Immunoregulation of KangAi injection combined with chemotherapy in advanced non-small cell lung cancer:a systematic review and meta-analysis
Liu Pu, Wei-Hao Chen, Kun-Ji Wu, Ji-Huan Lin, Cheng-Lu Li, Shu-Lian Chen, Lu-Xi Cao,Shi-Qi Wang, Shu-Jun Lin, Yi-Min Zhang,*, Ming-Min Zhu,*
1Traditional Chinese Medical College, Jinan University, 601 Huangpu West Avenue, Guangzhou,510632, China.2College of Stomatology, Jinan University, 601 Huangpu West Avenue, Guangzhou,510632, China.
# These authors contributed equally to this work
Abstract
Keywords:Non-small cell lung cancer, KangAi injection, Immunoregulation
Introduction
In the most recent WHO estimate, lung cancer remains the leading cause of cancer death globally [1, 2], with 18 million people diagnosed with lung cancer each year and 16 million of them dying of lung cancer.5-year survival rates vary from 4-17% depending on the stage and regional differences [3].NSCLC, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, is the most common type (80%) of lung cancer [4].The treatment of NSCLC mainly consists of chemotherapy, radiotherapy, and surgery.Unfortunately,a large number of diagnosed patients have not received a good treatment opportunity for surgery because of the poor medical technology in the past few years [5].Despite recent advances in treatment, the prognosis of patients diagnosed with advanced NSCLC remains poor[6, 7].Although the efficacy of chemotherapy is exact,its side effects cannot be ignored [8].Not only can Chemotherapy kill tumor cells, but they also damage normal human cells and impair immune function [9].Studies [10, 11] have shown that KA injection combined with chemo has a relatively clear auxiliary anti-cancer effect, mainly including enhancing and regulating the cellular immune function and reducing the side effects of chemotherapy.Nevertheless, there is still a lack of systematic evaluation criteria for the effects of KA injection combined with chemo on the immune function of advanced NSCLC.Therefore, the meta-analysis aimed to investigate whether KA injection plus chemo can regulate immunoregulation,improve clinical efficiency, enhance KPS score, and reduce adverse reactions in patients with advanced NSCLC compared with chemo alone.
Method
Search strategy and selection criteria
We searched seven databases, PubMed, EMBASE,ENTRAL, MEDLINE, CNKI, Wanfang data, and VIP database.The language of the studies was not limited.In addition, we also manually searched the literature,using the list of references in the main literature,published in Chinese or English.
Inclusion and Exclusion criteria Research Object
All patients, regardless of gender and age, were confirmed by histopathology and met the relevant criteria for NSCLC established by the International Union against Cancer (UICC) in 1997, excluding other diseases affecting the test results, and were able to receive chemotherapy.
Type of study and Interventions
Our study included all the randomized controlled trials(RCTs) comparing KA injection plus chemo with chemo alone in the treatment of NSCLC.In the RCTs,the control group was treated with chemo, and the experimental group was given KA injection on the basis of the control group.The experimental requirements of the experimental design reflected the individual effects of KA injection.There were no limits to the dose and duration of treatment in both groups.
Type of Outcome Measures
The primary outcome contained the immune function(CD3+, CD4+, CD8+,and CD4+/CD8+), clinical efficient,KPS (Karnofsky Performance Status) score and adverse events including white blood cell (WBC) reduction,thrombocytopenia, neutropenia, gastrointestinal reactions, myelosuppression, liver damage.It is determined by the WHO that CR (complete remission)and PR (partial remission) are considered clinically contributing.
Data Extraction
The data were extracted independently by two reviewers (Liu Pu and Weihao Chen).We initially excluded documents that did not meet the inclusion criteria by examining the title and abstract.Ultimately,according to the full text, we removed literature that cannot be judged by headlines and abstracts.During the evaluation process, the author's name and organization were hidden to prevent subjectivity.We resolved all the differences through discussions with the third commentator (Yimin Zhang).The following information was extracted:first author, year of publication, sample size, patient age, KPS score, and immunological function measurements including CD3+,CD4+, CD8+, and CD4+/CD8+(mean [SD]), adverse events.
