different mass fraction of MDEACP in the oil sample. It can be found that MDEACP, added into 400SN mineral oil at different concentration, could improve the BDI. Especially, at a MDEACP content of 1.0%, the BDI increased from 33.6% to 65.4%, indicating that MDEACP could promote markedly the biodegradation of 400SN mineral oil.Studies have shown that many compounds containing nitrogen and phosphorus elements can effectively enhance the biodegradation of hydrocarbons for remediation of the petroleum polluted water or soil[19-20].Hence, promotion of biodegradation of mineral base oil by MDEACP was evidentially possible. Moreover, the surface active MDEACP might also decrease interfacial tension of the oil and water, increase their interfacial area, thus making lubricant readily metabolized by microorganisms. When the concentration of MDEACP exceeded 1.0%, the BDI decreased. The reason might be that when interfacial tension of the oil and water was increased, their interfacial area was decreased to reduce the contact area of the oil and microbes,which could have an adverse effect on biodegradation of the base oil.
Figure 5 shows that the OD600of microbial culture medium with and without MDEACP varies with the biodegradation time. The results reveal a significantly higher OD600of microbial culture medium containing MDEACP in the exponential period of microbial growth.It testifies that MDEACP can increase the growth rate and quantity of microbes, which contributes to the accelerated degradation of 400SN mineral base oil.

Figure 4 Variation of BDI with different mass fraction of MDEACP
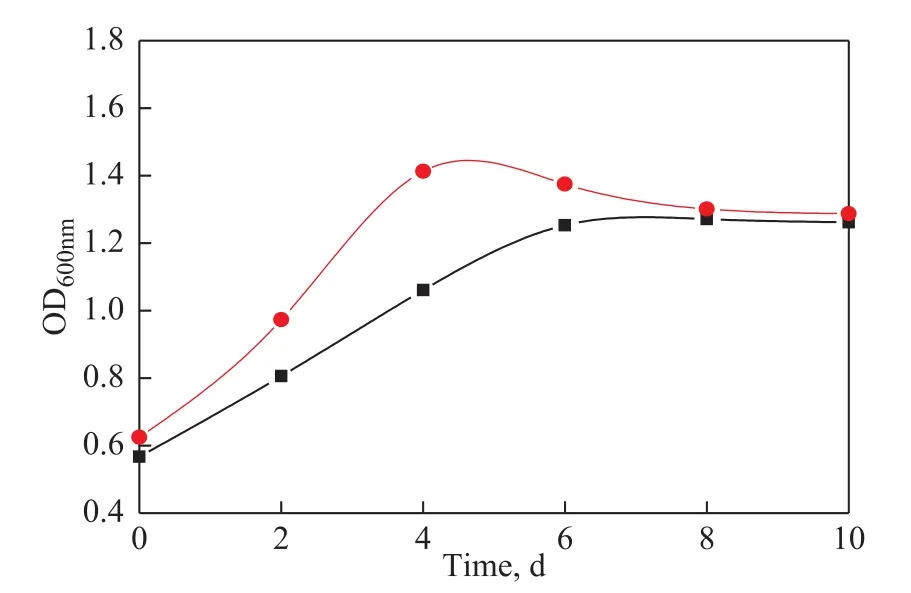
Figure 5 Variation of OD600 with biodegradation time
3.2 Anti-wear properties
Figure 6 displays the variation of WSDs with different contents of MDEACP in base oil under a rotary speed of 1 500 r/min and at a load of 392 N for 30 min. Figure 6 shows that MDEACP offers lower WSDs than those of the neat mineral oil. Especially at a MDEACP content of 1.0%, the WSD decreases from 0.64 mm to 0.42 mm. The results indicate that MDEACP obviously improves the anti-wear property of base oil which may be attributed to the protective films formed from a series of tribochemical reactions occurring during the friction process. With the concentration of MDEACP increasing to 1.0%—2.0%,the WSDs increase slowly because of corrosive wear of MDEACP.