Study quality evaluation
Two evaluators (Kun-ji Wu and Ji-huan Lin) assessed risk, including research bias based on the Cochrane bias risk tool.There are six aspects:(1) selection bias(random sequence generation and allocation concealment); (2) performance bias (participant and personnel constraints) (3) Detection bias (blind method for evaluation of results); (4) Consumption deviation(incomplete date of results); (5) Report bias (selective report); (6) Other biases (other potential bias).We have resolved all the differences by reaching a consensus with the third author (Yimin Zhang).
Data Analysis
We use Review Manager (RevMan) and Stata/SEversion14.0 software to aggregate and analyze data.We apply a 95% confidence interval (CI) to calculated mean differences (MD) and relative risk(RR), comparing successive and dichotomous variables,respectively.Calculate ways include Cochran's Q-statistics and I2-statistics.Heterogeneity in the study was tested by Cochran's Q-statistic and I2-statistic.If there was significant heterogeneity, the random effect models were used to synthesize the data.Otherwise, the fixed effect models were applied.If more than 10 studies were included, we use the funnel plot and the Egger or Harbord's modified test to evaluate the publication.
Results
Study Selection
We obtained 359 articles based on PubMed, EMBASE,ENTRAL, MEDLINE, CNKI, Wanfang, VIP database,and manual search.Excluding duplicated documents,there were 132 articles left.Firstly, we removed 95 articles by reading the title and abstract.The deleted literature included 5 animal studies, 38 theoretical studies, 39 reviews, and 13 in-NSCLC and KA injection.We then eliminate other 26 unqualified documents because of their incomplete data, unreasonable design,and non-RCTs after reading the full text.Finally, 11 eligible documents [12-22] were remained (Figure 1).
Study Characteristics
A total of 1060 patients were enrolled in the literature,including 539 in the experimental group and 531 in the control group.Because KA injection-based therapies were mainly used either in traditional Chinese medicine(TCM) or integrated Chinese and Western medicine,all researches were from China and published in Chinese.All the studies were RCTs, in which the experimental group was given a therapy of KA injection combined with chemo, and the control group was treated with chemo alone.The common chemotherapy regimens were as follows:GP regimen (gemcitabine +cisplatin), DP regimen (docetaxel + cisplatin), TP regimen (taxol + cisplatin), and NP regimen (navelbine + cisplatin).All mentioned studies reported immune function.Ten studies reported clinical effectiveness[12-18, 20-22], three studies described KPS [14, 15, 20]and seven articles discussed adverse effects [12, 14-19].The basic information and details about the characteristics of 11 studies were listed in Table 1.
Outcome measures
Immune Function
Nine studies containing 900 patients reported CD3+,one of the biomarkers of immune function.Tested by heterogeneity, there was a high degree of difference(Chi2=143.03, I2=94%,P<0.00001).The random-effects model was therefore adopted for analysis.The results showed that the combination of KA injection and chemo was more effective in increasing the expression of CD3+when compared with chemo alone (MD=7.14,95% CI:4.00-10.29,P<0.00001) (Figure 2).
There were nine studies involving 900 cases discussed CD4+.The heterogeneity test showed a statistically significant difference between the two groups(Chi2=125.08, I2=94%,P<0.00001).We chose a random effects model to analyze MD and 95%CI.The results indicated that compared with chemo alone, KA injection combined with chemo could significantly promote the expression level of CD4+(MD=7.13,95%CI:5.19-9.06,P<0.00001) (Figure 2).
The expression levels of CD8+ were described in seven studies, which included 288 cases.The results presented that there was significant evidence in heterogeneity between-study (Chi2=204.32, I2=97%,P<0.00001).Therefore, we used random-effects models to calculate MD and 95% CI.The results illustrated that there was a statistically significant difference between two groups and KA combined with chemo significantly decreased CD8+expression when compared with chemo alone(MD=-3.14, 95%CI:-6.00- -0.27,P=0.03) (Figure 2).Ten studies containing 990 participants reported CD4+/CD8+.Heterogeneity test exhibited high heterogeneity between studies (Chi2=265.74, I2=97%,P<0.00001).So, the random-effect models were applied to synthesize MD and 95%CI.The results indicated that there was a statistically significant difference between the group of KA injection combined with chemo and chemo group alone.Besides, the group with KA injection significantly increased CD4+/CD8+expression when compared with chemo alone (MD= 0.39, 95% CI:0.22-0.56,P<0.00001) (Figure 2)
NK, a parameter of immune function, was described in three studies involving 259 patients.The heterogeneity results showed strong differences in studies (Chi2=19.74, I2= 90%,P<0.00001).A random effects model was applied to assessed MD and 95%CI.The results revealed that there was a statistically significant difference between the chemo group and KA injection combined with chemo group, and KA injection combined with chemo significantly increased the expression level of NK (MD =6.34, 95%CI:3.61-9.07,P<0.00001) (Figure 2).