Figure 7 gives the impacts of different loads on WSDs of steel ball lubricated by base oil with and without 1.0% MDEACP at a rotary speed of 1 500 r/min for 30 min, while being subjected to different loads,namely, 196 N, 294 N, 392 N, and 490 N, respectively.Figure 7 shows, in comparison with the base oil,MDEACP provides smaller WSDs at an applied load ranging from 196 N to 490 N. The results suggest that MDEACP possesses good anti-wear properties in mineral base oil. Furthermore, WSDs increase with the increase of loads. The reason may be that, with the increase of loads, the molecular chains of MDEACP are decomposed sharply and become shortened. Therefore the strength of protective film of friction surface is weakened[21].
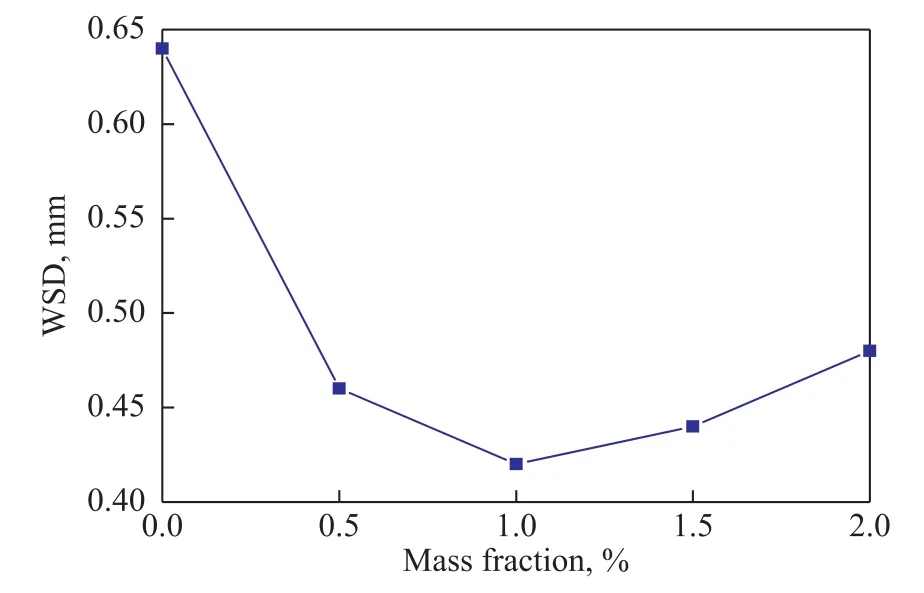
Figure 6 Variation of WSDs with different mass fraction of MDEACP
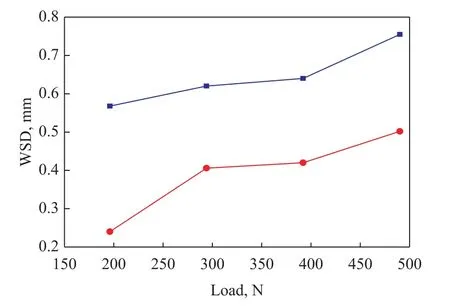
Figure 7 Variation of WSDs with different loads
3.3 Friction-reducing properties
Figure 8 shows the variation of friction coefficient with different contents of MDEACP at a rotary speed of 1 500 r/min and a load of 392 N for 30 min. It can be found that MDEACP, incorporated into base oil at different contents, can achieve smaller friction coefficient than the neat base oil. Especially when the concentration of MDEACP increases to 1.0%, the friction coefficient decreases sharply from 0.089 to 0.065. The results indicate that MDEACP boosts significantly the frictionreducing properties of 400SN base oil.
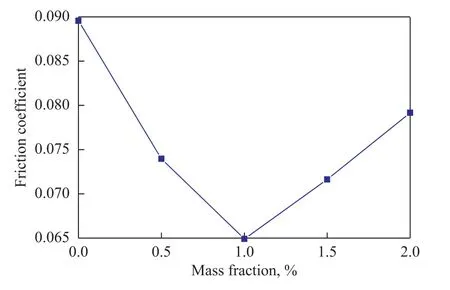
Figure 8 Variation of friction coefficient with different mass fraction of MDEACP
3.4 Extreme pressure properties
The maximum non-seizure load (PB) and the welding load (PD) are the important characteristics of extreme pressure properties. Figure 9 and Figure 10 display that thePBvalues andPDvalues vary with different mass fraction of MDEACP, respectively. It can be seen from Figure 9 and Figure 10 that thePBvalues andPDvalues increase with an increasing content of MDEACP in the range of 0.5% to 2.0%. When the content of MDEACP increases to 2.0%, thePBvalue grows from 495 N to 726 N, and consequently thePDvalues rises from 1 236 N to 1 961 N. The results reveal that MDEACP boosts the extreme pressure properties of 400SN base oil.
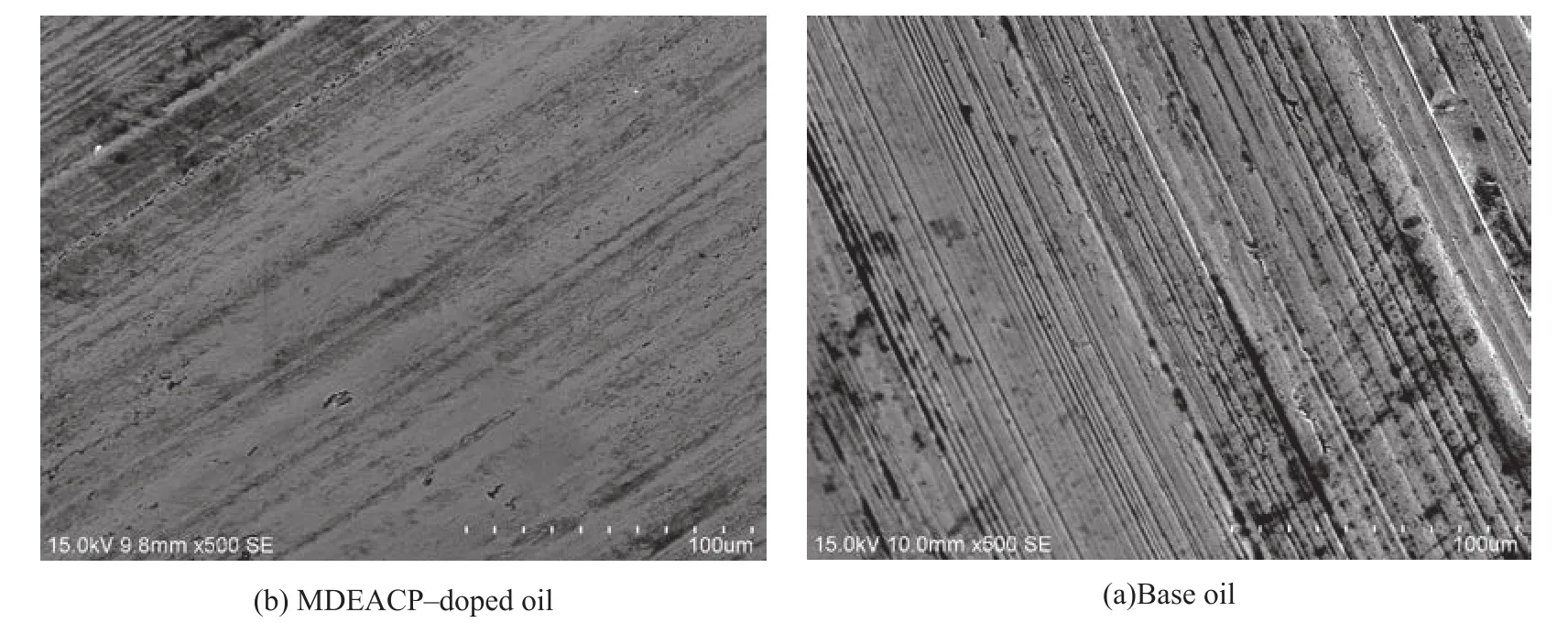
Figure 11 SEM images of the worn surfaces lubricated with base oil and MDEACP-doped oil
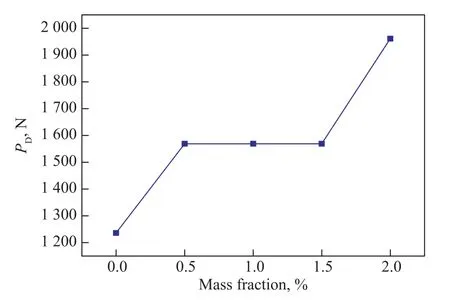
Figure 10 Variation of PD values with different mass fraction of MDEACP
3.5 SEM analysis
Figure 11 shows the SEM morphology of the worn surfaces of steel balls lubricated by the 400SN base oil and the oil containing 1.0% of MDEACP under a load of 392 N and at a rotary speed of 1500 r/min for 30 min.It can be discovered that compared with the neat base
oil (Figure 11(a)), the worn surface lubricated with the MDEACP-doped oil (Figure 11(b)) is smoother and the furrow is shallower. The results are consistent with better anti-wear and friction-reducing properties of the oil containing MDEACP referred to in Sections 3.2 and 3.3.