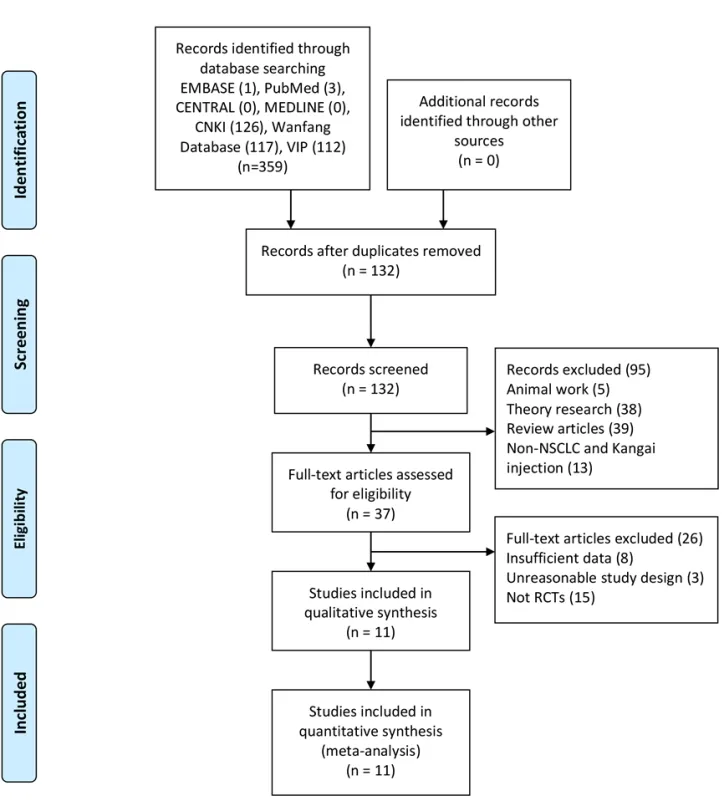
Figure 1 Flow diagram of the study selection
All the studies, covering 1060 cases, reported immune function.There was significant evidence of heterogeneity between studies (Chi2=24875.76,I2=100%,P<0.00001).

Table 1 Characteristics of the 11 included studies
Clinical efficiency
There were clinically effective reports in 10 studies,with 959 participants contained.Heterogeneity results showed nothing difference (Chi2=6.16, I2=0%,P=0.88).We therefore chose a fixed effect model.The analysis showed that KA injection combined with chemo significantly enhanced clinical efficiency compared with chemo alone in patients underwent NSCLC(RR=1.37, 95%CI:1.19-1.56,P<0.00001) (Figure 3).
KPS Score
Three studies facing 240 patients evaluated KPS scores.Based on the heterogeneity results, a fixed effect model was selected for analysis.The results of the analysis showed that there was a statistically significant difference between the two groups (Chi2=0.26,I2=0%,P=0.88).Analysis results indicated that KA injection combined with chemo significantly improved KPS score in patients with NSCLC when compared with the chemo alone (RR=1.86, 95%CI:1.35-2.57,P=0.0002)(Figure 4).
The random effects models were applied to the analysis.The results showed a statistically significant difference between the two groups.Compared with chemo alone,KA combined with chemo significantly improved immune function in patients with NSCLC (MD=3.18,95%CI:2.36-4.00,P<0.00001) (Figure 2).
Adverse event
Seven studies with 709 cases reported adverse gastrointestinal reactions.No heterogeneity between-study was observed (Chi2=9.94,I2=40%,P=0.13).The results showed that KA injection combined with chemo could significantly alleviate gastrointestinal discomfort in patients with NSCLC compared with chemo alone (RR =0.63, 95% CI:0.49-0.80,P=0.0002) (Figure 5).