3.6 XPS analysis
Figure 12 shows the XPS spectra of worn surfaces lubricated with the oil containing 1.0% of MDEACP at a rotary speed of 1 500 r/min and under a load of 392 N for 30 min. The XPS spectrum of C1s displays a peak in the binding energy range of 284.6 eV-288.6 eV, which is ascribed to the C-H and COO- species and suggests that MDEACP is adsorbed on the friction surface. The peak of O1s at the binding energy of 531.9 eV may be attributed to P=O or iron oxide. In the spectrum of Fe2p,the peak at the binding energy of 710.7 eV indicates that the iron is oxidized into Fe2O3or Fe3O4. The peak of N1s around the binding energy of 399.5 eV may be attributed to the organic nitrogen-containing compounds, which can further testify the adsorption of MDEACP on the friction surface. The peak of P2p at the binding energy of 133.2 eV may be ascribed to Fe3(PO4)2or FePO4,showing that MDEACP reacts tribochemically with the friction surface.The results show that the improvements in anti-wear and friction-reducing properties are caused by the formation of a complex boundary lubrication films composed of the adsorption film and tribochemical species such as Fe2O3,Fe3O4, FePO4and Fe3(PO4)2resulted from the tribochemical reaction of MDEACP taking place on the worn surface.
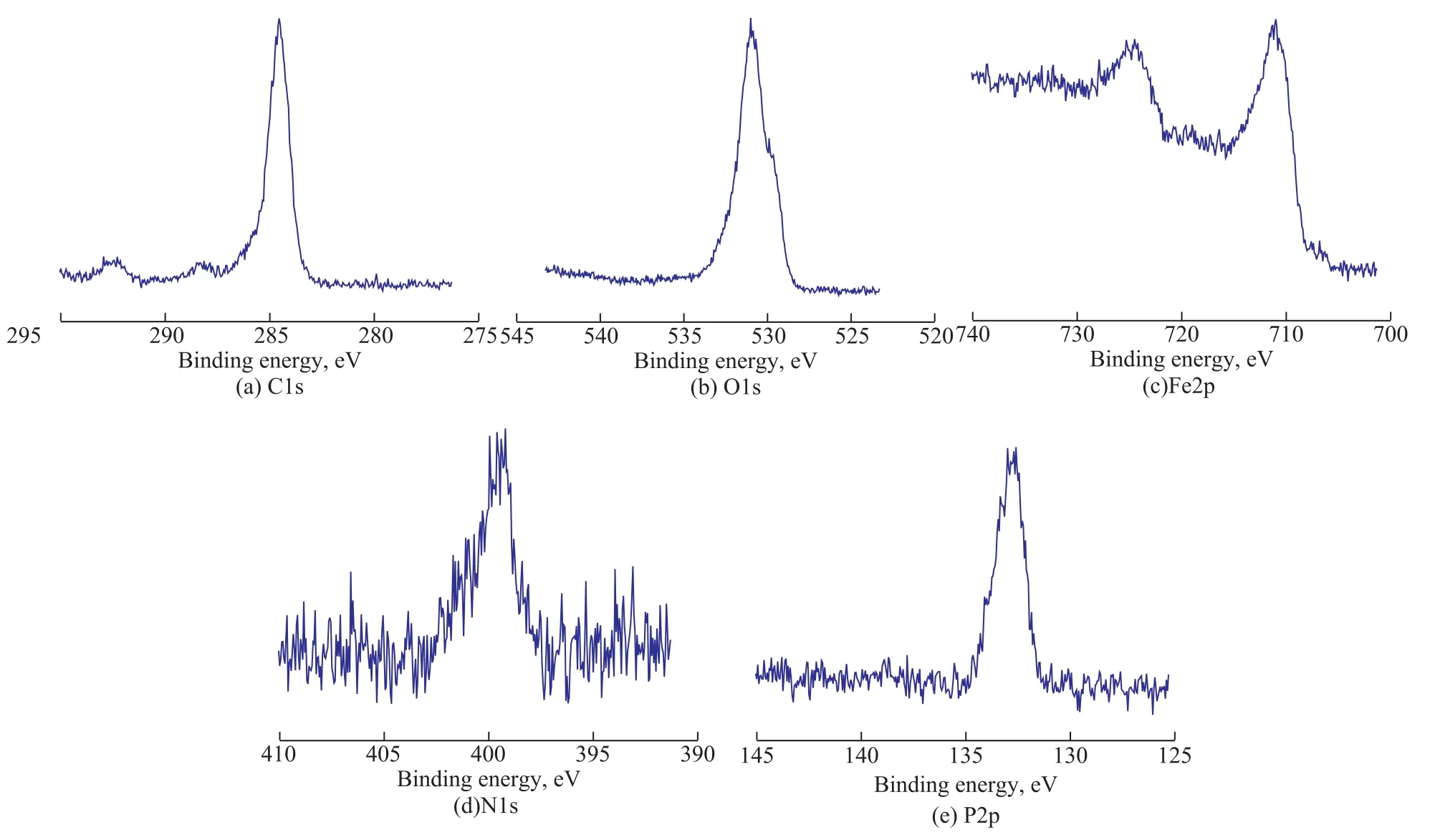
Figure 12 XPS spectra of typical elements on worn surfaces lubricated with MDEACP-doped oil
4 Conclusions