Four studies involving 386 participants discussed thrombopenia.No heterogeneity was detected between the two groups (Chi2=4.14,I2=27%,P=0.25).And the results revealed that compared with chemo alone, KA injection combined with chemo significantly improved thrombopenia on patients suffering from NSCLC(RR=0.50, 95%CI:0.37-0.67,P<0.00001) (Figure 5).
Five studies with 487 described white blood cell(WBC) reaction.The results showed that there were some heterogeneity between the two groups(Chi2=11.77, I2=66%,P=0.02).
As the results reflected, there was a statistically significant difference between two groups and KA injection combined with chemo significantly regulated the WBC reduction in patients troubled by NSCLC when compared with chemo alone (RR =0.58, 95%CI:0.44-0.77;P=0.001) (Figure 5).
Adverse effects about bone marrow suppression were reported in 2 studies, including 222 cases.No heterogeneity between-study was discovered (Chi2=0.20,I2=0%,P=0.65).As is shown in the results, there was an obvious statistically difference between the two groups.Moreover, compared with chemo alone KA injection combined with chemo effectively adjusted the bone marrow suppression in patients with NSCLC (RR=0.48,95%CI:0.28-0.82,P=0.007) (Figure 5).Four studies including 407 patients discussed liver function.There was no heterogeneity between-study(Chi2=0.70,I2=0%,P=0.87).Also, the results indicated that there was a statistically significant difference between two- group and KA injection combined with chemo significantly enhanced the liver function on patients with NSCLC when compared with chemo alone(RR=0.50, 95%CI:0.32-0.78,P=0.002) (Figure 5)
Seven studies, with a total of 707 participants were enrolled.Heterogeneity between studies was not observed (Chi2=33.28, I2=37%,P=0.04).The results showed that KA injection combined with chemo significantly reduced the incidence of side-effect events in patients with NSCLC compared with chemo alone(RR = 0.57, 95% CI:0.50-0.65,P<0.00001) (Figure 5).

Figure 2 Forest plot of immune function
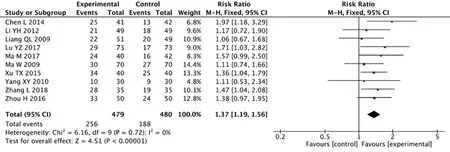
Figure 3 Forest plot of improved clinical response rate
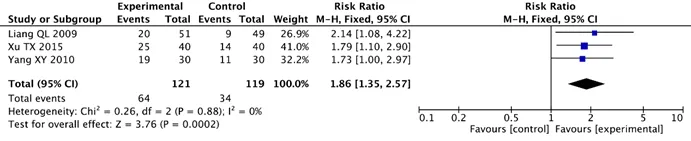
Figure 4 Forest plots of KPS score
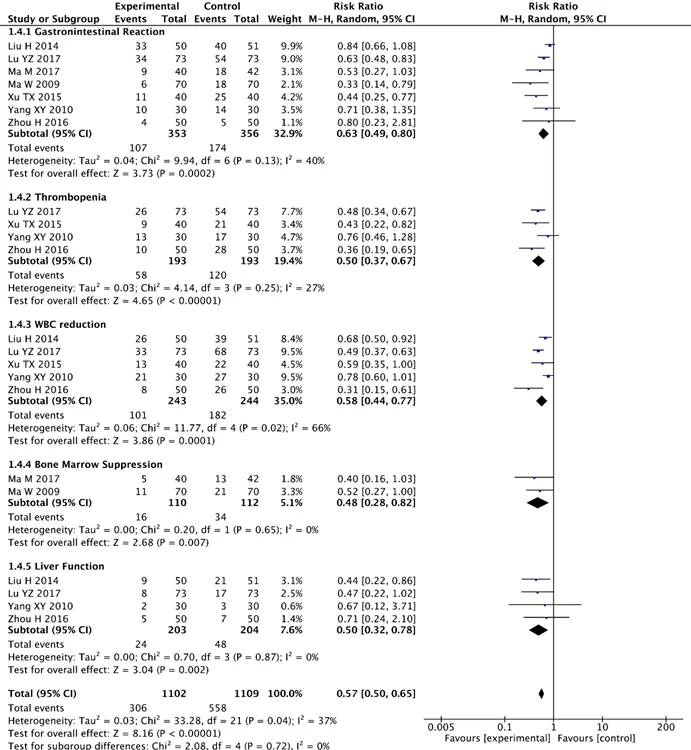
Figure 5 Forest plots of adverse effects
Risk of bias
Randomization was mentioned in all studies, three of which reported specific random methods and were classified as low-risk bias.However, eight studies did not describe specific stochastic methods and were therefore classified as unknown risks.No studies reported allocation concealment, participant blinding,and assessment of personnel and outcomes.All studies provided complete results data.All included studies with no selective reports and other sources of bias were classified as low-risk biases (Figure 6 and 7).