Caprylic methyl diethanolamine phosphate ester(MDEACP) is prepared, with its chemical structure characterized by the FT-IR spectroscopy. MDEACP accelerates the biodegradation of 400SN base oil and the BDI is improved by 31.8% at an optimum MDEACP content of 1.0%. Furthermore, MDEACP boosts the antiwear properties, the friction-reducing properties, and the extreme pressure properties of the base oil. At an optimum dosage of MDEACP, the WSD decreases from 0.64 mm to 0.42 mm, thePBvalue increases from 495 N to 726 N, and thePDvalue rises from 1 236 N to 1 961 N.The improvements in anti-wear and friction-reducing performance of the MDEACP-doped oil may be ascribed to the formation of the complex boundary lubrication film made of adsorbed MDEACP and tribochemical species such as Fe2O3, Fe3O4, FePO4, and Fe3(PO4)2.
Acknowledgments:The authors are grateful to the financial support from the National Defense Science Technology Foundation (Project No.3604003), the National Natural Science Foundation of China (Project No.51375491), the Natural Science Foundation of Chongqing (Project No. CSTC,2014JCYJAA50021), the Postgraduate Research and the Innovation Project of Chongqing (No. CYB 18128), and the Natural Science Foundation of Chongqing (Project No. CSTC, 2017JCYJAX0058).
- 中國煉油與石油化工的其它文章
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Numerical Simulation of Optimization of Mixing Tank for Residue Upgrading Reactor
- Enhanced Anti-Wear Property of Low Viscosity Engine Oil for Sequence IVB Engine Test Meeting the GF-6 Specification
- Study on Flue Gas Desulfurization Process with Selective SO2 Removal by N-formylmorpholine
- Research and Application of Image Recognition Technology in Microscopy Diagnosis of Catalytic Cracking Catalysts
- Evaluation of Inocula and Packing Material for Acceleration of Start-Up in Biofilters