Publication bias
The funnel plot was used to analyze the publication bias of the included literature.The results of Harbord's modified test of immune function showed no potential publication bias (P=0.296>0.05).The eager analysis revealed that there was no publication bias in clinical efficacy (P=0.8286>0.05), KPS score (P=0.373>0.05),and adverse events (P=0.146>0.05) (Figure 8).

Figure 6 Risk of bias graph
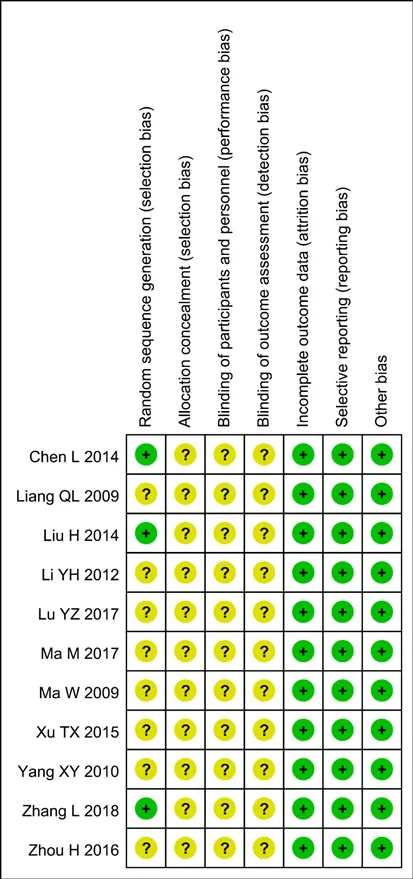
Figure 7 Risk of bias summary of included studies
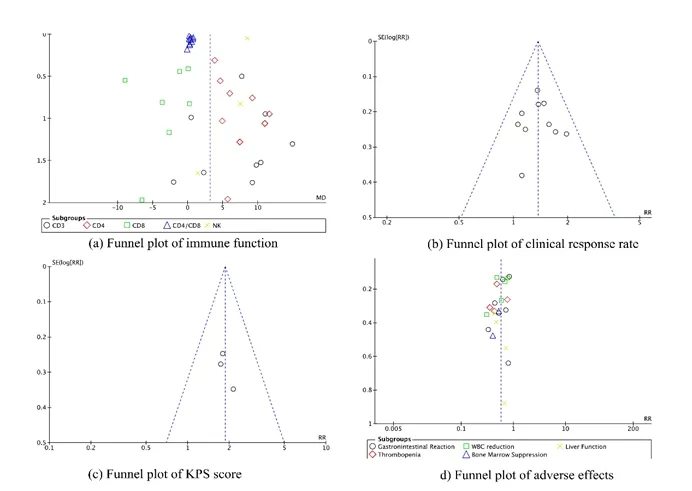
Figure 8 Funnel plot
Discussion
Despite progress in effective chemotherapeutic agents and driving mutation targeting agents, the prognosis remains frustrating [23].The immune system is the first line of defense against cancer [24].It has been reported that CD4+and CD8+T cells play a central role in the study of adaptive immune responses that protect against tumors [25].As demonstrated in our results, compared with chemo alone, KA combination group indeed improved immune function of patients with NSCLC,with an increase in the expression levels of CD3+, CD4+,and NK, and a reduction in CD8+.Furthermore,compared to a separate chemo regimen, KA injection combined with chemo also enhanced clinical efficiency;increased KPS score, and reduced the occurrence of adverse events.Security thereby, was improved.
Chinese herbal medicine against cancer has received extensive attention because of its low drug resistance and toxicity [26].KA injection, combined with first-line platinum-based chemotherapy drugs for the treatment of advanced NSCLC has been widely used [27].The State Food and Drug Administration approved it.(Approval No.WS-11222 (ZD-1222)-2002-2012Z).In modern research, several biological mechanisms can explain the protective effects of KA injection on advanced NSCLC.Pharmacological studies have shown that KA injection contains a variety of chemical components, refined and extracted by modern scientific methods, such as astragalus polysaccharide, ginsenoside, and matrine.
Astragalus polysaccharide works by inhibiting tumor cell proliferation [28], inducing tumor cell apoptosis[29], and regulating cell cycle growth [30].Studies have discovered that Astragalus root extract can markedly enhance the tumoricidal activity of peritoneal macrophages.At the same time, it can be used as an initiator of TNF production in some tumor-bearing mice to restore the suppressed immune function, induce the production of anti-tumor cells in vitro and enhance the body's immune function [31, 32].The immune function of the body is closely related to the occurrence and development of tumors [33].CD4+is a helper T cell that plays a major role in assisting and inducing the immune response of the body, while CD8+ exerts an inhibitory effect.When the body is in a normal state, CD4+/ CD8+are relatively stable.In most cancers, the balance between CD4+and CD8+is broken, and the immune function of the body cannot function [34, 35].Moreover,studies revealed that astragalus polysaccharide could induce the occurrence of dendritic cells (DC) and stimulate the proliferation of allogeneic peripheral blood T lymphocytes, suggesting that astragalus polysaccharide can promote maturation and antigen presentation of DC and specificity activate T cells to produce cellular immunity as well.Macrophages are effector cells, which play an important role in the body's non-specific immune defense.Studies discovered that astragalus polysaccharide could stimulate the release of cytokines (TNF-α, IFN-γ,et al.) by activating macrophages, thereby exerting anti-tumor effects[36-38].Furthermore, Studies showed that the ethyl acetate fraction of astragalus membranaceus has the strongest inhibitory effect on the cell survival and proliferation in NSCLC cell lines with different genetic backgrounds,and expresses anticancer activity induced by apoptosis in NSCLC cells [39].The ginsenosides successfully isolated from ginseng contain more than forty kinds, of which the ginsenoside Rg3 monomer has a better effect on tumors.Matrine is one of the pure natural alkaloids, and its pharmacological effects are wide, especially in the field of anti-tumor.Rg3 and matrine may inhibit tumors by reducing tumor cell proliferation activity [40, 41], promoting tumor cell apoptosis [35, 42], slowing the rate of angiogenesis in tumors [43, 44].
The meta-analysis was the first systematic review of the effects of KA injection combined with chemo on immunoregulation in patients with NSCLC.The advantages of our meta-analysis included many specific outcomes of immune function (CD3+, CD4+, CD8+, and CD4+/CD8+, NK), clinical response rate, KPS scores,and adverse effects (gastrointestinal reaction,thrombocytopenia, WBC reduction, bone marrow suppression, and liver function) for the comparison between KA injection plus chemo and chemo alone.Imperfectly, our meta-analysis had several limitations.Firstly, the methodological quality of the research was generally poor.Although most of the studies described are random, only two studies describe specific random methods, and one study describes the wrong approach to random sequence generation.None of the studies included described participant allocation concealment and blind methods, as well as personnel and outcome assessment.Secondly, all the included studies are published in Chinese, which may lead to racial prejudice.However, it is necessary to incorporate more diverse population samples into this meta-analysis and to obtain richer and more reliable results.Therefore,given the limitations of this study, randomized controlled trials with high methodological quality, good experimental design, and large sample size are needed to investigate the clinical efficacy and safety of KA injection in the treatment of NSCLC.
- Cancer Advances的其它文章
- Traditional Chinese Medicine for Treatment of Apatinib-induced Hand and Foot Skin Reaction:A case report
- Therapeutic effect of Liyan Baidu decoction combined with oxygen spray on radioactive stomatitis in patients with nasopharyngeal carcinoma
- Anti-tumor mechanism of Angelica and its compound preparations
- Research progress on radiation-induced bystander effect
- Study on the technical specification of Miao doctor's medicinal acupuncture therapy for interventional cancer pain

